Endoscopic vs. External Dacryocystorhinostomy in Granulomatosis with Polyangiitis: A Scoping Review of the Literature and Our Experience with Endoscopic Dacryocystorhinostomy
Abstract
1. Introduction
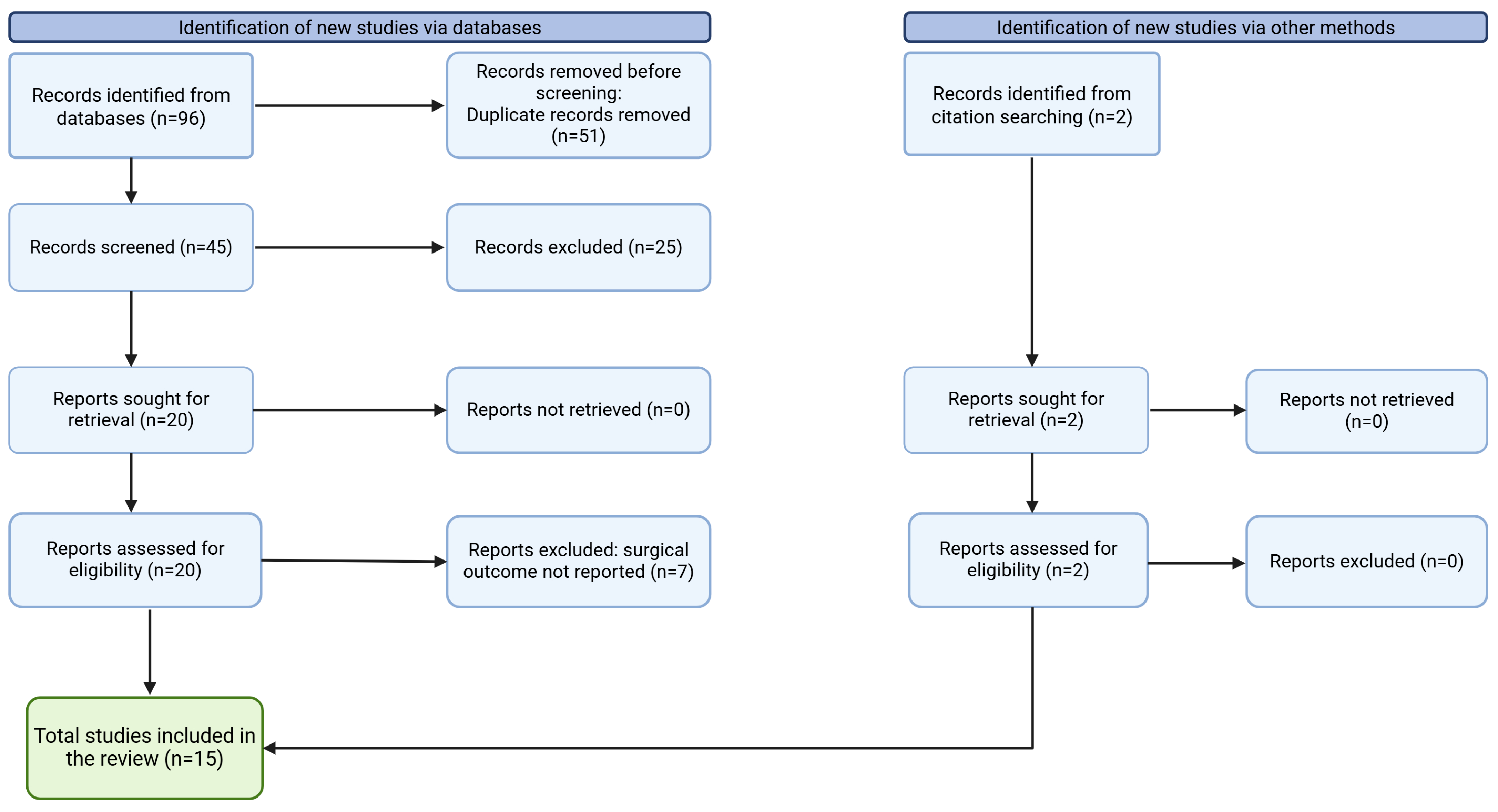
2. Materials and Methods
3. Results
3.1. External Dacryocystorhinostomy
3.2. Endoscopic Dacryocystorhinostomy
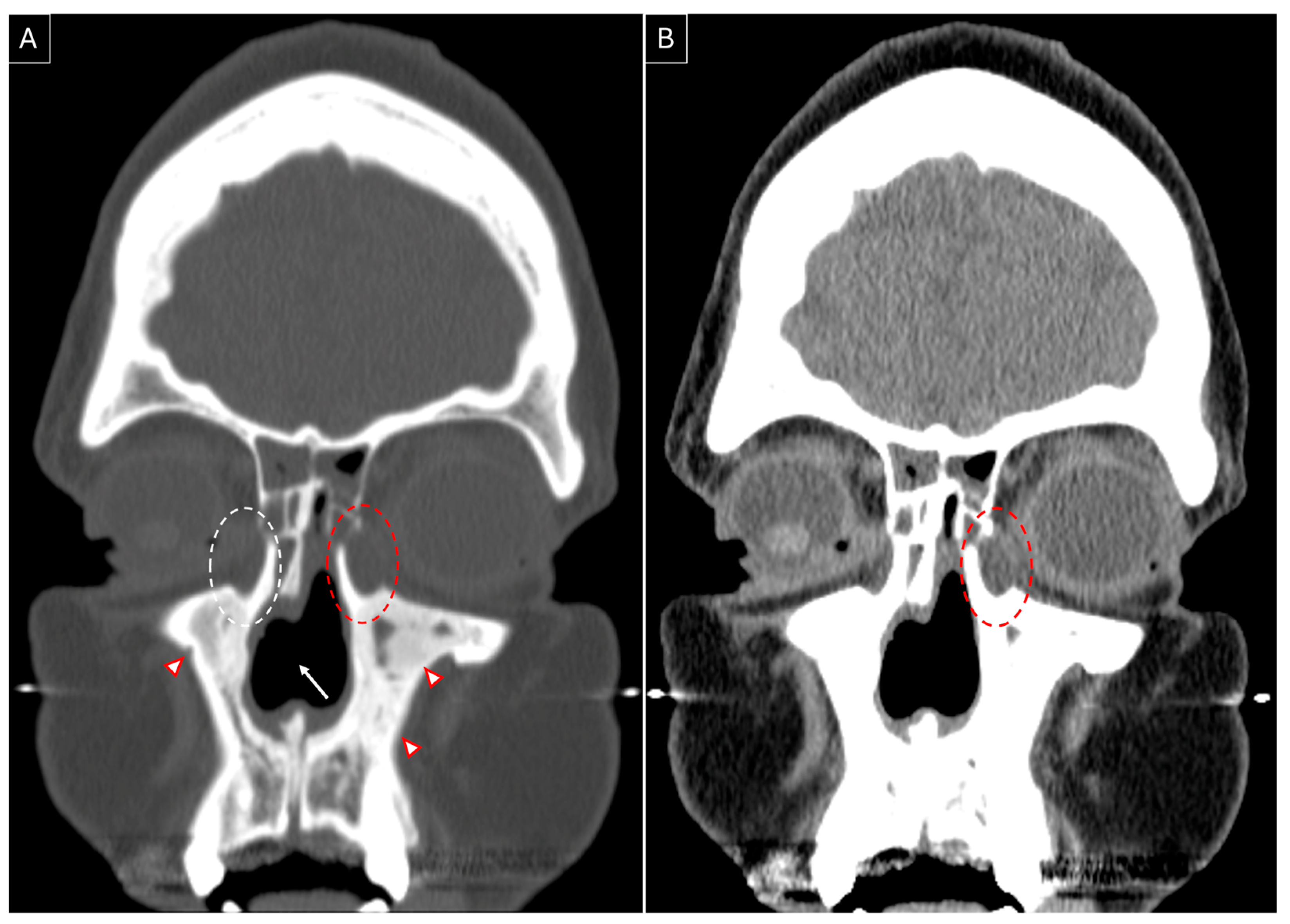
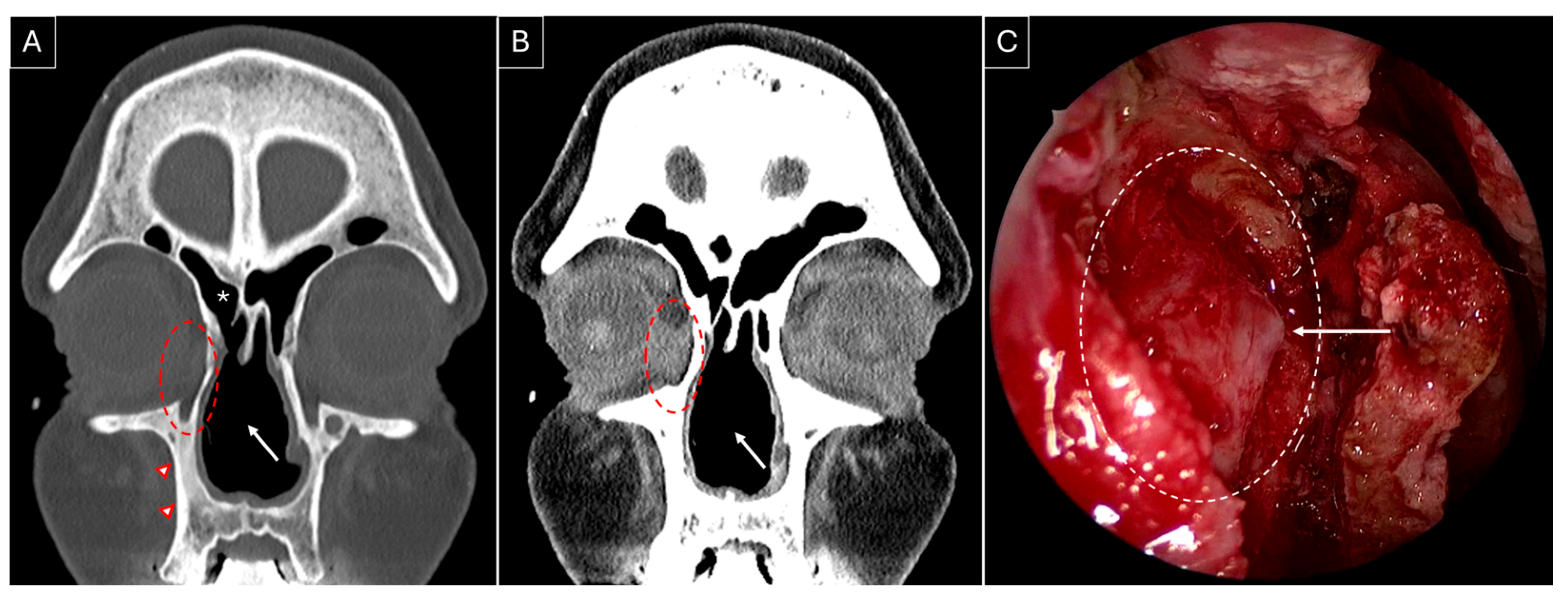
3.3. Our Experience: Two Patients of GPA Who Underwent Endoscopic DCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| DCR | Dacryocystorhinostomy |
| NLDO | Nasolacrimal duct obstruction |
| GPA | Granulomatosis with polyangiitis |
| PRISMA-ScR | Preferred Reporting Items for Systematic Review and Meta-Analysis extension for Scoping Review |
| cANCA | Cytoplasmic antineutrophilic cytoplasmic antibodies |
| pANCA | Perinuclear antineutrophilic cytoplasmic antibodies |
| CT | Computed tomography |
| DCT | Dacryocystectomy |
References
- Huang, J.; Malek, J.; Chin, D.; Snidvongs, K.; Wilcsek, G.; Tumuluri, K.; Sacks, R.; Harvey, R.J. Systematic review and meta-analysis on outcomes for endoscopic versus external dacryocystorhinostomy. Orbit 2014, 33, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.R.; Miller, D.; Anderson, R.L. Wound Necrosis Following Dacryocystorhinostomy in Patients with Wegener’s Granulomatosis. Ophthalmic Surg. 1987, 18, 800–803. [Google Scholar] [CrossRef]
- Hardwig, P.W.; Bartley, G.B.; Garrity, J.A. Surgical Management of Nasolacrimal Duct Obstruction in Patients with Wegener’s Granulomatosis. Ophthalmology 1992, 99, 133–139. [Google Scholar] [CrossRef]
- Glatt, H.J.; Putterman, A.M. Dacryocystorhinostomy in Wegener’s granulomatosis. Ophthalmic Plast. Reconstr. Surg. 1990, 6, 207–210. [Google Scholar] [CrossRef]
- Morris, D.S.; Selva, D.; Dolman, P.J. Endonasal dacryocystorhinostomy in Wegener granulomatosis. Arch. Ophthalmol. 2010, 128, 1212–1214. [Google Scholar] [CrossRef][Green Version]
- Eloy, P.; Leruth, E.; Bertrand, B.; Rombaux, P.H. Successful endonasal dacryocystorhinostomy in a patient with Wegener’s granulomatosis. Clin. Ophthalmol. 2009, 3, 651–656. [Google Scholar] [CrossRef][Green Version]
- Wong, R.J.; Gliklich, R.E.; Rubin, P.A.; Goodman, M. Bilateral nasolacrimal duct obstruction managed with endoscopic techniques. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 703–706. [Google Scholar] [CrossRef]
- Ishio, K.; Sugasawa, M.; Tayama, N.; Kaga, K. Clinical usefulness of endoscopic intranasal dacryocystorhinostomy. Acta Otolaryngol. 2007, 127 (Suppl. S559), 95–102. [Google Scholar] [CrossRef]
- Cannady, S.B.; Batra, P.S.; Koening, C.; Lorenz, R.R.; Citardi, M.J.; Langford, C.; Hoffman, G.S. Sinonasal Wegener granulomatosis: A single-institution experience with 120 cases. Laryngoscope 2009, 119, 757–761. [Google Scholar] [CrossRef]
- Sweeney, A.R.; Davis, G.E.; Chang, S.H.; Amadi, A.J. Outcomes of Endoscopic Dacryocystorhinostomy in Secondary Acquired Nasolacrimal Duct Obstruction: A Case-Control Study. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 20–25. [Google Scholar] [CrossRef]
- Kwan, A.S.L.; Rose, G.E. Lacrimal drainage surgery in Wegener’s granulomatosis. Br. J. Ophthalmol. 2000, 84, 329–331. [Google Scholar] [CrossRef][Green Version]
- Knoch, D.W.; Lucarelli, M.J.; Dortzbach, R.K.; Smith, M.E. Limited Wegener granulomatosis with 40 years of follow-up. Arch. Ophthalmol. 2003, 121, 1640–1642. [Google Scholar] [CrossRef][Green Version]
- Ghanem, R.C.; Chang, N.; Aoki, L.; Santo, R.M.; Matayoshi, S. Vasculitis of the lacrimal sac wall in Wegener granulomatosis. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Nelson, C.C.; Lewis, C.D.; Perry, J.D. External dacryocystorhinostomy surgery in patients with Wegener granulomatosis. Ophthalmic Plast. Reconstr. Surg. 2012, 28, 389–392. [Google Scholar] [CrossRef]
- De Castro, D.K.; Santiago, Y.M.B.; Cunningham, M.; Gray, S.T.; Metson, R.; Fay, A. A modified lacrimal sac implant for high-risk dacryocystorhinostomy. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 367–372. [Google Scholar] [CrossRef]
- Stewart, C.M.; Rose, G.E. External dacryocystorhinostomy in patients with granulomatous polyangiitis. Eye 2020, 34, 1382–1385. [Google Scholar] [CrossRef]
- Sfiniadaki, E.; Tsiara, I.; Theodossiadis, P.; Chatziralli, I. Ocular Manifestations of Granulomatosis with Polyangiitis: A Review of the Literature. Ophthalmol. Ther. 2019, 8, 227–234. [Google Scholar] [CrossRef]
- Wormald, P.J.; Kew, J.; Van Hasselt, A. Intranasal anatomy of the nasolacrimal sac in endoscopic dacryocystorhinostomy. Otolaryngol. Head Neck Surg. 2000, 123, 307–310. [Google Scholar] [CrossRef]
- Hurtikainen, J.; Grenman, R.; Puukka, P.; Seppgii, H. Prospective Randomized Comparison of External Dacryocystorhinostomy and Endonasal Laser Dacryocystorhinostomy. Ophthalmology 1998, 105, 1106–1113. [Google Scholar] [CrossRef]
- Bullen, C.L.; Liesegang, T.J.; McDonald, T.J.; DeRemee, R.A. Ocular Complications of Wegener’s Granulomatosis. Ophthalmology 1983, 90, 279–290. [Google Scholar] [CrossRef]
- Baddeley, P.A.; Lewis, G.D.; Lane, C.M. A novel technique to facilitate dacryocystectomy using viscoelastic substances. Orbit 2011, 30, 158–159. [Google Scholar] [CrossRef] [PubMed]
| Author Name | Year | No. of Surgeries for GPA Patients | Outcome | Follow-Up (Months) |
|---|---|---|---|---|
| External dacryocystorhinostomy | ||||
| Jordan, et al. [2] | 1987 | 2 | Wound necrosis, nasocutaneous fistula in both | |
| Glatt, et al. [4] | 1990 | 2 | Successful | 8, 16 |
| Hardwig, et al. [3] | 1992 | 10 | 6 successful, recurrence in 4 | 11–66 |
| Kwan, et al. [11] | 2000 | 15 | 13 successful, 1 resolved after revision surgery, 1 failed | 3–96 |
| Knoch, et al. [12] | 2003 | 2 | Successful | 108 |
| Ghanem, et al. [13] | 2004 | 1 | Successful | 12 |
| Lee, et al. [14] | 2012 | 18 (16 primary, 2 revision) | Successful | 10–72 |
| De Castro, et al. [15] | 2013 | 2 | Successful | 30 (mean) |
| Stewart, et al. [16] | 2019 | 70 | 64 successful, rest resolved after revision surgery | 3–14 |
| Endoscopic dacryocystorhinostomy | ||||
| Wong, et al. [7] | 1998 | 2 | Successful | 5 |
| Ishio, et al. [8] | 2007 | 1 | Successful, recurrence of symptoms resolved after short endonasal procedure | 16 |
| Eloy, et al. [6] | 2009 | 1 | Successful | 6 |
| Cannady, et al. [9] | 2009 | 7 | Successful | 50 (mean) |
| Morris, et al. [5] | 2010 | 5 | Successful | 9–60 |
| Sweeney, et al. [10] | 2018 | 6 | Successful | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Marks, L.A.; Lança Gomes, P.; Lal, D. Endoscopic vs. External Dacryocystorhinostomy in Granulomatosis with Polyangiitis: A Scoping Review of the Literature and Our Experience with Endoscopic Dacryocystorhinostomy. J. Pers. Med. 2025, 15, 278. https://doi.org/10.3390/jpm15070278
Kumar N, Marks LA, Lança Gomes P, Lal D. Endoscopic vs. External Dacryocystorhinostomy in Granulomatosis with Polyangiitis: A Scoping Review of the Literature and Our Experience with Endoscopic Dacryocystorhinostomy. Journal of Personalized Medicine. 2025; 15(7):278. https://doi.org/10.3390/jpm15070278
Chicago/Turabian StyleKumar, Nitish, Lisa A. Marks, Pedro Lança Gomes, and Devyani Lal. 2025. "Endoscopic vs. External Dacryocystorhinostomy in Granulomatosis with Polyangiitis: A Scoping Review of the Literature and Our Experience with Endoscopic Dacryocystorhinostomy" Journal of Personalized Medicine 15, no. 7: 278. https://doi.org/10.3390/jpm15070278
APA StyleKumar, N., Marks, L. A., Lança Gomes, P., & Lal, D. (2025). Endoscopic vs. External Dacryocystorhinostomy in Granulomatosis with Polyangiitis: A Scoping Review of the Literature and Our Experience with Endoscopic Dacryocystorhinostomy. Journal of Personalized Medicine, 15(7), 278. https://doi.org/10.3390/jpm15070278








