Efficacy and Safety of Anti-CD38 Monoclonal Antibodies in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Endpoints and Subgroup Analysis
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
3. Results
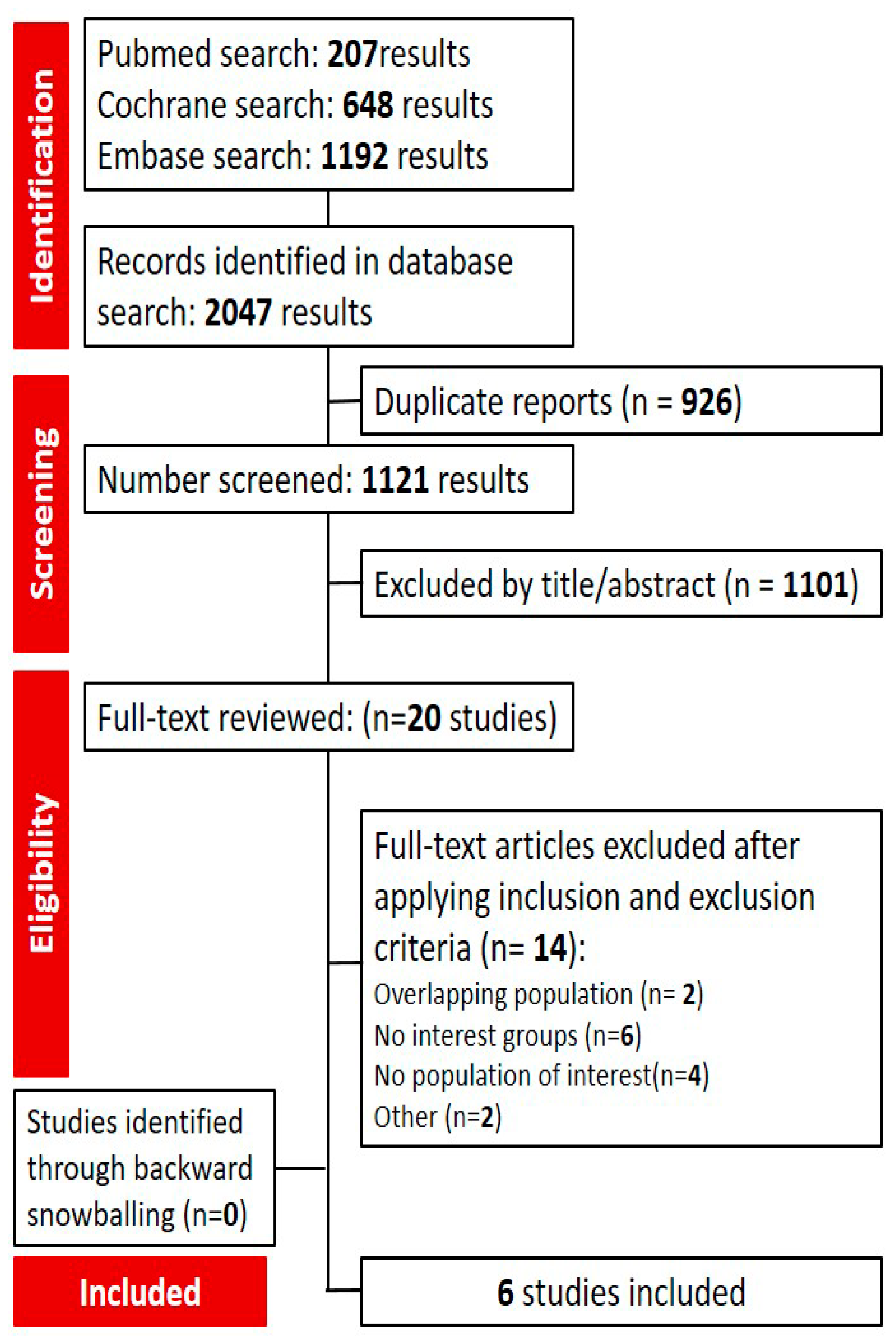
3.1. Search Results and Characteristics of Included Studies
3.2. Results Based on Outcome
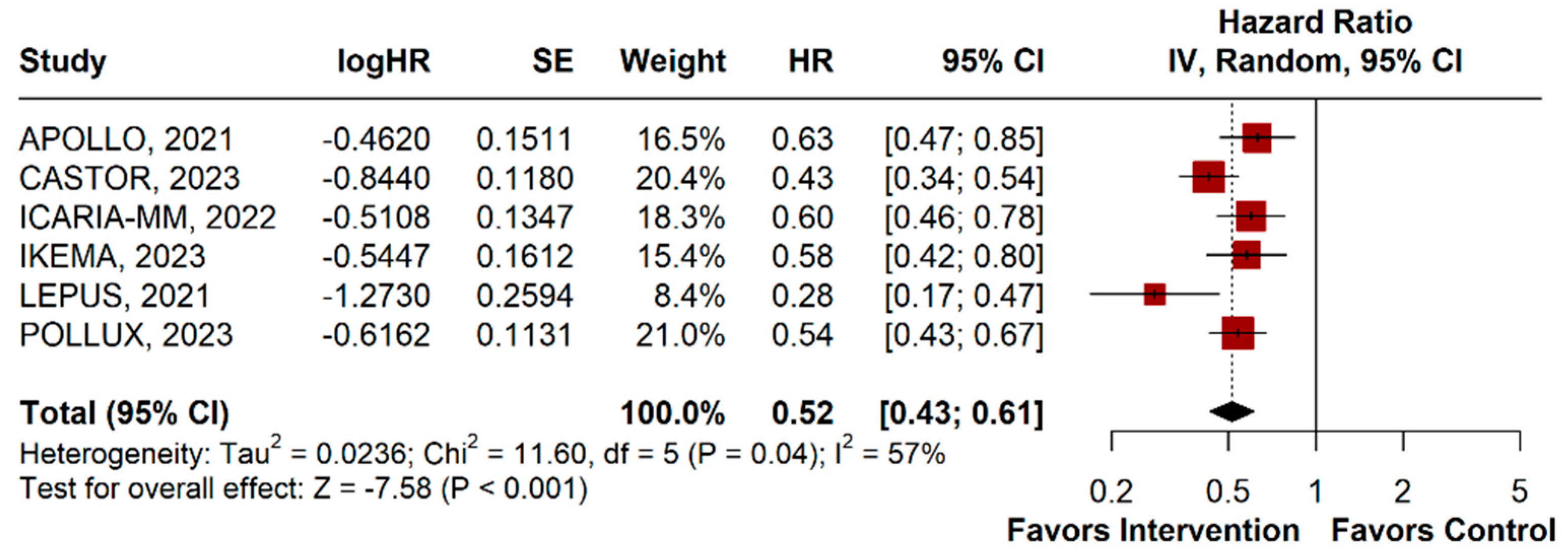
3.2.1. Progression-Free Survival
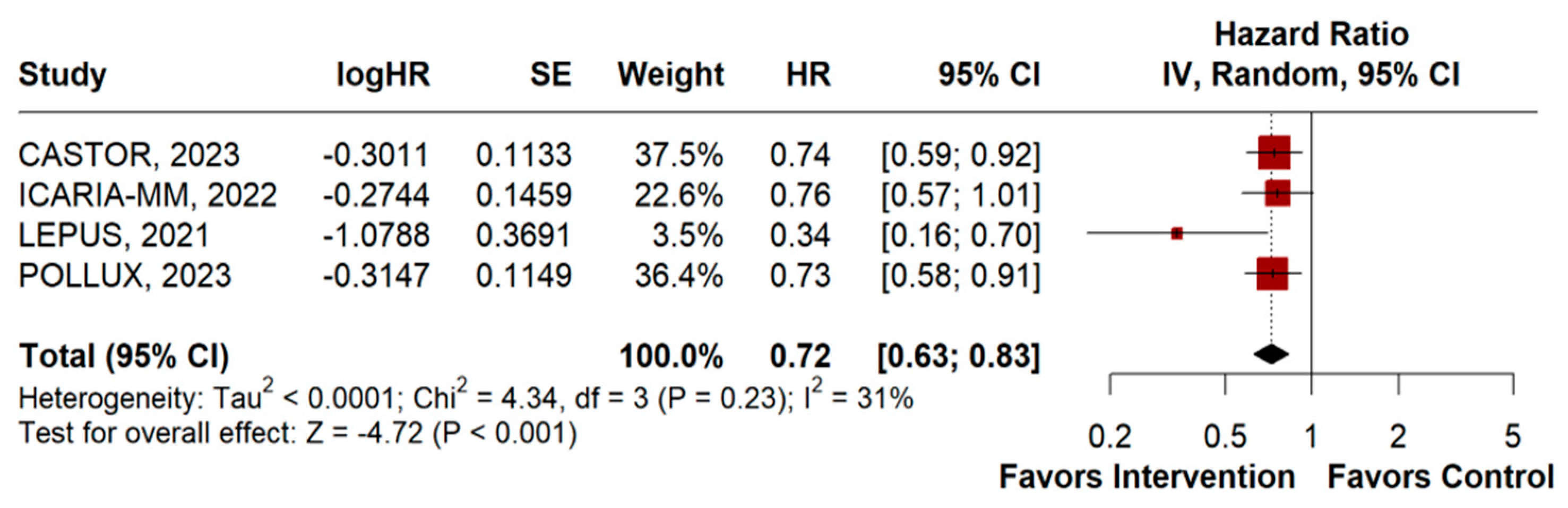
3.2.2. Overall Survival
3.2.3. Adverse Events
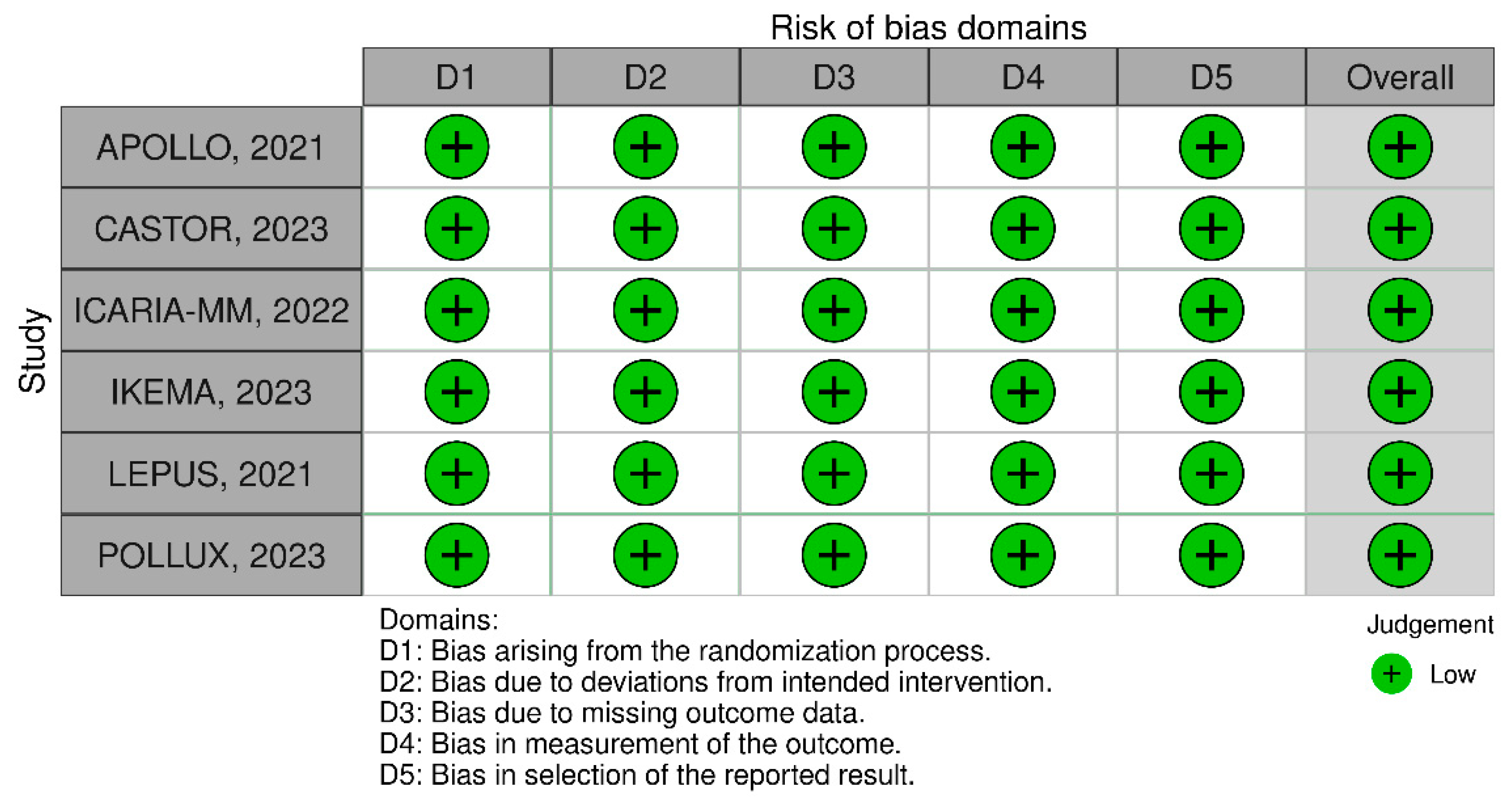
3.2.4. Sensitivity Analysis and Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morandi, F.; Horenstein, A.L.; Costa, F.; Giuliani, N.; Pistoia, V.; Malavasi, F. CD38: A Target for Immunotherapeutic Approaches in Multiple Myeloma. Front. Immunol. 2018, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, W.M.; Bergsagel, P.L. Multiple Myeloma: Evolving Genetic Events and Host Interactions. Nat. Rev. Cancer 2002, 2, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; De Veirman, K.; De Becker, A.; Vanderkerken, K.; Van Riet, I. Mesenchymal Stem Cells in Multiple Myeloma: A Therapeutical Tool or Target? Leukemia 2018, 32, 1500–1514. [Google Scholar] [CrossRef]
- Salomon-Perzyński, A.; Jamroziak, K.; Głodkowska-Mrówka, E. Clonal Evolution of Multiple Myeloma—Clinical and Diagnostic Implications. Diagnostics 2021, 11, 1534. [Google Scholar] [CrossRef] [PubMed]
- Yavorkovsky, L.L. Smoldering Multiple Myeloma 40 Years Later: A Story of Unintended Disease. Expert Rev. Hematol. 2021, 14, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Callander, N. Diagnosis and Management of Monoclonal Gammopathy and Smoldering Multiple Myeloma. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- International Myeloma Working Group. Criteria for the Classification of Monoclonal Gammopathies, Multiple Myeloma and Related Disorders: A Report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal Gammopathy of Undetermined Significance (MGUS) Consistently Precedes Multiple Myeloma: A Prospective Study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef] [PubMed]
- Zingone, A.; Kuehl, W.M. Pathogenesis of Monoclonal Gammopathy of Undetermined Significance (MGUS) and Progression to Multiple Myeloma. Semin. Hematol. 2011, 48, 4–12. [Google Scholar] [CrossRef]
- Kyle, R.A.; Durie, B.G.M.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kröger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering (Asymptomatic) Multiple Myeloma: IMWG Consensus Perspectives Risk Factors for Progression and Guidelines for Monitoring and Management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Abramson, H.N. Immunotherapy of Multiple Myeloma: Current Status as Prologue to the Future. Int. J. Mol. Sci. 2023, 24, 15674. [Google Scholar] [CrossRef]
- Bianchi, G.; Ghobrial, I.M. Does My Patient with a Serum Monoclonal Spike Have Multiple Myeloma? Hematol. Oncol. Clin. N. Am. 2012, 26, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Phekoo, K.J.; Schey, S.A.; Richards, M.A.; Bevan, D.H.; Bell, S.; Gillett, D.; Møller, H. Consultant Haematologists, South Thames Haematology Specialist Committee A Population Study to Define the Incidence and Survival of Multiple Myeloma in a National Health Service Region in UK. Br. J. Haematol. 2004, 127, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple Myeloma: 2020 Update on Diagnosis, Risk-Stratification and Management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Attal, M.; Roussel, M. Shifts in the Therapeutic Paradigm for Patients Newly Diagnosed with Multiple Myeloma: Maintenance Therapy and Overall Survival. Clin. Cancer Res. 2011, 17, 1253–1263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, Z.M.; Vohra, S.N.; Jensen, C.E.; Nyrop, K.A.; Deal, A.M.; Heiling, H.M.; Mangieri, N.J.; Grant, S.J.; Lichtman, E.I.; Rubinstein, S.M.; et al. Prevalence and Clinical Correlates of Cognitive Impairment in Adults with Plasma Cell Disorders. J. Geriatr. Oncol. 2022, 13, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, Q.; Wei, G.; Wang, L.; Huang, Y.; Hu, K.; Hu, Y.; Huang, H. Measuring the Global, Regional, and National Burden of Multiple Myeloma from 1990 to 2019. BMC Cancer 2021, 21, 606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved Survival in Multiple Myeloma and the Impact of Novel Therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Kazandjian, D. Multiple Myeloma Epidemiology and Survival: A Unique Malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Abouzaid, S.; Bonafede, M.; Cai, Q.; Parikh, K.; Cosler, L.; Richardson, P. Trends in Overall Survival and Costs of Multiple Myeloma, 2000–2014. Leukemia 2017, 31, 1915–1921. [Google Scholar] [CrossRef]
- Moreau, P.; Zamagni, E.; Mateos, M.-V. Treatment of Patients with Multiple Myeloma Progressing on Frontline-Therapy with Lenalidomide. Blood Cancer J. 2019, 9, 38. [Google Scholar] [CrossRef]
- Ho, M.; Bianchi, G.; Anderson, K.C. Proteomics-Inspired Precision Medicine for Treating and Understanding Multiple Myeloma. Expert Rev. Precis. Med. Drug Dev. 2020, 5, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Gondos, A.; Pulte, D. Recent Major Improvement in Long-Term Survival of Younger Patients with Multiple Myeloma. Blood 2008, 111, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 Antibodies in Multiple Myeloma: Back to the Future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, S.; Sanchorawala, V. Treatment Options For Relapsed/Refractory Systemic Light-Chain (AL) Amyloidosis: Current Perspectives. J. Blood Med. 2019, 10, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Kloock, C.; Comenzo, R. Relapsed/Refractory Multiple Myeloma: A Review of Available Therapies and Clinical Scenarios Encountered in Myeloma Relapse. Curr. Oncol. 2023, 30, 2322–2347. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.G.; Corzo, K.; Chiron, M.; van de Velde, H.; Abbadessa, G.; Campana, F.; Solanki, M.; Meng, R.; Lee, H.; Wiederschain, D.; et al. Therapeutic Opportunities with Pharmacological Inhibition of CD38 with Isatuximab. Cells 2019, 8, 1522. [Google Scholar] [CrossRef]
- Chillemi, A.; Quarona, V.; Antonioli, L.; Ferrari, D.; Horenstein, A.L.; Malavasi, F. Roles and Modalities of Ectonucleotidases in Remodeling the Multiple Myeloma Niche. Front. Immunol. 2017, 8, 305. [Google Scholar] [CrossRef]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef] [PubMed]
- Munshi, C.; Aarhus, R.; Graeff, R.; Walseth, T.F.; Levitt, D.; Lee, H.C. Identification of the Enzymatic Active Site of CD38 by Site-Directed Mutagenesis. J. Biol. Chem. 2000, 275, 21566–21571. [Google Scholar] [CrossRef]
- Lapietra, G.; Fazio, F.; Petrucci, M.T. Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment. Biomolecules 2022, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 9 February 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman Method for Random Effects Meta-Analysis Is Straightforward and Considerably Outperforms the Standard DerSimonian-Laird Method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Delimpasi, S.; Beksac, M.; Katodritou, E.; Moreau, P.; Baldini, L.; Symeonidis, A.; Bila, J.; et al. Daratumumab plus Pomalidomide and Dexamethasone versus Pomalidomide and Dexamethasone Alone in Previously Treated Multiple Myeloma (APOLLO): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Perrot, A.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; Huang, J.S.-Y.; et al. Isatuximab plus Pomalidomide and Low-Dose Dexamethasone versus Pomalidomide and Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma (ICARIA-MM): Follow-up Analysis of a Randomised, Phase 3 Study. Lancet Oncol. 2022, 23, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, Carfilzomib, and Dexamethasone in Patients with Relapsed Multiple Myeloma: Updated Results from IKEMA, a Randomized Phase 3 Study. Blood Cancer J. 2023, 13, 72. [Google Scholar] [CrossRef]
- Lu, J.; Fu, W.; Li, W.; Hu, J.; An, G.; Wang, Y.; Fu, C.; Chen, L.; Jin, J.; Cen, X.; et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Chinese Patients with Relapsed or Refractory Multiple Myeloma: Phase 3 LEPUS (MMY3009) Study. Clin. Lymphoma Myeloma Leuk. 2021, 21, e699–e709. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Suzuki, K.; Plesner, T.; et al. Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.-V.; et al. Overall Survival With Daratumumab, Bortezomib, and Dexamethasone in Previously Treated Multiple Myeloma (CASTOR): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Overdijk, M.B.; Jansen, J.H.M.; Nederend, M.; Lammerts van Bueren, J.J.; Groen, R.W.J.; Parren, P.W.H.I.; Leusen, J.H.W.; Boross, P. The Therapeutic CD38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death via Fcγ Receptor-Mediated Cross-Linking. J. Immunol. 2016, 197, 807–813. [Google Scholar] [CrossRef]
- Gozzetti, A.; Ciofini, S.; Simoncelli, M.; Santoni, A.; Pacelli, P.; Raspadori, D.; Bocchia, M. Anti CD38 Monoclonal Antibodies for Multiple Myeloma Treatment. Hum. Vaccines Immunother. 2022, 18, 2052658. [Google Scholar] [CrossRef]
- Melis, J.P.M.; Strumane, K.; Ruuls, S.R.; Beurskens, F.J.; Schuurman, J.; Parren, P.W.H.I. Complement in Therapy and Disease: Regulating the Complement System with Antibody-Based Therapeutics. Mol. Immunol. 2015, 67, 117–130. [Google Scholar] [CrossRef]
- Taylor, R.P.; Lindorfer, M.A. Cytotoxic Mechanisms of Immunotherapy: Harnessing Complement in the Action of Anti-Tumor Monoclonal Antibodies. Semin. Immunol. 2016, 28, 309–316. [Google Scholar] [CrossRef]
- Saltarella, I.; Desantis, V.; Melaccio, A.; Solimando, A.G.; Lamanuzzi, A.; Ria, R.; Storlazzi, C.T.; Mariggiò, M.A.; Vacca, A.; Frassanito, M.A. Mechanisms of Resistance to Anti-CD38 Daratumumab in Multiple Myeloma. Cells 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Usmani, S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018, 9, 2134. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E.; Gessner, J.E. Fc Receptors and Their Interaction with Complement in Autoimmunity. Immunol. Lett. 2005, 100, 56–67. [Google Scholar] [CrossRef]
- de Weers, M.; Tai, Y.-T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.H.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a Novel Therapeutic Human CD38 Monoclonal Antibody, Induces Killing of Multiple Myeloma and Other Hematological Tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef]
- Tay, M.Z.; Wiehe, K.; Pollara, J. Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses. Front. Immunol. 2019, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, C.; Macagno, M.; Sangiolo, D.; Bertolaccini, L.; Aglietta, M.; Merlano, M.C. NK-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity in Solid Tumors: Biological Evidence and Clinical Perspectives. Ann. Transl. Med. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Pérez, A.; Oñate, C.; Andrés-Tovar, A.; Garzón-Tituaña, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228. [Google Scholar] [CrossRef] [PubMed]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Payer, Á.R.; Gonzalez, S.; López-Soto, A. Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. Int. J. Mol. Sci. 2020, 21, 3726. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, A.M.; Bondza, S.; Evers, M.; Rösner, T.; Valerius, T.; ten Broeke, T. Potent Fc Receptor Signaling by IgA Leads to Superior Killing of Cancer Cells by Neutrophils Compared to IgG. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Zent, C.S.; Elliott, M.R. Maxed out Macs: Physiologic Cell Clearance as a Function of Macrophage Phagocytic Capacity. FEBS J. 2017, 284, 1021–1039. [Google Scholar] [CrossRef]
- Kamen, L.; Myneni, S.; Langsdorf, C.; Kho, E.; Ordonia, B.; Thakurta, T.; Zheng, K.; Song, A.; Chung, S. A Novel Method for Determining Antibody-Dependent Cellular Phagocytosis. J. Immunol. Methods 2019, 468, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Song, Z.; Wang, A.; Srinivasan, S.; Yang, G.; Greco, R.; Theilhaber, J.; Shehu, E.; Wu, L.; Yang, Z.-Y.; et al. Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front. Immunol. 2020, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Shen, W. Isatuximab in the Treatment of Multiple Myeloma: A Review and Comparison with Daratumumab. Technol. Cancer Res. Treat. 2022, 21, 15330338221106563. [Google Scholar] [CrossRef]
- Romano, A.; Storti, P.; Marchica, V.; Di Raimondo, F.; Giuliani, N. Mechanisms of Action of the New Antibodies in Use in Multiple Myeloma. Front. Oncol. 2021, 11, 684561. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Seitzler, S.; Finley-Oliver, E.; Simonelli, C.; Baz, R. Quality of Life in Multiple Myeloma: Considerations and Recommendations. Expert Rev. Hematol. 2019, 12, 419–424. [Google Scholar] [CrossRef]
- Fragola, M. Patient-Reported Outcomes and Assessment of Quality of Life: A Focus on Multiple Myeloma. J. Adv. Pract. Oncol. 2020, 11, 513–520. [Google Scholar] [CrossRef]
| Study | Designs | Sample Size | Intervention | Age † | Sex Male/Female | Race | ECOG Status, No (%) | Prior Lines of Therapy | International Staging System Disease Stage | Type of Measurable MM IgG/Non-IgG |
|---|---|---|---|---|---|---|---|---|---|---|
| APOLLO, 2021 [42] | RCT-phase III | IG:151 CG:153 | IG: Daratumumab + polamalidomide + dexamethasone CG: Pomalidomide + dexamethasone | IG: 67 (42–86) CG: 68 (35–90) | IG: 79/72 CG: 82/71 | IG: White-135 (89%); Non-white-16 (11%) CG: White-137 (90%); Non-white-16 (10%) | IG: 0–91 (60%); ≥1–60 (40%); CG: 0–77 (50%); ≥1–76 (50%) | IG: 1–16 (11%); 2/3–114 (75%); ≥4–21 (14%) CG: 1–18 (12%); 2/3–113 (74%); ≥4–22 (14%) | IG: I-68 (45%); II-50 (33%); III-33 (22%) CG: I-69 (45%); II-51 (33%); III-33 (22%) | IG: IgG-62 (41%); Non-IgG-89 (59%) CG: IgG-63 (41%); Non-IgG-90 (59%) |
| CASTOR, 2023 [47] | RCT-phase III | IG:251 CG:247 | IG: Daratumumab + bortezomib + dexamethasone CG: Bortezomib + dexamethasone | Overall:64 (30–88) | IG:137/114 CG:148/99 | IG: White-216 (86%); Non-white-35(14%) CG: White-219 (88%); Non-white-28 (12%) | IG: 0–106 (42%); ≥1–144 (58%); Not reported: 1 CG: 0–116 (47%); ≥1–131 (53%) | IG: 1–122 (48.6%); 2/3–107 (42.6%); ≥4–22 (9.2%) CG: 1–113 (45.7%); 2/3–106 (42.9%); ≥4–28 (11.4%) | IG: I-98 (39%); II-94 (37%); III-59 (24%) CG: I-96 (39%); II-100 (40%); III-42 (21%) | IG: IgG-125 (67%); Non-IgG-61 (33%); Unknown: 65 CG: IgG-138 (70%); Non-IgG-58 (30%); Unknown: 51 |
| ICARIA-MM, 2022 [43] | RCT-phase III | IG:154 CG:153 | IG: Isatuximab + pomalidomide + dexamethasone CG: Pomalidomide + dexamethasone | IG:68 CG:66 | IG:89/65 CG:70/83 | NA | NA | NA | IG: I-64 (42%); II-53 (34%); III-34 (22%); Unknown-3 (2%) CG: I-51 (33%); II-56 (37%); III-26 (14.5%); Unknown-1 (0.6%) | IG: IgG-102 (66%); Non-IgG-52 (34%) CG: IgG-100 (65%); Non-IgG-53 (35%) |
| IKEMA, 2023 [44] | RCT-phase III | IG:179 CG:123 | IG: Isatuximab + carfilzomib + dexamethasone CG: Carfilzomib + dexamethasone | IG:65 (37–86) CG:63 (33–90) | NA | NA | NA | IG: 1–79 (44.1%); 2/3–97 (55.9%); ≥4–0 CG: 1–55 (44.7%); 2/3–66 (55.3%); ≥4–0 | IG: I-89 (49.7%); II-63 (35.2%); III-34 (22%); Unknown-3 (2%) CG: I-71 (57.7%); II-31 (25.2%); III-20 (16.3%); Unknown-1 (0.8%) | NA |
| LEPUS, 2021 [45] | RCT-phase III | IG:141 CG:70 | IG: Daratumumab + bortezomib + dexamethasone CG: Bortezomib + dexamethasone | IG:61.0 (28–79) CG:61.0 (43–82) | IG:85/56 CG:42/28 | NA | IG: 0–64 (45.4%); ≥1–77 (54.6%); CG: 0–27 (38.6%); ≥1–43 (61.4%); | IG: 1–41 (29.1%); 2/3–70 (49.6%); ≥4–30 (21.3%) CG: 1–19 (27.1%); 2/3–33 (47.1%); ≥4–18 (25.7%) | IG: I-72 (51.1%); II-45 (31.9%); III-24 (17%) CG: I-34 (48.6%); II-22 (31.4%); III-14 (20%) | IG: IgG-52 (36.9%); Non-IgG-89 (63.1%) CG: IgG-28 (40%); Non-IgG-42 (60%) |
| POLLUX, 2023 [46] | RCT-phase III | IG:286 CG:283 | IG: Daratumumab + lenalidomide + dexamethasone CG: lenalidomide + dexamethasone | IG:65.0 (34–89) CG:65.0 (42–87) | IG:173/113 CG:164/119 | IG:: White-207 (72.4)%); Non-white-79 (27.6%) CG: White-186 (65.7%); Non-white-97 (34.3%) | IG: 0–139 (48.6%); ≥1–147 (51.4%); CG: 0–150 (53%); ≥1–133 (47%); | IG: 1–149 (52%); 2/3–123 (43%); ≥4–14 (5%) CG: 1–146 (51%); 2/3–118 (41.7%); ≥4–19 (7.3%) | IG: I-137 (48%); II-93 (32.5%); III-56 (19.5%) CG: I-140 (49.5%); II-86 (30.4%); III-57 (20.1%) | G: IgG-151 (73.6%); Non-IgG-54 (26.4%); Unknown: 81 CG: IgG-158 (74.9%); Non-IgG-53 (25.1%); Unknown: 72 |
| Adverse Events | Events/Total Intervention | Events/Total Control | RR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Hematological adverse events | |||||
| Anemia | 543/1144 | 434/1007 | 0.99 | 0.90–1.09 | 0.83 |
| Febrile neutropenia | 49/584 | 17/580 | 2.83 | 1.60–4.87 | <0.01 |
| Lymphopenia | 155/815 | 73/736 | 1.62 | 0.96–2.74 | 0.07 |
| Neutropenia | 606/1144 | 376/1007 | 1.41 | 1.26–1.58 | <0.01 |
| Thrombocytopenia | 607/1144 | 425/1007 | 1.14 | 1.02–1.27 | 0.02 |
| Non-hematological adverse events | |||||
| Arthralgia | 180/855 | 101/789 | 1.69 | 1.07–2.69 | 0.03 |
| Asthenia | 181/1004 | 168/939 | 1.00 | 0.81–1.24 | 0.97 |
| Back pain | 205/855 | 135/789 | 1.38 | 1.05–1.82 | 0.02 |
| Bronchitis | 187/855 | 97/789 | 1.89 | 1.30–2.75 | <0.01 |
| Constipation | 199/818 | 164/735 | 1.01 | 0.71–1.43 | 0.96 |
| Cough | 246/843 | 98/708 | 2.19 | 1.77–2.70 | <0.01 |
| Diarrhea | 458/1144 | 281/1007 | 1.41 | 1.23–1.63 | <0.01 |
| Dyspnea | 198/855 | 102/789 | 1.72 | 1.38–2.13 | <0.01 |
| Fatigue | 303/1004 | 218/939 | 1.36 | 0.97–1.91 | 0.08 |
| Hypertension | 124/560 | 54/427 | 2.38 | 0.81–6.99 | 0.11 |
| Insomnia | 198/843 | 139/708 | 1.20 | 0.99–1.45 | 0.07 |
| Nausea | 147/678 | 94/667 | 1.55 | 1.23–1.95 | <0.01 |
| Peripheral edema | 151/678 | 88/667 | 1.70 | 1.27–2.28 | <0.01 |
| Pneumonia | 263/1144 | 175/1007 | 1.34 | 1.13–1.59 | <0.01 |
| Pyrexia | 222/967 | 122/885 | 1.63 | 1.33–1.99 | <0.01 |
| Upper respiratory tract infection | 423/1144 | 224/1007 | 1.64 | 1.43–1.89 | <0.01 |
| Adverse Events | Events/Total Intervention | Events/Total Control | RR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Hematological adverse events | |||||
| Anemia | 205/1144 | 173/1007 | 1.00 | 0.81–1.24 | 0.99 |
| Febrile neutropenia | 49/584 | 17/580 | 2.83 | 1.65–4.87 | <0.01 |
| Lymphopenia | 121/815 | 43/736 | 2.13 | 1.24–3.64 | <0.01 |
| Neutropenia | 455/1144 | 276/1007 | 1.64 | 1.33–2.01 | <0.01 |
| Thrombocytopenia | 330/1144 | 225/1007 | 1.25 | 1.08–1.44 | <0.01 |
| Non-hematological adverse events | |||||
| Arthralgia | 15/855 | 7/789 | 1.62 | 0.65–4.04 | 0.30 |
| Asthenia | 29/1004 | 23/939 | 1.08 | 0.52–2.22 | 0.84 |
| Back pain | 23/855 | 11/789 | 1.98 | 0.97–4.04 | 0.06 |
| Bronchitis | 27/855 | 14/789 | 1.78 | 0.83–3.84 | 0.14 |
| Constipation | 4/818 | 4/735 | 0.88 | 0.10–7.90 | 0.91 |
| Cough | 1/843 | 2/708 | 0.52 | 0.02–14.73 | 0.70 |
| Diarrhea | 63/1144 | 25/1007 | 1.95 | 1.10–3.47 | 0.02 |
| Dyspnea | 42/855 | 7/789 | 5.32 | 2.39–11.84 | <0.01 |
| Fatigue | 71/1004 | 46/939 | 1.86 | 1.19–2.91 | <0.01 |
| Hypertension | 75/560 | 32/427 | 2.94 | 0.62–13.96 | 0.18 |
| Insomnia | 20/843 | 12/708 | 1.30 | 0.62–2.71 | 0.48 |
| Nausea | 8/678 | 2/667 | 3.31 | 0.81–13.56 | 0.10 |
| Peripheral edema | 6/678 | 4/667 | 1.25 | 0.36–4.27 | 0.73 |
| Pneumonia | 173/1144 | 117/1007 | 1.31 | 1.06–1.63 | 0.01 |
| Pyrexia | 19/967 | 12/885 | 1.47 | 0.73–2.98 | 0.28 |
| Upper respiratory tract infection | 44/1144 | 22/1007 | 1.97 | 1.02–3.79 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, F.C.A.d.; Sano, V.K.T.; Lôbo, A.d.O.M.; Kelly, F.A.; Morbach, V.; Pasqualotto, E.; Burbano, R.M.R. Efficacy and Safety of Anti-CD38 Monoclonal Antibodies in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Randomized Clinical Trials. J. Pers. Med. 2024, 14, 360. https://doi.org/10.3390/jpm14040360
Moraes FCAd, Sano VKT, Lôbo AdOM, Kelly FA, Morbach V, Pasqualotto E, Burbano RMR. Efficacy and Safety of Anti-CD38 Monoclonal Antibodies in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Randomized Clinical Trials. Journal of Personalized Medicine. 2024; 14(4):360. https://doi.org/10.3390/jpm14040360
Chicago/Turabian StyleMoraes, Francisco Cezar Aquino de, Vitor Kendi Tsuchiya Sano, Artur de Oliveira Macena Lôbo, Francinny Alves Kelly, Victória Morbach, Eric Pasqualotto, and Rommel Mario Rodríguez Burbano. 2024. "Efficacy and Safety of Anti-CD38 Monoclonal Antibodies in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Randomized Clinical Trials" Journal of Personalized Medicine 14, no. 4: 360. https://doi.org/10.3390/jpm14040360
APA StyleMoraes, F. C. A. d., Sano, V. K. T., Lôbo, A. d. O. M., Kelly, F. A., Morbach, V., Pasqualotto, E., & Burbano, R. M. R. (2024). Efficacy and Safety of Anti-CD38 Monoclonal Antibodies in Patients with Relapsed or Refractory Multiple Myeloma: A Meta-Analysis of Randomized Clinical Trials. Journal of Personalized Medicine, 14(4), 360. https://doi.org/10.3390/jpm14040360









