Stored Intestinal Biopsies in Inflammatory Bowel Disease Research: A Danish Nationwide Population-Based Register Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Data Sources
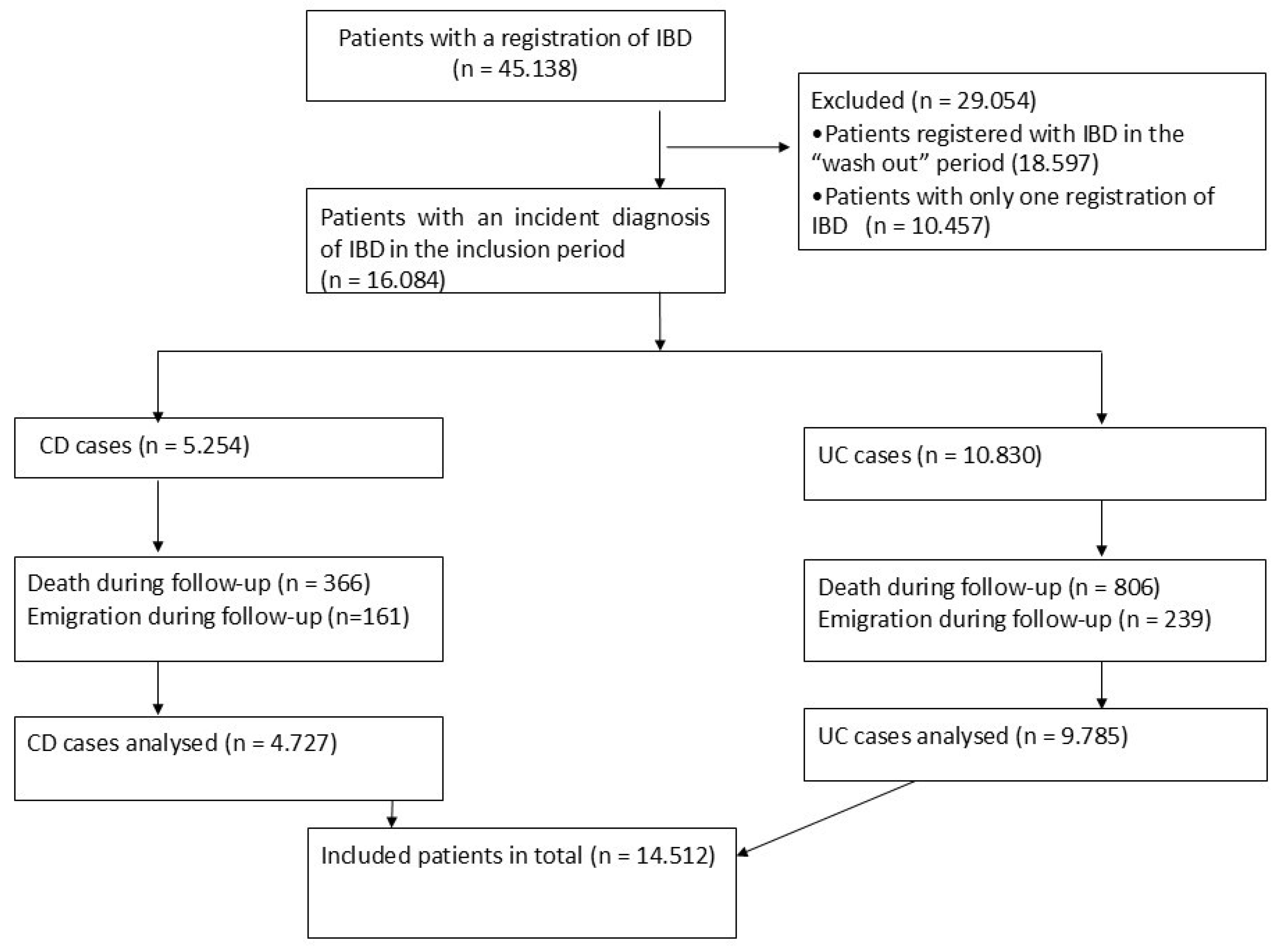
2.3. Study Population
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Proportion of Patients Having Biopsies Taken and Number of Biopsies
3.3. Gastrointestinal Location of Biopsies
3.4. Severe Disease Course and Number of Biopsy Visits
4. Discussion
4.1. Comparison with Other Similar Studies
4.2. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| CRS | Civil Registration System |
| DNPR | Danish National Patient Register |
| DPR | Danish Pathology Register |
| IQR | interquartile ranges |
| IRR | Incidence rate ratios |
References
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st Century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 56–66. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hibi, T. Improving IBD outcomes in the era of many treatment options. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 79–80. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Le Berre, C. Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. N. Engl. J. Med. 2021, 385, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gao, Z.; Li, K. Controversy of Preoperative Exposure to Tumor Necrosis Factor Inhibitors in Surgical and Infectious Complications of Inflammatory Bowel Disease. Gastroenterology 2023, 164, 307–308. [Google Scholar] [CrossRef]
- Singh, S.; Ananthakrishnan, A.N.; Nguyen, N.H.; Cohen, B.L.; Velayos, F.S.; Weiss, J.M.; Sultan, S.; Siddique, S.M.; Adler, J.; Chachu, K.A. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology 2023, 164, 344–372. [Google Scholar] [CrossRef]
- Agrawal, M.; Spencer, E.A.; Colombel, J.F.; Ungaro, R.C. Approach to the Management of Recently Diagnosed Inflammatory Bowel Disease Patients: A User’s Guide for Adult and Pediatric Gastroenterologists. Gastroenterology 2021, 161, 47–65. [Google Scholar] [CrossRef]
- Andersen, V.; Bennike, T.B.; Bang, C.; Rioux, J.D.; Hébert-Milette, I.; Sato, T.; Hansen, A.K.; Nielsen, O.H. Investigating the Crime Scene-Molecular Signatures in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 11217. [Google Scholar] [CrossRef]
- Anderson, B.R.; Gotlieb, E.G.; Hill, K.; McHugh, K.E.; Scheurer, M.A.; Mery, C.M.; Pelletier, G.J.; Kaltman, J.R.; White, O.J.; Trachtenberg, F.L.; et al. Registry-based trials: A potential model for cost savings? Cardiol. Young 2020, 30, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Pedersen, L.; Sorensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.J.; Adelborg, K.; Sundboll, J.; Laugesen, K.; Ehrenstein, V.; Sorensen, H.T. The Danish health care system and epidemiological research: From health care contacts to database records. Clin. Epidemiol. 2019, 11, 563–591. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sorensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef]
- Bjerregaard, B.; Larsen, O.B. The Danish Pathology Register. Scand. J. Public Health 2011, 39, 72–74. [Google Scholar] [CrossRef]
- Patobank. Available online: https://www.patobank.dk (accessed on 19 November 2024).
- NOMESCO. NOMESCO Classification of Surgical Procedures (NCSP), version 1.16, NOMESCO: Oslo, Norway, 2011. Available online: https://nhwstat.org/publications/ncsp-classification-surgical-procedures (accessed on 19 November 2024).
- Bernstein, C.N.; Fried, M.; Krabshuis, J.H.; Cohen, H.; Eliakim, R.; Fedail, S.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; et al. World Gastroenterology Organization Practice Guidelines for the Diagnosis and Management of IBD in 2010. Inflamm. Bowel Dis. 2010, 16, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, N.F.; Green, A.; Allin, K.H.; Iversen, A.T.; Madsen, G.I.; Pedersen, A.K.; Wolff, D.L.; Jess, T.; Andersen, V. Clinical procedures used to diagnose inflammatory bowel disease: Real-world evidence from a Danish nationwide population-based study. BMJ Open Gastroenterol. 2022, 9, e000958. [Google Scholar] [CrossRef] [PubMed]
- Donczo, B.; Guttman, A. Biomedical analysis of formalin-fixed, paraffin-embedded tissue samples: The Holy Grail for molecular diagnostics. J. Pharm. Biomed. Anal. 2018, 155, 125–134. [Google Scholar] [CrossRef]
- Bennike, T.B.; Kastaniegaard, K.; Padurariu, S.; Gaihede, M.; Birkelund, S.; Andersen, V.; Stensballe, A. Proteome stability analysis of snap frozen, RNAlater preserved, and formalin-fixed paraffin-embedded human colon mucosal biopsies. Data Brief 2016, 6, 942–947. [Google Scholar] [CrossRef]
- Longuespée, R.; Baiwir, D.; Mazzucchelli, G.; Smargiasso, N.; De Pauw, E. Laser Microdissection-Based Microproteomics of Formalin-Fixed and Paraffin-Embedded (FFPE) Tissues. Methods Mol. Biol. 2018, 1723, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Fairley, J.A.; Gilmour, K.; Walsh, K. Making the most of pathological specimens: Molecular diagnosis in formalin-fixed, paraffin embedded tissue. Curr. Drug Targets 2012, 13, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- 10xGenomics. HD Spatial Gene Expression. Available online: https://www.10xgenomics.com/support/spatial-gene-expression-hd (accessed on 19 November 2024).
- 10xGenomics. Map the Whole Transcriptome Within the Tissue Context. Available online: https://www.10xgenomics.com/products/spatial-gene-expression (accessed on 19 November 2024).
- Leko, V.; Groh, E.; Levi, S.T.; Copeland, A.R.; White, B.S.; Gasmi, B.; Li, Y.; Hill, V.; Gurusamy, D.; Levin, N.; et al. Utilization of primary tumor samples for cancer neoantigen discovery. J. Immunother. Cancer 2025, 13, e010993. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, L.C.; Daasnes, C.; Thaulow, I.; Brønnum-Hansen, H. Introduction to Danish (nationwide) registers on health and social issues: Structure, access, legislation, and archiving. Scand. J. Public Health 2011, 39, 12–16. [Google Scholar] [CrossRef]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Sauar, J.; Schulz, T.; Stray, N.; Vatn, M.H.; Moum, B.; Group, T.I.S. Change of diagnosis during the first five years after onset of inflammatory bowel disease: Results of a prospective follow-up study (the IBSEN Study). Scand. J. Gastroenterol. 2006, 41, 1037–1043. [Google Scholar] [CrossRef]
- The Danish National Center for Ethics. Registry Research Projects Without the Use of Biological Material. Available online: https://researchethics.dk/information-for-researchers/reporting-to-the-regional-research-ethics-committees/registry-research-projects-without-the-use-of-biological-material (accessed on 21 February 2025).
- OpenAI. ChatGPT (GPT-4) [Large Language Model]. 2024. Available online: https://openai.com (accessed on 19 November 2024).

| IBD | CD | UC | p-Value | |
|---|---|---|---|---|
| Enrolled participants, n (%) | 14,512 | 4727 (32) | 9785 (68) | |
| Females, n (%) | 7675 (53) | 2605 (55) | 5070 (52) | <0.001 |
| Age at IBD diagnosis (median, IQR) | 38 (25–54) | 32 (21–49) | 40 (27–56) | <0.001 |
| Patients with at least one biopsy visit, n (%) | 13,936 (96) | 4463 (94) | 9473 (97) | <0.001 |
| Patients having their first biopsy visit | <0.001 | |||
| Patients with no biopsy visit, n (%) | 576 (4) | 264 (6) | 312 (3) | |
| In the period of diagnostic process, n (%) | 13,598 (94) | 4329 (92) | 9269 (95) | |
| >1 ≤2 years after their first registration of IBD, n (%) | 144 (1) | 59 (1) | 85 (<1) | |
| >2 ≤3 years after their first registration of IBD, n (%)) | 94 (<1) | 40 (<1) | 54 (<1) | |
| >3 ≤4 years after their first registration of IBD, n (%) | 53 (<1) | 15 (<1) | 38 (<1) | |
| >4 ≤5 years after their first registration of IBD, n (%) | 47 (<1) | 20 (<1) | 27 (<1) | |
| Accumulated biopsy visits | <0.001 | |||
| 0, n (%) | 576 (4) | 264 (6) | 312 (3) | |
| 1, n (%) | 4452 (31) | 1295 (27) | 3157 (32) | |
| 2, n (%) | 3843 (27) | 1294 (27) | 2549 (26) | |
| 3, n (%) | 2381 (16) | 849 (18) | 1532 (16) | |
| >3, n (%) | 3260 (23) | 1025 (22) | 2235 (23) | |
| Patients with biopsies from different gastrointestinal locations † | <0.001 | |||
| Upper gastrointestinal tract, n (%) | 579 (4) | 244 (5) | 335 (3) | |
| Small intestine, n (%) | 1725 (12) | 1107 (23) | 618 (6) | |
| Colon, n (%) | 11,463 (82) | 2863 (61) | 8600 (88) | |
| Multiple locations, n (%) | 6668 (46) | 3051 (65) | 3617 (37) | |
| Patients with non-severe disease course | 9136 (63) | 2195 (46) | 6941 (71) | <0.001 |
| Patients with severe disease course, yes, n (%) | 5376 (37) | 2532 (54) | 2844 (29) | <0.001 |
| Patients with severe disease course because of: † | ||||
| Initiating biologics, n (%) | 3059 (21) | 1518 (32) | 1541 (16) | <0.001 |
| IBD related hospitalization ≥7 days, n (%) | 3410 (24) | 1342 (28) | 2068 (21) | <0.001 |
| IBD related surgery, n (%) | 1747 (12) | 988 (21) | 759 (8) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Søfelt, H.L.; Pingel, J.; Wolff, D.L.; Møllegaard, K.M.; Overgaard, S.H.; Green, A.; Madsen, G.I.; Qvist, N.; Petersen, S.R.; Andresen, T.; et al. Stored Intestinal Biopsies in Inflammatory Bowel Disease Research: A Danish Nationwide Population-Based Register Study. J. Pers. Med. 2025, 15, 129. https://doi.org/10.3390/jpm15040129
Søfelt HL, Pingel J, Wolff DL, Møllegaard KM, Overgaard SH, Green A, Madsen GI, Qvist N, Petersen SR, Andresen T, et al. Stored Intestinal Biopsies in Inflammatory Bowel Disease Research: A Danish Nationwide Population-Based Register Study. Journal of Personalized Medicine. 2025; 15(4):129. https://doi.org/10.3390/jpm15040129
Chicago/Turabian StyleSøfelt, Heidi Lynge, Jessica Pingel, Donna Lykke Wolff, Karen Mai Møllegaard, Silja Hvid Overgaard, Anders Green, Gunvor Iben Madsen, Niels Qvist, Sofie Ronja Petersen, Trine Andresen, and et al. 2025. "Stored Intestinal Biopsies in Inflammatory Bowel Disease Research: A Danish Nationwide Population-Based Register Study" Journal of Personalized Medicine 15, no. 4: 129. https://doi.org/10.3390/jpm15040129
APA StyleSøfelt, H. L., Pingel, J., Wolff, D. L., Møllegaard, K. M., Overgaard, S. H., Green, A., Madsen, G. I., Qvist, N., Petersen, S. R., Andresen, T., Franke, A., Marcussen, N., Christensen, R., & Andersen, V. (2025). Stored Intestinal Biopsies in Inflammatory Bowel Disease Research: A Danish Nationwide Population-Based Register Study. Journal of Personalized Medicine, 15(4), 129. https://doi.org/10.3390/jpm15040129






