The Uncommon Phenomenon of Short QT Syndrome: A Scoping Review of the Literature
Abstract
1. Introduction
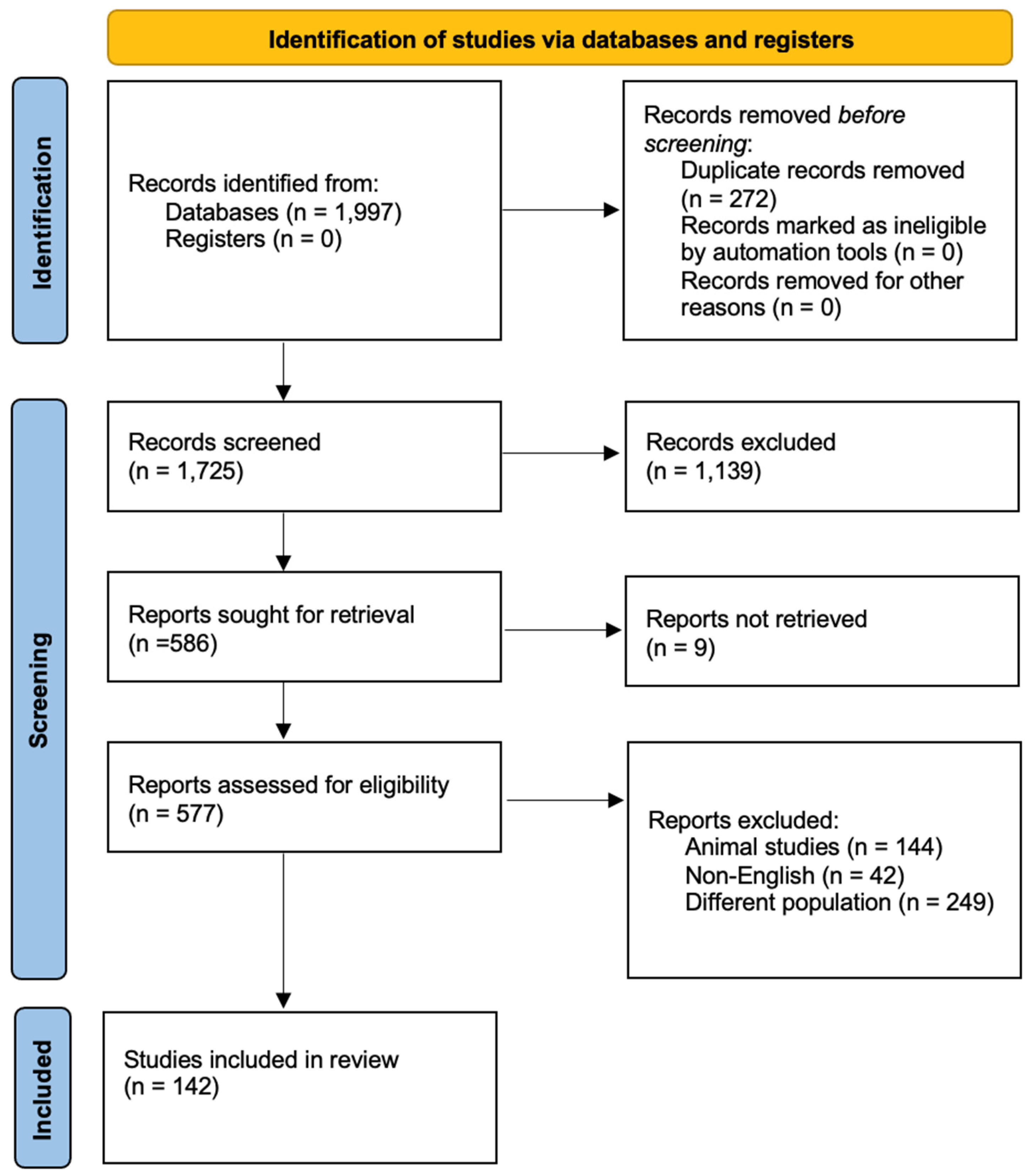
2. Materials and Methods
3. Results
3.1. Case Reports
3.2. Case Series
3.3. Studies
3.3.1. Studies Screening Healthy Populations or Medical Databases for SQTS
3.3.2. Genetic Testing in Patients with SQTS
3.3.3. Studies Assessing Outcomes of Patients with SQTS
3.3.4. Drug Therapy in Patients with SQTS
3.3.5. Studies Assessing Families of Sudden Cardiac Death Victims for the Presence of SQTS
3.3.6. Studies Demonstrating an Association Between SQT Intervals and Other Clinical Conditions
3.3.7. Findings of Various Diagnostic Modalities Among Patients with SQTS
3.3.8. Other Advances in Research on SQTS
3.4. Summary of Findings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SQTS | Short QT syndrome |
| QTc | Corrected QT |
| ECG | Electrocardiogram |
| SCD | Sudden cardiac death |
| VF | Ventricular fibrillation |
| ESC | European Society of Cardiology |
| ICD | Implantable cardioverter defibrillator |
| SQT | Short QT |
| AF | Atrial fibrillation |
| ERP | Early repolarization |
| LVEF | Left ventricular ejection fraction |
| BrS | Brugada syndrome |
| AI | Artificial intelligence |
References
- Gussak, I.; Brugada, P.; Brugada, J.; Wright, R.S.; Kopecky, S.L.; Chaitman, B.R.; Bjerregaard, P. Idiopathic short QT interval: A new clinical syndrome? Cardiology 2000, 94, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Moss, A.J.; Vincent, G.M.; Crampton, R.S. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993, 88, 782–784. [Google Scholar] [CrossRef]
- Gollob, M.H.; Redpath, C.J.; Roberts, J.D. The short QT syndrome: Proposed diagnostic criteria. J. Am. Coll. Cardiol. 2011, 57, 802–812. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; A Blom, N.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar]
- Thorsen, K.; Dam, V.S.; Kjaer-Sorensen, K.; Pedersen, L.N.; Skeberdis, V.A.; Jurevičius, J.; Treinys, R.; Petersen, I.M.B.S.; Nielsen, M.S.; Oxvig, C.; et al. Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat. Commun. 2017, 8, 1696. [Google Scholar] [CrossRef]
- Raschwitz, L.S.; El-Battrawy, I.; Schlentrich, K.; Besler, J.; Veith, M.; Roterberg, G.; Liebe, V.; Schimpf, R.; Lang, S.; Wolpert, C.; et al. Differences in Short QT Syndrome Subtypes: A Systematic Literature Review and Pooled Analysis. Front. Genet. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Ploneda-Valencia, R.G.; Ortiz-Solis, W.A.; Ruiz-Gonzalez, G.; Santiago-Garcia, A.K.; Rivera-Rodríguez, L.; Nava-Townsend, S.; Márquez, M.F.; Levinstein-Jacinto, M. Supraventricular tachyarrhythmia and sinus node dysfunction as a first manifestation of short QT syndrome in a pediatric patient. Case Report. J. Electrocardiol. 2022, 74, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Grytsay, O.N.; Skybchyk, Y.V.; Shorikova, D.V.; Shorikov, E.I. Clinical Cases of Life—Threatening Arrhythmias: Long and Short Qt Syndromes. Wiad. Lek. 2022, 75, 1805–1812. [Google Scholar] [CrossRef]
- Ramoğlu, M.G.; Karagözlü, S.; Uçar, T.; Tutar, E. Aborted cardiac arrest during sport activity in a teenager diagnosed with short QT syndrome. Cardiol. Young. 2020, 30, 886–889. [Google Scholar]
- Endres, D.; Decher, N.; Röhr, I.; Vowinkel, K.; Domschke, K.; Komlosi, K.; Tzschach, A.; Gläser, B.; Schiele, M.A.; Runge, K.; et al. New Cav1.2 Channelopathy with High-Functioning Autism, Affective Disorder, Severe Dental Enamel Defects, a Short QT Interval, and a Novel CACNA1C Loss-Of-Function Mutation. Int. J. Mol. Sci. 2020, 21, 8611. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, W.; Jiang, C.; Fu, G.; Sun, Y.; Hu, D. Implantable cardioverter defibrillator replacement guided by T wave safety margin in a short QT syndrome patient. Pacing Clin. Electrophysiol. 2019, 42, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.N.; Reiffel, J.A. Implantable Cardioverter-defibrillator Therapy for Syncope: An Educational Example of a Multicomponent Electrocardiographic Differential Diagnosis and the Application of Clinical Trial Data to an Individual Patient. J. Innov. Card. Rhythm Manag. 2019, 10, 3860–3864. [Google Scholar] [CrossRef]
- Morimoto, Y.; Watanabe, A.; Morita, H.; Nishii, N.; Nakamura, K.; Ito, H. Successful radiofrequency catheter ablation of a premature ventricular contraction triggering ventricular fibrillation in a patient with short QT syndrome. Heart Case Rep. 2019, 5, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Sun, Y.; Wang, X.; Wang, H.; Wang, J.; Gong, T.; Chen, X.; Zhang, P.; Su, L.; Fu, G.; et al. Patient-Specific and Gene-Corrected Induced Pluripotent Stem Cell-Derived Cardiomyocytes Elucidate Single-Cell Phenotype of Short QT Syndrome. Circ. Res. 2019, 124, 66–78. [Google Scholar] [CrossRef]
- Wakatsuki, D.; Iso, Y.; Mase, H.; Kurata, M.; Kyuno, E.; Shimojima, H.; Asano, T.; Sambe, T.; Suzuki, H. Sudden cardiac arrest during marathon training in a young adult with short QT syndrome. Int. J. Cardiol. Heart Vasc. 2018, 18, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Di Stolfo, G.; Palumbo, P.; Castellana, S.; Mastroianno, S.; Biagini, T.; Palumbo, O.; Leone, M.P.; De Luca, G.; Potenza, D.R.; Mazza, T.; et al. Sudden cardiac death in J wave syndrome with short QT associated to a novel mutation in Na(v) 1.8 coding gene SCN10A: First case report for a possible pharmacogenomic role. J. Electrocardiol. 2018, 51, 809–813. [Google Scholar] [CrossRef]
- Binda, A.; Rivolta, I.; Villa, C.; Chisci, E.; Beghi, M.; Cornaggia, C.M.; Giovannoni, R.; Combi, R. A Novel KCNJ2 Mutation Identified in an Autistic Proband Affects the Single Channel Properties of Kir2.1. Front. Cell. Neurosci. 2018, 12, 76. [Google Scholar] [CrossRef]
- Chen, Y.; Barajas-Martinez, H.; Zhu, D.; Wang, X.; Chen, C.; Zhuang, R.; Shi, J.; Wu, X.; Tao, Y.; Jin, W.; et al. Novel trigenic CACNA1C/DES/MYPN mutations in a family of hypertrophic cardiomyopathy with early repolarization and short QT syndrome. J. Transl. Med. 2017, 15, 78. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.K.; Awasthy, N. Bystander Cardio Pulmonary Resuscitation Saves Life in a Patient with Short QT Syndrome. Indian Pediatr. 2016, 53, 933–934. [Google Scholar]
- Righi, D.; Silvetti, M.S.; Drago, F. Sinus bradycardia, junctional rhythm, and low-rate atrial fibrillation in Short QT syndrome during 20 years of follow-up: Three faces of the same genetic problem. Cardiol. Young. 2016, 26, 589–592. [Google Scholar] [CrossRef]
- Ergül, Y.; Özyılmaz, İ.; Onan, S.H.; Güzeltaş, A. Short QT syndrome in a 14-year-old patient: The first pediatric case from Turkey. Anatol. J. Cardiol. 2015, 15, 590–591. [Google Scholar] [CrossRef][Green Version]
- Sadeghian, S.; Bozorgi, A.; Safkhani, Z. Short QT syndrome and idiopathic ventricular tachycardia in a 28-year-old young man: A potential disease-specific link? Europace 2014, 16, 1645. [Google Scholar] [CrossRef] [PubMed]
- Pavão, M.L.; Ono, V.C.; Arfelli, E.; Simões, M.V.; Marin Neto, J.A.; Schmidt, A. Sudden cardiac death and short QT syndrome. Arq. Bras. Cardiol. 2014, 103, e37–e40. [Google Scholar] [CrossRef] [PubMed]
- Maltret, A.; Wiener-Vacher, S.; Denis, C.; Extramiana, F.; Morisseau-Durand, M.P.; Fressart, V.; Bonnet, D.; Chabbert, C. Type 2 short QT syndrome and vestibular dysfunction: Mirror of the Jervell and Lange-Nielsen syndrome? Int. J. Cardiol. 2014, 171, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Fuglsang-Frederiksen, A.; Brugada, R.; Pedersen, B.; Rubboli, G.; Johansen, P.; Beniczky, S. Heart rate variability analysis indicates preictal parasympathetic overdrive preceding seizure-induced cardiac dysrhythmias leading to sudden unexpected death in a patient with epilepsy. Epilepsia 2014, 55, e67–e71. [Google Scholar] [CrossRef]
- Deo, M.; Ruan, Y.; Pandit, S.V.; Shah, K.; Berenfeld, O.; Blaufox, A.; Cerrone, M.; Noujaim, S.F.; Denegri, M.; Jalife, J.; et al. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc. Natl. Acad. Sci. USA 2013, 110, 4291–4296. [Google Scholar] [CrossRef]
- Hong, K.; Hu, J.; Yu, J.; Brugada, R. Concomitant Brugada-like and short QT electrocardiogram linked to SCN5A mutation. Eur. J. Hum. Genet. 2012, 20, 1189–1192. [Google Scholar] [CrossRef]
- Hattori, T.; Makiyama, T.; Akao, M.; Ehara, E.; Ohno, S.; Iguchi, M.; Nishio, Y.; Sasaki, K.; Itoh, H.; Yokode, M.; et al. A novel gain-of-function KCNJ2 mutation associated with short-QT syndrome impairs inward rectification of Kir2.1 currents. Cardiovasc. Res. 2012, 93, 666–673. [Google Scholar] [CrossRef]
- Chinushi, M.; Sato, A.; Iijima, K.; Suzuki, K.; Hiroshi, F.; Izumi, D.; Watanabe, H.; Kanae, H.; Aizawa, Y. Exercise-related QT interval shortening with a peaked T wave in a healthy boy with a family history of sudden cardiac death. Pacing Clin. Electrophysiol. 2012, 35, e239–e242. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Rougier, J.S.; Baumer, A.; Kaplan, V.; Albesa, M.; Sticht, H.; Rauch, A.; Puleo, C.; Hu, D. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur. Heart J. 2011, 32, 1077–1088. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Li, X.; Guo, J. Inappropriate ICD discharge due to T-wave oversensing in a patient with short QT syndrome. Pacing Clin. Electrophysiol. 2010, 33, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Villafane, J.; Young, M.L.; Maury, P.; Wolpert, C.; Anttonen, O.; Hamilton, R.; Kannankeril, P.J.; Fischbach, P.S. Short QT syndrome in a pediatric patient. Pediatr. Cardiol. 2009, 30, 846–850. [Google Scholar] [CrossRef]
- Rooryck, C.; Stef, M.; Burgelin, I.; Simon, D.; Souakri, N.; Thambo, J.B.; Chateil, J.F.; Lacombe, D.; Arveiler, B. 2.3 Mb terminal deletion in 12p13.33 associated with oculoauriculovertebral spectrum and evaluation of WNT5B as a candidate gene. Eur. J. Med. Genet. 2009, 52, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Sakaguchi, T.; Ashihara, T.; Ding, W.G.; Nagaoka, I.; Oka, Y.; Nakazawa, Y.; Yao, T.; Jo, H.; Ito, M.; et al. A novel KCNH2 mutation as a modifier for short QT interval. Int. J. Cardiol. 2009, 137, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Efremidis, M.; Letsas, K.P.; Weber, R.; Gavrielatos, G.; Filippatos, G.S.; Sideris, A.; Kardaras, F. Recurrent syncope associated with a distinct ECG pattern consisting of short QT interval, early repolarization and atrioventricular block. Clin. Res. Cardiol. 2009, 98, 807–810. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Enjoji, Y.; Yamamoto, R.; Ono, T.; Funatsu, A.; Kambayashi, D.; Kobayashi, T.; Nakamura, S. Nifekalant and disopyramide in a patient with short QT syndrome: Evaluation of pharmacological effects and electrophysiological properties. Pacing Clin. Electrophysiol. 2008, 31, 1229–1232. [Google Scholar] [CrossRef]
- Fichet, J.; Genee, O.; Pierre, B.; Babuty, D. Fatal QT interval. Am. J. Emerg. Med. 2008, 26, 739.e5-6. [Google Scholar] [CrossRef]
- Schimpf, R.; Bauersfeld, U.; Gaita, F.; Wolpert, C. Short QT syndrome: Successful prevention of sudden cardiac death in an adolescent by implantable cardioverter-defibrillator treatment for primary prophylaxis. Heart Rhythm 2005, 2, 416–417. [Google Scholar] [CrossRef]
- Kirilmaz, A.; Ulusoy, R.E.; Kardesoglu, E.; Ozmen, N.; Demiralp, E. Short QT interval syndrome: A case report. J. Electrocardiol. 2005, 38, 371–374. [Google Scholar] [CrossRef]
- Hong, K.; Piper, D.R.; Diaz-Valdecantos, A.; Brugada, J.; Oliva, A.; Burashnikov, E.; Santos-de-Soto, J.; Grueso-Montero, J.; Diaz-Enfante, E.; Brugada, P.; et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc. Res. 2005, 68, 433–440. [Google Scholar] [CrossRef]
- Bellocq, C.; van Ginneken, A.C.; Bezzina, C.R.; Alders, M.; Escande, D.; Mannens, M.M.; Baró, I.; Wilde, A.A. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004, 109, 2394–2397. [Google Scholar] [CrossRef]
- Zienciuk-Krajka, A.; Kukla, P.; Stec, S.; Raczak, G. Short QT syndrome presenting with atrial fibrillation and LV hypertrophy. Int. J. Cardiol. 2012, 156, e9. [Google Scholar] [CrossRef] [PubMed]
- van Schie, M.S.; Ramdat Misier, N.L.; van Leeuwen, W.J.; Taverne, Y.J.H.J.; de Groot, N.M.S. An unexpected finding by epicardial mapping: Atrial fibrillation in a 14-month-old patient with short QT syndrome. Heart Case Rep. 2023, 9, 219–221. [Google Scholar] [CrossRef]
- Terlemez, S.; Çil, E.; Kula, S.; Oǧuz, A.D.; Tunaoǧlu, F.S. A diagnosis that escapes our attention: Short QT syndrome. Gazi Med. J. 2018, 29, 246–248. [Google Scholar]
- Spartalis, M.; Livanis, E.; Spartalis, E.; Tsoutsinos, A. Electrical storm in an acquired short QT syndrome successfully treated with quinidine. Clin. Case Rep. 2019, 7, 1617–1618. [Google Scholar] [CrossRef] [PubMed]
- Portugal, G.; Martins Oliveira, M.; Silva Cunha, P.; Ferreira, F.; Lousinha, A.; Fiarresga, A.; Nogueira Da Silva, M.; Cruz Ferreira, R. Short QT syndrome presenting as syncope: How short is too short? Rev. Port. Cardiol. 2014, 33, 649.e1–e6. [Google Scholar] [CrossRef]
- Morphet, J.A.M. The short QT syndrome and sudden infant death syndrome. Can. J. Cardiol. 2007, 23, 105. [Google Scholar] [CrossRef]
- Farag, M.J.; Atallah, J. Use of topical lidocaine in eliminating mechanically stimulated ventricular fibrillation in a patient with short QT syndrome. Heart Case Rep. 2019, 5, 152–154. [Google Scholar] [CrossRef]
- Chevalier, P.; Moreau, A.; Richard, S.; Janin, A.; Millat, G.; Bessière, F.; Delinière, A. Short QT interval as a harbinger of an arrhythmogenic cardiomyopathy. Heart Case Rep. 2021, 7, 734–738. [Google Scholar] [CrossRef]
- Akdis, D.; Saguner, A.M.; Medeiros-Domingo, A.; Schaller, A.; Balmer, C.; Steffel, J.; Brunckhorst, C.; Duru, F. Multiple clinical profiles of families with the short QT syndrome. Europace 2018, 20, f113–f121. [Google Scholar] [CrossRef]
- Ambrosini, E.; Sicca, F.; Brignone, M.S.; D’Adamo, M.C.; Napolitano, C.; Servettini, I.; Moro, F.; Ruan, Y.; Guglielmi, L.; Pieroni, S.; et al. Genetically induced dysfunctions of Kir2.1 channels: Implications for short QT3 syndrome and autism-epilepsy phenotype. Hum. Mol. Genet. 2014, 23, 4875–4886. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, O.; Väänänen, H.; Junttila, J.; Huikuri, H.V.; Viitasalo, M. Electrocardiographic transmural dispersion of repolarization in patients with inherited short QT syndrome. Ann. Noninvasive. Electrocardiol. 2008, 13, 295–300. [Google Scholar] [CrossRef]
- Basarici, I. Delayed diagnosis of short QT syndrome concealed by pacemaker implant due to sick sinus syndrome. Anatol. J. Cardiol. 2020, 23, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Bohora, S.; Namboodiri, N.; Tharakan, J.; Vk, A.K.; Nayyar, S. Dilated cardiomyopathy with short QT interval: Is it a new clinical entity? Pacing Clin. Electrophysiol. 2009, 32, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Brugada, R.; Hong, K.; Dumaine, R.; Cordeiro, J.; Gaita, F.; Borggrefe, M.; Menendez, T.M.; Brugada, J.; Pollevick, G.D.; Wolpert, C.; et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004, 109, 30–35. [Google Scholar] [CrossRef]
- Bun, S.S.; Maury, P.; Giustetto, C.; Deharo, J.C. Electrical storm in short-QT syndrome successfully treated with Isoproterenol. J. Cardiovasc. Electrophysiol. 2012, 23, 1028–1030. [Google Scholar] [CrossRef]
- Chinushi, M.; Sato, A.; Izumi, D.; Furushima, H. Nifekalant enlarged the transmural activation-recovery interval difference as well as the peak-to-end interval on surface ECG in a patient with short-QT syndrome. J. Cardiovasc. Electrophysiol. 2012, 23, 877–880. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Besler, J.; Liebe, V.; Schimpf, R.; Tülümen, E.; Rudic, B.; Lang, S.; Wolpert, C.; Zhou, X.; Akin, I.; et al. Long-Term Follow-Up of Patients With Short QT Syndrome: Clinical Profile and Outcome. J. Am. Heart Assoc. 2018, 7, e010073. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Lan, H.; Cyganek, L.; Zhao, Z.; Li, X.; Buljubasic, F.; Lang, S.; Yücel, G.; Sattler, K.; Zimmermann, W.H.; et al. Modeling Short QT Syndrome Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Am. Heart Assoc. 2018, 7, e007394. [Google Scholar] [CrossRef]
- Gaita, F.; Giustetto, C.; Bianchi, F.; Wolpert, C.; Schimpf, R.; Riccardi, R.; Grossi, S.; Richiardi, E.; Borggrefe, M. Short QT Syndrome: A familial cause of sudden death. Circulation 2003, 108, 965–970. [Google Scholar] [CrossRef]
- Giustetto, C.; Scrocco, C.; Giachino, D.; Rapezzi, C.; Mognetti, B.; Gaita, F. The lack of effect of sotalol in short QT syndrome patients carrying the T618I mutation in the KCNH2 gene. Heart Case Rep. 2015, 1, 373–378. [Google Scholar] [CrossRef]
- Harrell, D.T.; Ashihara, T.; Ishikawa, T.; Tominaga, I.; Mazzanti, A.; Takahashi, K.; Oginosawa, Y.; Abe, H.; Maemura, K.; Sumitomo, N.; et al. Genotype-dependent differences in age of manifestation and arrhythmia complications in short QT syndrome. Int. J. Cardiol. 2015, 190, 393–402. [Google Scholar] [CrossRef]
- Hong, K.; Bjerregaard, P.; Gussak, I.; Brugada, R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J. Cardiovasc. Electrophysiol. 2005, 16, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.X.; Zhou, W.; Zhang, X.; Cao, Q.; Yu, K.; Zhu, C. Short QT syndrome: A case report and review of literature. Resuscitation 2006, 71, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Moriya, M.; Seto, S.; Yano, K.; Akahoshi, M. Two cases of short QT interval. Pacing Clin. Electrophysiol. 2007, 30, 1522–1526. [Google Scholar] [CrossRef]
- Pirro, E.; De Francia, S.; Banaudi, E.; Riggi, C.; De Martino, F.; Piccione, F.M.; Giustetto, C.; Racca, S.; Agnoletti, G.; Di Carlo, F. Short QT syndrome in infancy. Therapeutic drug monitoring of hydroquinidine in a newborn infant. Br. J. Clin. Pharmacol. 2011, 72, 982–984. [Google Scholar] [CrossRef][Green Version]
- Priori, S.G.; Pandit, S.V.; Rivolta, I.; Berenfeld, O.; Ronchetti, E.; Dhamoon, A.; Napolitano, C.; Anumonwo, J.; di Barletta, M.R.; Gudapakkam, S.; et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ. Res. 2005, 96, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Sarquella-Brugada, G.; Campuzano, O.; Iglesias, A.; Grueso, J.; Bradley, D.J.; Kerst, G.; Shmorhun, D.; Brugada, J.; Brugada, R. Short QT and atrial fibrillation: A KCNQ1 mutation-specific disease. Late follow-up in three unrelated children. Heart Case Rep. 2015, 1, 193–197. [Google Scholar] [CrossRef]
- Sun, Y.; Quan, X.Q.; Fromme, S.; Cox, R.H.; Zhang, P.; Zhang, L.; Guo, D.; Guo, J.; Patel, C.; Kowey, P.R.; et al. A novel mutation in the KCNH2 gene associated with short QT syndrome. J. Mol. Cell. Cardiol. 2011, 50, 433–441. [Google Scholar] [CrossRef]
- Suzuki, H.; Hoshina, S.; Ozawa, J.; Sato, A.; Minamino, T.; Aizawa, Y.; Saitoh, A. Short QT syndrome in a boy diagnosed on screening for heart disease. Pediatr. Int. 2014, 56, 774–776. [Google Scholar] [CrossRef]
- Wisten, A.; Boström, I.M.; Mörner, S.; Stattin, E.L. Mutation analysis of cases of sudden unexplained death, 15 years after death: Prompt genetic evaluation after resuscitation can save future lives. Resuscitation 2012, 83, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Parrott, A.; Spar, D.; Knilans, T.; Czosek, R.; Miller, E.; Anderson, J. A novel variant in KCNQ1 associated with short QT syndrome. Heart Case Rep. 2021, 7, 650–654. [Google Scholar] [CrossRef]
- Peters, S.; Trümmel, M.; Koehler, B. Shorter-than-normal QT interval and provocable right precordial ST segment elevation in three patients with suspicious arrhythmogenic right ventricular cardiomyopathy. J. Fur Kardiol. 2011, 18, 326–328. [Google Scholar]
- Giustetto, C.; Gaita, F. Syncope in a Patient with a Short QT Interval. Syncope Cases 2007, 177–179. [Google Scholar] [CrossRef]
- Gimeno, J.R.; Lacunza, J.; García-Molina, E.; Oliva-Sandoval, M.J.; Valdes, M. Short QT and dilated cardiomyopathy. A phenotype with a good prognosis? Int. J. Cardiol. 2011, 151, 356–357. [Google Scholar] [CrossRef]
- Babaoǧlu, K.; Binnetoǧlu, K.; Altun, G.; Tuzcu, V. A 13-year-old boy with a short QT interval—Case report. Anatol. J. Cardiol. 2012, 12, 275. [Google Scholar]
- Wu, Z.J.; Huang, Y.; Fu, Y.C.; Zhao, X.J.; Zhu, C.; Zhang, Y.; Xu, B.; Zhu, Q.L.; Li, Y. Characterization of a Chinese KCNQ1 mutation (R259H) that shortens repolarization and causes short QT syndrome 2. J. Geriatr. Cardiol. 2015, 12, 394–401. [Google Scholar] [PubMed]
- Wolpert, C.; Schimpf, R.; Giustetto, C.; Antzelevitch, C.; Cordeiro, J.; Dumaine, R.; Brugada, R.; Hong, K.; Bauersfeld, U.; Gaita, F.; et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J. Cardiovasc. Electrophysiol. 2005, 16, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Makiyama, T.; Koyama, T.; Kannankeril, P.J.; Seto, S.; Okamura, K.; Oda, H.; Itoh, H.; Okada, M.; Tanabe, N.; et al. High prevalence of early repolarization in short QT syndrome. Heart Rhythm 2010, 7, 647–652. [Google Scholar] [CrossRef]
- Villafañe, J.; Atallah, J.; Gollob, M.H.; Maury, P.; Wolpert, C.; Gebauer, R.; Watanabe, H.; Horie, M.; Anttonen, O.; Kannakeril, P.; et al. Long-term follow-up of a pediatric cohort with short QT syndrome. J. Am. Coll. Cardiol. 2013, 61, 1183–1191. [Google Scholar] [CrossRef]
- Tülümen, E.; Giustetto, C.; Wolpert, C.; Maury, P.; Anttonen, O.; Probst, V.; Blanc, J.J.; Sbragia, P.; Scrocco, C.; Rudic, B.; et al. PQ segment depression in patients with short QT syndrome: A novel marker for diagnosing short QT syndrome? Heart Rhythm 2014, 11, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Horie, M.; Ozawa, J.; Sumitomo, N.; Ohno, S.; Hoshino, K.; Ehara, E.; Takahashi, K.; Maeda, Y.; Yoshinaga, M.; et al. Novel electrocardiographic criteria for short QT syndrome in children and adolescents. Europace 2021, 23, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Stępień-Wojno, M.; Ponińska, J.; Rydzanicz, M.; Bilińska, M.; Truszkowska, G.; Baranowski, R.; Lutyńska, A.; Biernacka, E.K.; Stępińska, J.; Kowalik, I.; et al. Sudden cardiac arrest in patients without overt heart disease: A limited value of next generation sequencing. Pol. Arch. Intern. Med. 2018, 128, 721–730. [Google Scholar] [CrossRef]
- Schimpf, R.; Wolpert, C.; Bianchi, F.; Giustetto, C.; Gaita, F.; Bauersfeld, U.; Borggrefe, M. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: Inherent risk for inappropriate shock delivery. J. Cardiovasc. Electrophysiol. 2003, 14, 1273–1277. [Google Scholar] [CrossRef]
- Schimpf, R.; Antzelevitch, C.; Haghi, D.; Giustetto, C.; Pizzuti, A.; Gaita, F.; Veltmann, C.; Wolpert, C.; Borggrefe, M. Electromechanical coupling in patients with the short QT syndrome: Further insights into the mechanoelectrical hypothesis of the U wave. Heart Rhythm 2008, 5, 241–245. [Google Scholar] [CrossRef]
- Rudic, B.; Tülümen, E.; Berlin, V.; Röger, S.; Stach, K.; Liebe, V.; El-Battrawy, I.; Dösch, C.; Papavassiliu, T.; Akin, I.; et al. Low Prevalence of Inappropriate Shocks in Patients With Inherited Arrhythmia Syndromes With the Subcutaneous Implantable Defibrillator Single Center Experience and Long-Term Follow-Up. J. Am. Heart Assoc. 2017, 6, e006265. [Google Scholar] [CrossRef] [PubMed]
- Rollin, A.; Gandjbakhch, E.; Giustetto, C.; Scrocco, C.; Fourcade, C.; Monteil, B.; Mondoly, P.; Cardin, C.; Maupain, C.; Gaita, F.; et al. Shortening of the Short Refractory Periods in Short QT Syndrome. J. Am. Heart Assoc. 2017, 6, e005684. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Tang, J.K.K. The Short QTc Is a Marker for the Development of Atrial Flutter and Atrial Fibrillation. Cardiol. Res. Pract. 2020, 2020, 2858149. [Google Scholar] [CrossRef]
- Providência, R.; Karim, N.; Srinivasan, N.; Honarbakhsh, S.; Vidigal Ferreira, M.J.; Gonçalves, L.; Marijon, E.; Lambiase, P.D. Impact of QTc formulae in the prevalence of short corrected QT interval and impact on probability and diagnosis of short QT syndrome. Heart 2018, 104, 502–508. [Google Scholar] [CrossRef]
- Pickham, D.; Zarafshar, S.; Sani, D.; Kumar, N.; Froelicher, V. Comparison of three ECG criteria for athlete pre-participation screening. J. Electrocardiol. 2014, 47, 769–774. [Google Scholar] [CrossRef]
- Pasero, E.; Gaita, F.; Randazzo, V.; Meynet, P.; Cannata, S.; Maury, P.; Giustetto, C. Artificial Intelligence ECG Analysis in Patients with Short QT Syndrome to Predict Life-Threatening Arrhythmic Events. Sensors 2023, 23, 8900. [Google Scholar] [CrossRef] [PubMed]
- Panicker, G.K.; Manohar, D.; Karnad, D.R.; Salvi, V.; Kothari, S.; Lokhandwala, Y. Early repolarization and short QT interval in healthy subjects. Heart Rhythm 2012, 9, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.H.; Heiran, A.; Mashayekh, F.; Rezaianzadeh, A.; Shiravani, A.; Azadian, F. A descriptive report on short QT interval in Kherameh branch of the PERSIAN cohort study. Sci. Rep. 2022, 12, 2898. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Hayashi, H.; Yoshino, T.; Kawaguchi, T.; Taniguchi, A.; Itoh, H.; Sugimoto, Y.; Itoh, M.; Makiyama, T.; Xue, J.Q.; et al. Clinical and electrocardiographic characteristics of patients with short QT interval in a large hospital-based population. Heart Rhythm 2012, 9, 66–74. [Google Scholar] [CrossRef]
- Migliore, F.; Silvano, M.; Zorzi, A.; Bertaglia, E.; Siciliano, M.; Leoni, L.; De Franceschi, P.; Iliceto, S.; Corrado, D. Implantable cardioverter defibrillator therapy in young patients with cardiomyopathies and channelopathies: A single Italian centre experience. J. Cardiovasc. Med. 2016, 17, 485–493. [Google Scholar] [CrossRef]
- Mazzanti, A.; Maragna, R.; Vacanti, G.; Kostopoulou, A.; Marino, M.; Monteforte, N.; Bloise, R.; Underwood, K.; Tibollo, V.; Pagan, E.; et al. Hydroquinidine Prevents Life-Threatening Arrhythmic Events in Patients With Short QT Syndrome. J. Am. Coll. Cardiol. 2017, 70, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, A.; Kanthan, A.; Monteforte, N.; Memmi, M.; Bloise, R.; Novelli, V.; Miceli, C.; O’Rourke, S.; Borio, G.; Zienciuk-Krajka, A.; et al. Novel insight into the natural history of short QT syndrome. J. Am. Coll. Cardiol. 2014, 63, 1300–1308. [Google Scholar] [CrossRef]
- Maury, P.; Extramiana, F.; Giustetto, C.; Cardin, C.; Rollin, A.; Duparc, A.; Mondoly, P.; Denjoy, I.; Delay, M.; Messali, A.; et al. Microvolt T-wave alternans in short QT syndrome. Pacing Clin. Electrophysiol. 2012, 35, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Lubart, E.; Segal, R.; Yearovoi, A.; Fridenson, A.; Baumoehl, Y.; Leibovitz, A. QT interval disturbances in hospitalized elderly patients. Isr. Med. Assoc. J. 2009, 11, 147–150. [Google Scholar]
- Li, J.; Seyler, C.; Wiedmann, F.; Schmidt, C.; Schweizer, P.A.; Becker, R.; Katus, H.A.; Thomas, D. Anti-KCNQ1 K⁺ channel autoantibodies increase IKs current and are associated with QT interval shortening in dilated cardiomyopathy. Cardiovasc. Res. 2013, 98, 496–503. [Google Scholar] [CrossRef]
- Kumar, S.; Peters, S.; Thompson, T.; Morgan, N.; Maccicoca, I.; Trainer, A.; Zentner, D.; Kalman, J.M.; Winship, I.; Vohra, J.K. Familial cardiological and targeted genetic evaluation: Low yield in sudden unexplained death and high yield in unexplained cardiac arrest syndromes. Heart Rhythm 2013, 10, 1653–1660. [Google Scholar] [CrossRef]
- Kim, D.Y.; Uhm, J.S.; Kim, M.; Kim, I.S.; Jin, M.N.; Yu, H.T.; Kim, T.H.; Kim, J.Y.; Joung, B.; Pak, H.N.; et al. Long-term prognosis of short QT interval in Korean patients: A multicenter retrospective cohort study. BMC Cardiovasc. Disord. 2021, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Kaiser-Nielsen, L.V.; Tischer, S.G.; Prescott, E.B.; Rasmusen, H.K. Symptoms, diagnoses, and sporting consequences among athletes referred to a Danish sports cardiology clinic. Scand. J. Med. Sci. Sports 2017, 27, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, I.N.; Skakkebaek, A.; Andersen, N.H.; Pedersen, L.N.; Hougaard, D.M.; Bojesen, A.; Trolle, C.; Gravholt, C.H. Short QTc interval in males with klinefelter syndrome-influence of CAG repeat length, body composition, and testosterone replacement therapy. Pacing Clin. Electrophysiol. 2015, 38, 472–482. [Google Scholar] [CrossRef]
- Jiménez-Jáimez, J.; Peinado, R.; Grima, E.Z.; Segura, F.; Moriña, P.; Sánchez Muñoz, J.J.; Mazuelos, F.; Cózar, R.; Gimeno, J.R.; Heras, R.P.; et al. Diagnostic Approach to Unexplained Cardiac Arrest (from the FIVI-Gen Study). Am. J. Cardiol. 2015, 116, 894–899. [Google Scholar] [CrossRef]
- Iribarren, C.; Round, A.D.; Peng, J.A.; Lu, M.; Klatsky, A.L.; Zaroff, J.G.; Holve, T.J.; Prasad, A.; Stang, P. Short QT in a cohort of 1.7 million persons: Prevalence, correlates, and prognosis. Ann. Noninvasive. Electrocardiol. 2014, 19, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, Y.; Zhang, J.; Pfeiffer, R.; Gollob, M.H.; Healey, J.; Harrell, D.T.; Makita, N.; Abe, H.; Sun, Y.; et al. The Phenotypic Spectrum of a Mutation Hotspot Responsible for the Short QT Syndrome. JACC Clin. Electrophysiol. 2017, 3, 727–743. [Google Scholar] [CrossRef]
- Guerrier, K.; Kwiatkowski, D.; Czosek, R.J.; Spar, D.S.; Anderson, J.B.; Knilans, T.K. Short QT Interval Prevalence and Clinical Outcomes in a Pediatric Population. Circ. Arrhythm. Electrophysiol. 2015, 8, 1460–1464. [Google Scholar] [CrossRef]
- Giustetto, C.; Scrocco, C.; Schimpf, R.; Maury, P.; Mazzanti, A.; Levetto, M.; Anttonen, O.; Dalmasso, P.; Cerrato, N.; Gribaudo, E.; et al. Usefulness of exercise test in the diagnosis of short QT syndrome. Europace 2015, 17, 628–634. [Google Scholar] [CrossRef]
- Giustetto, C.; Schimpf, R.; Mazzanti, A.; Scrocco, C.; Maury, P.; Anttonen, O.; Probst, V.; Blanc, J.J.; Sbragia, P.; Dalmasso, P.; et al. Long-term follow-up of patients with short QT syndrome. J. Am. Coll. Cardiol. 2011, 58, 587–595. [Google Scholar] [CrossRef]
- Giustetto, C.; Di Monte, F.; Wolpert, C.; Borggrefe, M.; Schimpf, R.; Sbragia, P.; Leone, G.; Maury, P.; Anttonen, O.; Haissaguerre, M.; et al. Short QT syndrome: Clinical findings and diagnostic-therapeutic implications. Eur. Heart J. 2006, 27, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Garvey, E.M.; Papez, A.L.; Notrica, D.M.; Egan, J.C.; Molitor, M.; Cohen, M.I.; van Leeuwen, K. Thoracoscopic Cardiac Sympathetic Denervation: Adjunct Therapy for Secondary Prevention of Life-Threatening Ventricular Arrhythmias in Children. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 1387–1392. [Google Scholar] [CrossRef]
- Gaita, F.; Giustetto, C.; Bianchi, F.; Schimpf, R.; Haissaguerre, M.; Calò, L.; Brugada, R.; Antzelevitch, C.; Borggrefe, M.; Wolpert, C. Short QT syndrome: Pharmacological treatment. J. Am. Coll. Cardiol. 2004, 43, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Funada, A.; Hayashi, K.; Ino, H.; Fujino, N.; Uchiyama, K.; Sakata, K.; Masuta, E.; Sakamoto, Y.; Tsubokawa, T.; Yamagishi, M. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin. Cardiol. 2008, 31, 270–274. [Google Scholar] [CrossRef]
- Fukuyama, M.; Ohno, S.; Wang, Q.; Kimura, H.; Makiyama, T.; Itoh, H.; Ito, M.; Horie, M. L-type calcium channel mutations in Japanese patients with inherited arrhythmias. Circ. J. 2013, 77, 1799–1806. [Google Scholar] [CrossRef]
- Frea, S.; Giustetto, C.; Capriolo, M.; Scrocco, C.; Fornengo, C.; Benedetto, S.; Bianchi, F.; Pidello, S.; Morello, M.; Gaita, F. New echocardiographic insights in short QT syndrome: More than a channelopathy? Heart Rhythm 2015, 12, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Extramiana, F.; Maury, P.; Maison-Blanche, P.; Duparc, A.; Delay, M.; Leenhardt, A. Electrocardiographic biomarkers of ventricular repolarisation in a single family of short QT syndrome and the role of the Bazett correction formula. Am. J. Cardiol. 2008, 101, 855–860. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Besler, J.; Li, X.; Lan, H.; Zhao, Z.; Liebe, V.; Schimpf, R.; Lang, S.; Wolpert, C.; Zhou, X.; et al. Impact of Antiarrhythmic Drugs on the Outcome of Short QT Syndrome. Front. Pharmacol. 2019, 10, 771. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Besler, J.; Ansari, U.; Liebe, V.; Schimpf, R.; Tülümen, E.; Rudic, B.; Lang, S.; Odening, K.; Cyganek, L.; et al. Long-term follow-up of implantable cardioverter-defibrillators in Short QT syndrome. Clin. Res. Cardiol. 2019, 108, 1140–1146. [Google Scholar] [CrossRef]
- Dhutia, H.; Malhotra, A.; Parpia, S.; Gabus, V.; Finocchiaro, G.; Mellor, G.; Merghani, A.; Millar, L.; Narain, R.; Sheikh, N.; et al. The prevalence and significance of a short QT interval in 18,825 low-risk individuals including athletes. Br. J. Sports Med. 2016, 50, 124–129. [Google Scholar] [CrossRef]
- Christiansen, M.K.; Kjær-Sørensen, K.; Clavsen, N.C.; Dittmann, S.; Jensen, M.F.; Guldbrandsen, H.; Pedersen, L.N.; Sørensen, R.H.; Lildballe, D.L.; Müller, K.; et al. Genetic analysis identifies the SLC4A3 anion exchanger as a major gene for short QT syndrome. Heart Rhythm 2023, 20, 1136–1143. [Google Scholar] [CrossRef]
- Blancard, M.; Debbiche, A.; Kato, K.; Cardin, C.; Sabrina, G.; Gandjbakhch, E.; Probst, V.; Haissaguerre, M.; Extramiana, F.; Hocini, M.; et al. An African loss-of-function CACNA1C variant p.T1787M associated with a risk of ventricular fibrillation. Sci. Rep. 2018, 8, 14619. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, O.; Junttila, M.J.; Rissanen, H.; Reunanen, A.; Viitasalo, M.; Huikuri, H.V. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 2007, 116, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, O.; Junttila, J.; Giustetto, C.; Gaita, F.; Linna, E.; Karsikas, M.; Seppänen, T.; Perkiömäki, J.S.; Mäkikallio, T.H.; Brugada, R.; et al. T-Wave morphology in short QT syndrome. Ann. Noninvasive Electrocardiol. 2009, 14, 262–267. [Google Scholar] [CrossRef]
- Adler, A.; Sadek, M.M.; Chan, A.Y.; Dell, E.; Rutberg, J.; Davis, D.; Green, M.S.; Spears, D.A.; Gollob, M.H. Patient Outcomes From a Specialized Inherited Arrhythmia Clinic. Circ. Arrhythm. Electrophysiol. 2016, 9, e003440. [Google Scholar] [CrossRef] [PubMed]
- Viskin, S.; Zeltser, D.; Ish-Shalom, M.; Katz, A.; Glikson, M.; Justo, D.; Tekes-Manova, D.; Belhassen, B. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm 2004, 1, 587–591. [Google Scholar] [CrossRef]
- Teh, H.S.; Tan, H.J.; Loo, C.Y.; Raymond, A.A. Short QTc in epilepsy patients without cardiac symptoms. Med. J. Malays. 2007, 62, 104–108. [Google Scholar]
- Schimpf, R.; Veltmann, C.; Giustetto, C.; Gaita, F.; Borggrefe, M.; Wolpert, C. In vivo effects of mutant HERG K+ channel inhibition by disopyramide in patients with a short QT-1 Syndrome: A pilot study. J. Cardiovasc. Electrophysiol. 2007, 18, 1157–1160. [Google Scholar] [CrossRef]
- Robinson, J.A.; Lapage, M.J.; Atallah, J.; Webster, G.; Miyake, C.Y.; Ratnasamy, C.; Ollberding, N.J.; Mohan, S.; Von Bergen, N.H.; Johnsrude, C.L.; et al. Outcomes of Pediatric Patients With Defibrillators Following Initial Presentation With Sudden Cardiac Arrest. Circ. Arrhythmia Electrophysiol. 2021, 14, E008517. [Google Scholar] [CrossRef]
- Makarov, L.M.; Kisileva, I.I.; Dolgikh, V.V.; Bimbaev, A.B.Z.; Bairova, T.A.; Drozdova, A.I. Assesment of parameters of QT interval in children and adolescents. Kardiologiya 2006, 46, 37–41. [Google Scholar]
- Makarov, L.M.; Chuprova, S.N.; Kiseleva, I.I. QT interval shortening in families with history of sudden death at young age. Kardiologiia 2004, 44, 51–56. [Google Scholar]
- Lubart, E.; Segal, R.; Megid, S.; Yarovoy, A.; Leibovitz, A. QT interval disturbances in elderly residents of long-term care facilities. Isr. Med. Assoc. J. 2012, 14, 244–246. [Google Scholar] [PubMed]
- Kobza, R.; Roos, M.; Niggli, B.; Abächerli, R.; Lupi, G.A.; Frey, F.; Schmid, J.J.; Erne, P. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm 2009, 6, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Hazeki, D.; Ninomiya, Y.; Ueno, K.; Yoshinaga, M. Tentative screening criteria for short QT interval in children and adolescents. Circ. J. 2018, 82, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.M.; Magliano, G.; Yap, Y.G.; Padula, M.; Morgia, V.; Postorino, C.; Liberato, F.; Leo, R.; Borzi, M.; Romeo, F. Distribution and Prognostic Significance of QT Intervals in the Lowest Half Centile in 12,012 Apparently Healthy Persons. Am. J. Cardiol. 2006, 98, 933–935. [Google Scholar] [CrossRef]
- Burashnikov, E.; Pfeiffer, R.; Barajas-Martinez, H.; Delpón, E.; Hu, D.; Desai, M.; Borggrefe, M.; Hissaguerre, M.; Kanter, R.; Pollevick, G.D.; et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm 2010, 7, 1872–1882. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Pollevick, G.D.; Cordeiro, J.M.; Casis, O.; Sanguinetti, M.C.; Aizawa, Y.; Guerchicoff, A.; Pfeiffer, R.; Oliva, A.; Wollnik, B.; et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007, 115, 442–449. [Google Scholar] [CrossRef]
- Anttonen, O.; Junttila, M.J.; Maury, P.; Schimpf, R.; Wolpert, C.; Borggrefe, M.; Giustetto, C.; Gaita, F.; Sacher, F.; Haissaguerre, M.; et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm 2009, 6, 267–271. [Google Scholar] [CrossRef]
- Ahmadi-Renani, S.; Soltani, D.; Farshbafnadi, M.; Shafiee, A.; Jalali, A.; Mohammadi, M.; Golestanian, S.; Kamalian, E.; Alaeddini, F.; Saadat, S.; et al. Prevalence and associated factors of ECG abnormality patterns indicative of cardiac channelopathies among adult general population of Tehran, Iran: A report from the Tehran Cohort Study (TeCS). BMC Cardiovasc. Disord. 2024, 24, 566. [Google Scholar] [CrossRef]
- Conte, G.; Bergonti, M.; Probst, V.; Morita, H.; Tfelt-Hansen, J.; Behr, E.R.; Kengo, K.; Arbelo, E.; Crotti, L.; Sarquella-Brugada, G.; et al. aTrial arrhythmias in inhEriTed aRrhythmIa Syndromes: Results from the TETRIS study. Europace 2024, 26, euae288. [Google Scholar] [CrossRef]
- Mazzanti, A.; O’Rourke, S.; Ng, K.; Miceli, C.; Borio, G.; Curcio, A.; Esposito, F.; Napolitano, C.; Priori, S.G. The usual suspects in sudden cardiac death of the young: A focus on inherited arrhythmogenic diseases. Expert Rev. Cardiovasc. Ther. 2014, 12, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.P.; Dharmadjati, B.B. Short QT syndrome: The current evidences of diagnosis and management. J. Arrhythm. 2020, 36, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Gollob, M.H. Short QT syndrome: Advancing our understanding of genetics and cardiac physiology. Heart Rhythm 2023, 20, 1144–1145. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.S.; Jick, H.; Cohen, S.I. Adverse reactions to quinidine in hospitalized patients: Findings based on data from the Boston Collaborative Drug Surveillance Program. Prog. Cardiovasc. Dis. 1977, 20, 151–163. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Lodewyckx, P.; Issa, J.; Gaschignard, M.; Lamireau, D.; De Lonlay, P.; Servais, A.; Barth, M.; Courapied, S.; Morin, G.; Benbrik, N.; et al. Systemic primary carnitine deficiency induces severe arrhythmia due to shortening of QT interval. Mol. Genet. Metab. 2023, 140, 107733. [Google Scholar] [CrossRef]
- John, T.J.; John, K.; Jansen Van Rensburg, R.; Kyriakakis, C. Hypercalcaemia and a short QT interval. QJM 2020, 113, 55–56. [Google Scholar] [CrossRef]
- Holbrook, M.; Malik, M.; Shah, R.R.; Valentin, J.P. Drug induced shortening of the QT/QTc interval: An emerging safety issue warranting further modelling and evaluation in drug research and development? J. Pharmacol. Toxicol. Methods 2009, 59, 21–28. [Google Scholar] [CrossRef]
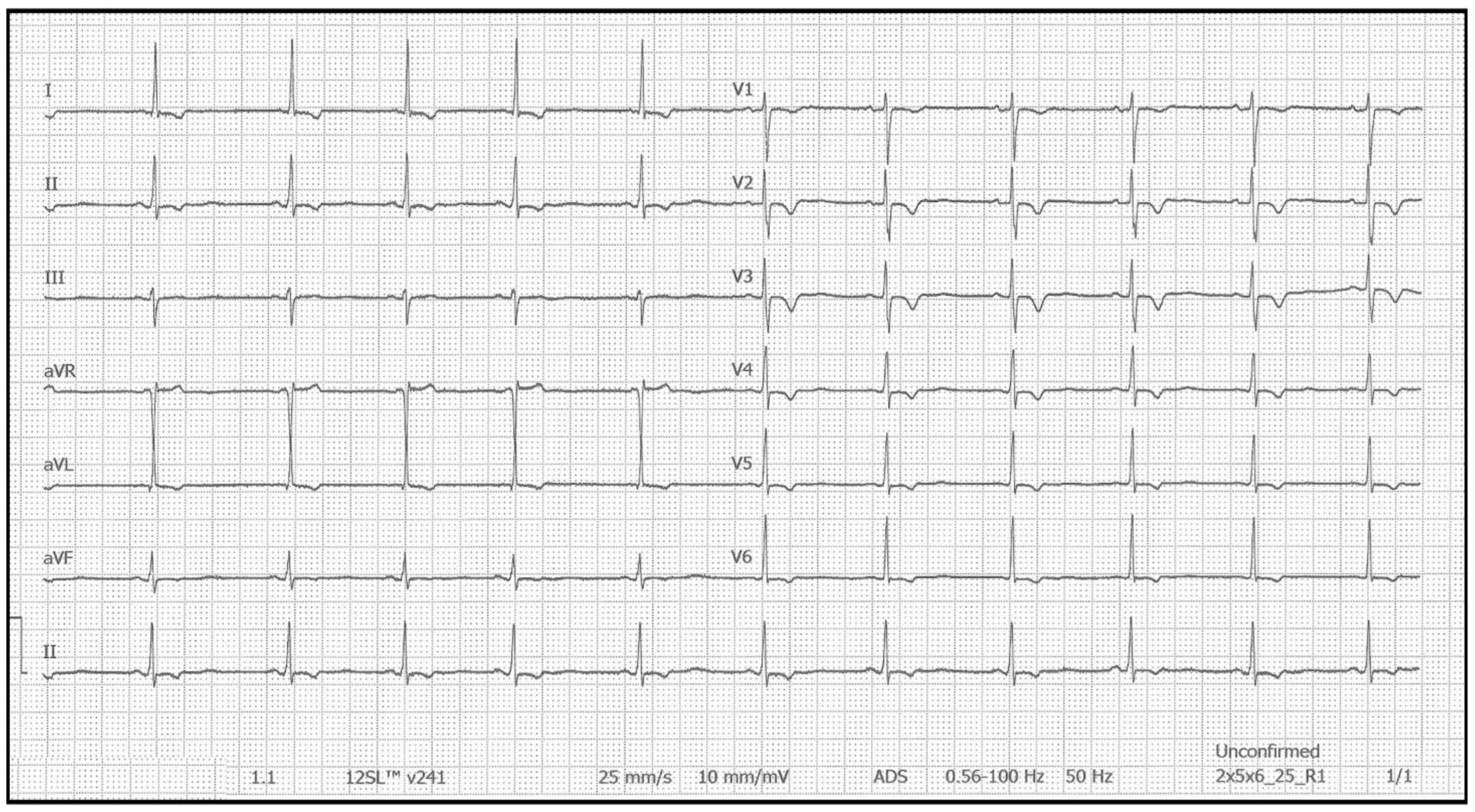
| Parameter | Summary of Findings |
|---|---|
| Age at diagnosis | Mean age: ~25 years (range: infancy to late adulthood) [1,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,93,108,120,141,142] |
| Gender distribution | SQTS has been reported in both males and females, though some studies suggest a slight male predominance (Supplementary Tables S2 and S3, [104,126,133]) |
| Diagnostic criteria | QTc ≤ 360 ms and pathogenic mutation or family history of SQTS or survival of VT/VF in the absence of heart disease; diagnosis should be considered when QTc is less than 320 ms, or in the presence of QTc >= 320 ms and =< 360 ms and arrhythmic syncope or a family history of SD at an age younger than 40 years [4] |
| ICD implantation | Recommended for survivors of cardiac arrest or for patients with documented sustained VT or those with arrhythmic syncope; variable use in asymptomatic cases [3,4,80,112,119,126,129,143] |
| Pharmacological therapy | Hydroquinidine (most studied drug) is effective in QT prolongation and arrhythmia suppression; other drugs (e.g., sotalol, flecainide) have had variable success [3,4,78,113,143] |
| Outcomes | High risk of arrhythmic events, with some studies reporting inappropriate ICD shocks (up to 64% of ICD recipients); limited long-term survival data [1,3,4,65,106,108,112,120,141,142] |
| Shock-free survival | Median follow-up suggests high incidence of ICD interventions, including both appropriate and inappropriate shocks [4,65,80,93,106,108,119,120,131,143] |
| Genetic testing | Most common mutations: KCNH2, KCNJ2, and KCNQ1 (ClinGen-approved); weaker evidence of mutation in other genes (e.g., CACNA1C, CACNB2, CACNA2D1) [3,4,55,62,77,100,115,121,122,136,137] |
| Associated conditions | SQTS often coexists with cardiomyopathies, early repolarization syndrome, and other inherited arrhythmias [18,42,49,54,73,95,100,142] |
| Future directions | Need for multicenter registries, genetic studies, and RCTs to refine risk stratification and treatment approaches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulmpou, A.; Giannopoulos, A.; Papadopoulos, C.; Giannopoulos, G.; Papagiannis, I.; Zormpas, G.; Keivanidou, A.; Fidani, L.; Vassilikos, V. The Uncommon Phenomenon of Short QT Syndrome: A Scoping Review of the Literature. J. Pers. Med. 2025, 15, 105. https://doi.org/10.3390/jpm15030105
Boulmpou A, Giannopoulos A, Papadopoulos C, Giannopoulos G, Papagiannis I, Zormpas G, Keivanidou A, Fidani L, Vassilikos V. The Uncommon Phenomenon of Short QT Syndrome: A Scoping Review of the Literature. Journal of Personalized Medicine. 2025; 15(3):105. https://doi.org/10.3390/jpm15030105
Chicago/Turabian StyleBoulmpou, Aristi, Andreas Giannopoulos, Christodoulos Papadopoulos, Georgios Giannopoulos, Ioannis Papagiannis, Georgios Zormpas, Anastasia Keivanidou, Liana Fidani, and Vassilios Vassilikos. 2025. "The Uncommon Phenomenon of Short QT Syndrome: A Scoping Review of the Literature" Journal of Personalized Medicine 15, no. 3: 105. https://doi.org/10.3390/jpm15030105
APA StyleBoulmpou, A., Giannopoulos, A., Papadopoulos, C., Giannopoulos, G., Papagiannis, I., Zormpas, G., Keivanidou, A., Fidani, L., & Vassilikos, V. (2025). The Uncommon Phenomenon of Short QT Syndrome: A Scoping Review of the Literature. Journal of Personalized Medicine, 15(3), 105. https://doi.org/10.3390/jpm15030105






