Investigating ABO Blood Groups and Secretor Status in Relation to SARS-CoV-2 Infection and COVID-19 Severity
Abstract
1. Introduction
2. Methods
3. Results
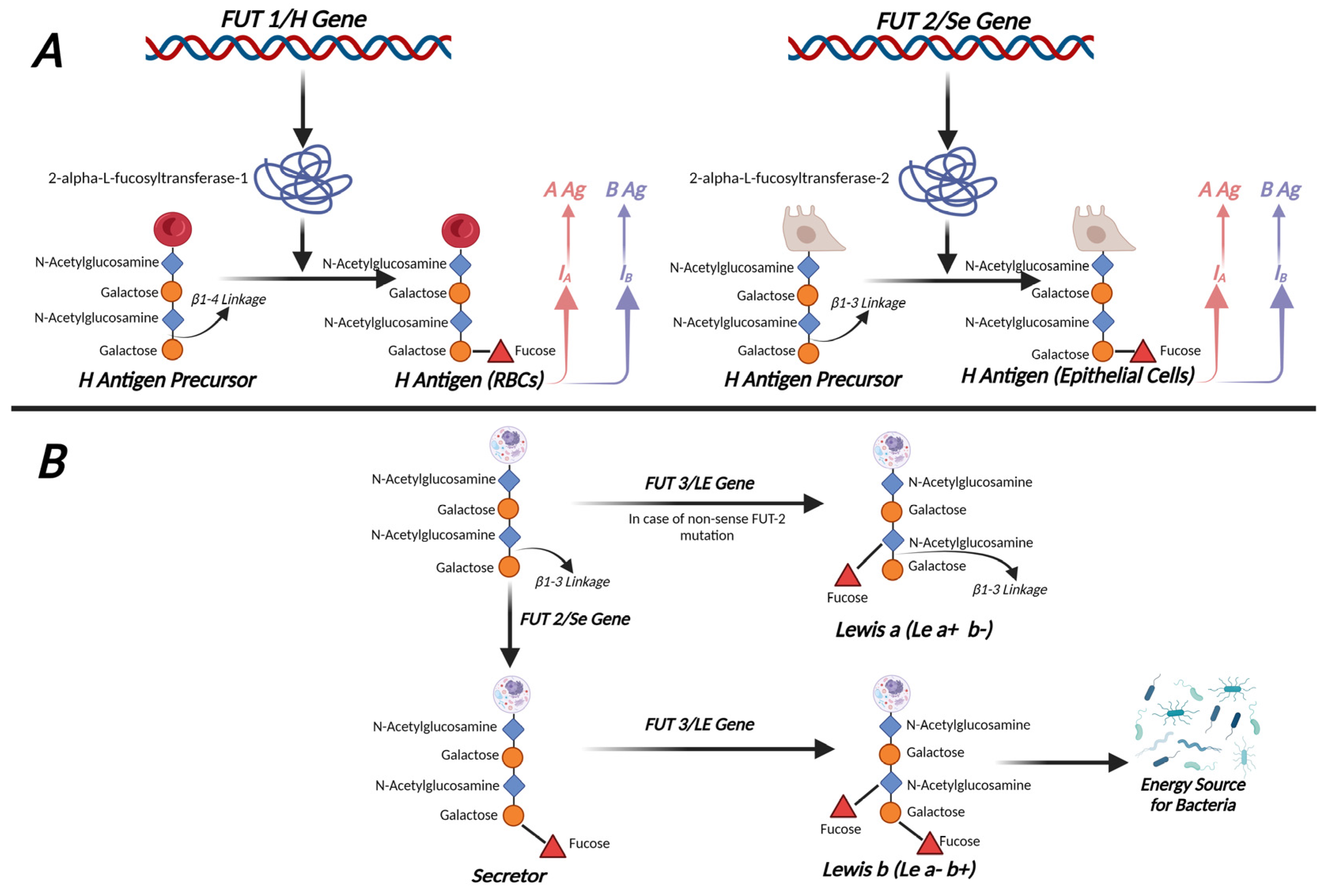
3.1. Overview of the ABO Blood Group, Lewis Antigens, and Secretor Systems
3.2. Reported Associations of SARS-CoV-2/COVID-19 with ABO Blood Groups, Lewis Antigens, and Secretor Systems
3.2.1. ABO Blood Groups and Secretor Status in Relation to COVID-19 Severity
3.2.2. Proposed Theories for Explaining the Potential Association between the A Blood Group and Increased COVID-19 Severity
3.2.3. ABO Blood Groups and Secretor Status in Relation to Long COVID
3.2.4. ABO Blood Groups and Secretor Status in Relation to COVID-19 Vaccine Efficacy and Safety
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ARDS | Acute respiratory distress syndrome |
| COVID-19 | Coronavirus disease 2019 |
| FUT1 (H gene) | Fucosyltransferase 1 |
| FUT2 (Secretor gene, Se) | Fucosyltransferase 2 |
| FUT3 (Lewis gene, Le) | Fucosyltransferase 3 |
| HBGAs | Histo-blood group antigens |
| Lea | Lewis antigen A |
| Leb | Lewis antigen B |
| RBCs | Red blood cells |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
References
- Karlsson, E.K.; Kwiatkowski, D.P.; Sabeti, P.C. Natural selection and infectious disease in human populations. Nat. Rev. Genet. 2014, 15, 379–393. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Disease associations of the ABO blood group. Acta Genet. Stat. Med. 1956, 6, 561–563. [Google Scholar] [CrossRef]
- Lindesmith, L.; Moe, C.; Marionneau, S.; Ruvoen, N.; Jiang, X.; Lindblad, L.; Stewart, P.; LePendu, J.; Baric, R. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003, 9, 548–553. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Ahmed, T.; Rahman, M.A.; Chowdhury, F.; Rashu, R.; Khan, A.I.; LaRocque, R.C.; Harris, J.B.; Bhuiyan, T.R.; Ryan, E.T.; et al. Individuals with Le(a+b-) blood group have increased susceptibility to symptomatic vibrio cholerae O1 infection. PLoS Negl. Trop. Dis. 2011, 5, e1413. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Dickson, D.M.; Decamp, A.C.; Colgate, E.R.; Diehl, S.A.; Uddin, M.I.; Sharmin, S.; Islam, S.; Bhuiyan, T.R.; Alam, M.; et al. Histo-Blood Group Antigen Phenotype Determines Susceptibility to Genotype-Specific Rotavirus Infections and Impacts Measures of Rotavirus Vaccine Efficacy. J. Infect. Dis. 2018, 217, 1399–1407. [Google Scholar] [CrossRef]
- Dixon, R.E. Economic costs of respiratory tract infections in the United States. Am. J. Med. 1985, 78, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Coimbra, J.; Sarda, C.; Rello, J. Burden of Community-Acquired Pneumonia and Unmet Clinical Needs. Adv. Ther. 2020, 37, 1302–1318. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, WHO. COVID-19 Epidemiological Update—19 January 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---19-january-2024 (accessed on 8 February 2024).
- Boufidou, F.; Medić, S.; Lampropoulou, V.; Siafakas, N.; Tsakris, A.; Anastassopoulou, C. SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. Int. J. Mol. Sci. 2023, 24, 12962. [Google Scholar] [CrossRef]
- Marionneau, S.; Cailleau-Thomas, A.; Rocher, J.; Le Moullac-Vaidye, B.; Ruvoën, N.; Clément, M.; Le Pendu, J. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 2001, 83, 565–573. [Google Scholar] [CrossRef]
- Dean, L. Blood Groups and Red Cell Antigens [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2005; Chapter 6; The Hh Blood Group. Available online: https://www.ncbi.nlm.nih.gov/books/NBK2268/ (accessed on 30 November 2023).
- Koda, Y.; Soejima, M.; Liu, Y.; Kimura, H. Molecular basis for secretor type alpha(1,2)-fucosyltransferase gene deficiency in a Japanese population: A fusion gene generated by unequal crossover responsible for the enzyme deficiency. Am. J. Hum. Genet. 1996, 59, 343–350. [Google Scholar]
- Oriol, R.; Candelier, J.J.; Mollicone, R. Molecular genetics of H. Vox Sang. 2000, 78 (Suppl. S2), 105–108. [Google Scholar] [PubMed]
- Mollicone, R.; Cailleau, A.; Oriol, R. Molecular genetics of H, Se, Lewis and other fucosyltransferase genes. Transfus. Clin. Biol. 1995, 2, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gupta, M.; Sagar, V. FUT2 gene as a genetic susceptible marker of infectious diseases: A Review. Int. J. Mol. Epidemiol. Genet. 2022, 13, 1–14. [Google Scholar]
- Ravn, V.; Dabelsteen, E. Tissue distribution of histo-blood group antigens. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2000, 108, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, R. AB para-Bombay phenotype: A rare blood group variant and its clinical significance. Hematol. Transfus. Cell Ther. 2018, 40, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Saboor, M.; Ullah, A.; Qamar, K.; Mir, A. Frequency of ABH secretors and non secretors: A cross sectional study in Karachi. Pak. J. Med. Sci. 2014, 30, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Yang, Y.H.; Broadberry, R.E.; Chen, Y.H.; Chan, Y.S.; Lin, M. Correlation of a missense mutation in the human Secretor alpha 1,2-fucosyltransferase gene with the Lewis(a+b+) phenotype: A potential molecular basis for the weak Secretor allele (Sew). Biochem. J. 1995, 312 Pt 2, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Soejima, M.; Koda, Y. Molecular mechanisms of Lewis antigen expression. Leg. Med. 2005, 7, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Mollicone, R.; Reguigne, I.; Kelly, R.; Fletcher, A.; Watt, J.; Chatfield, S.; Aziz, A.; Cameron, H.; Weston, B.; Lowe, J. Molecular basis for Lewis alpha(1,3/1,4)-fucosyltransferase gene deficiency (FUT3) found in Lewis-negative Indonesian pedigrees. J. Biol. Chem. 1994, 269, 20987–20994. [Google Scholar] [CrossRef]
- Subramaniyan, R. Serological characteristics of Lewis antibodies and their clinical significance—A case series. Hematol. Transfus. Cell Ther. 2023, 45, 159–164. [Google Scholar] [CrossRef]
- May, S.J.; Blackwell, C.C.; Weir, D.M. Lewis a blood group antigen of non-secretors: A receptor for candida blastospores. FEMS Microbiol. Immunol. 1989, 1, 407–409. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Wu, S.-C.; Zheng, L.; Jajosky, A.N.; Jajosky, P.G.; Josephson, C.D.; Hollenhorst, M.A.; Sackstein, R.; Cummings, R.D.; Arthur, C.M.; et al. ABO blood group antigens and differential glycan expression: Perspective on the evolution of common human enzyme deficiencies. iScience 2022, 26, 105798. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Pagliano, P.; Sellitto, C.; Conti, V.; Ascione, T.; Esposito, S. Characteristics of viral pneumonia in the COVID-19 era: An update. Infection 2021, 49, 607–616. [Google Scholar] [CrossRef]
- Griffin, D.O.; Brennan-Rieder, D.; Ngo, B.; Kory, P.; Confalonieri, M.; Shapiro, L.; Iglesias, J.; Dube, M.; Nanda, N.; In, G.K.; et al. The Importance of Understanding the Stages of COVID-19 in Treatment and Trials. AIDS Rev. 2021, 23, 40–47. [Google Scholar] [CrossRef]
- Mokhtari, T.; Hassani, F.; Ghaffari, N.; Ebrahimi, B.; Yarahmadi, A.; Hassanzadeh, G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J. Mol. Histol. 2020, 51, 613–628. [Google Scholar] [CrossRef]
- Kutsogiannis, D.J.; Alharthy, A.; Balhamar, A.; Faqihi, F.; Papanikolaou, J.; Alqahtani, S.A.; Memish, Z.A.; Brindley, P.G.; Brochard, L.; Karakitsos, D. Mortality and Pulmonary Embolism in Acute Respiratory Distress Syndrome From COVID-19 vs. Non-COVID-19. Front. Med. 2022, 9, 800241. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Spanakis, N.; Tsakris, A. SARS-CoV-2 transmission, the ambiguous role of children and considerations for the reopening of schools in the fall. Future Microbiol. 2020, 15, 1201–1206. [Google Scholar] [CrossRef]
- Fontanet, A.; Tondeur, L.; Grant, R.; Temmam, S.; Madec, Y.; Bigot, T.; Grzelak, L.; Cailleau, I.; Besombes, C.; Ungeheuer, M.-N.; et al. SARS-CoV-2 infection in schools in a northern French city: A retrospective serological cohort study in an area of high transmission, France, January to April 2020. Euro Surveill. 2021, 26, 2001695. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.C.; Biele, G.; Mühlemann, B.; Veith, T.; Schneider, J.; Beheim-Schwarzbach, J.; Bleicker, T.; Tesch, J.; Schmidt, M.L.; Sander, L.E.; et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021, 373, eabi5273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, E.D.; Engin, A. Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking. Environ. Toxicol. Pharmacol. 2020, 78, 103411. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Gkizarioti, Z.; Patrinos, G.P.; Tsakris, A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum. Genom. 2020, 14, 40. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Cai, Y.; Deng, A.; Yang, M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br. J. Haematol. 2020, 190, 24–27. [Google Scholar] [CrossRef]
- Soares, D.M.B.; Araújo, D.A.B.S.; Souza, J.L.d.B.d.; Maurício, R.B.; Soares, E.M.B.; Neto, F.d.C.A.; Pinheiro, M.S.N.; Gama, V.C.d.V.; Braga-Neto, P.; Nóbrega, P.R.; et al. Correlation between ABO blood type, susceptibility to SARS-CoV-2 infection and COVID-19 disease severity: A systematic review. Hematol. Transfus. Cell Ther. 2023, 45, 483–494. [Google Scholar] [CrossRef]
- Golinelli, D.; Boetto, E.; Maietti, E.; Fantini, M.P. The association between ABO blood group and SARS-CoV-2 infection: A meta-analysis. PLoS ONE 2020, 15, e0239508. [Google Scholar] [CrossRef] [PubMed]
- Pourali, F.; Afshari, M.; Alizadeh-Navaei, R.; Javidnia, J.; Moosazadeh, M.; Hessami, A. Relationship between blood group and risk of infection and death in COVID-19: A live meta-analysis. New Microbes New Infect. 2020, 37, 100743. [Google Scholar] [CrossRef] [PubMed]
- Kabrah, S.M.; Kabrah, A.M.; Flemban, A.F.; Abuzerr, S. Systematic review and meta-analysis of the susceptibility of ABO blood group to COVID-19 infection. Transfus. Apher. Sci. 2021, 60, 103169. [Google Scholar] [CrossRef]
- Latz, C.A.; DeCarlo, C.; Boitano, L.; Png, C.Y.M.; Patell, R.; Conrad, M.F.; Eagleton, M.; Dua, A. Blood type and outcomes in patients with COVID-19. Ann. Hematol. 2020, 99, 2113–2118. [Google Scholar] [CrossRef]
- Wu, B.B.; Gu, D.Z.; Yu, J.N.; Yang, J.; Shen, W.Q. Association between ABO blood groups and COVID-19 infection, severity and demise: A systematic review and meta-analysis. Infect. Genet. Evol. 2020, 84, 104485. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Jia, L.; Ai, J.; Yu, Y.; Wang, M.; Li, P. ABO blood group influence COVID-19 infection: A meta-analysis. J. Infect. Dev. Ctries. 2021, 15, 1801–1807. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, T.; Ma, L.; Zhang, H.; Wang, H.; Wei, W.; Pei, H.; Li, H. The impact of ABO blood group on COVID-19 infection risk and mortality: A systematic review and meta-analysis. Blood Rev. 2021, 48, 100785. [Google Scholar] [CrossRef]
- Abuawwad, M.T.; Taha, M.J.J.; Abu-Ismail, L.; Alrubasy, W.A.; Sameer, S.K.; Abuawwad, I.T.; Al-Bustanji, Y.; Nashwan, A.J. Effects of ABO blood groups and RH-factor on COVID-19 transmission, course and outcome: A review. Front. Med. 2023, 9, 1045060. [Google Scholar] [CrossRef]
- Bullerdiek, J.; Reisinger, E.; Rommel, B.; Dotzauer, A. ABO blood groups and the risk of SARS-CoV-2 infection. Protoplasma 2022, 259, 1381–1395. [Google Scholar] [CrossRef]
- Soo, K.M.; Chung, K.M.; Mohd Azlan, M.A.A.; Lam, J.Y.; Ren, J.W.X.; Arvind, J.J.; Wong, Y.P.; Chee, H.Y.; Amin-Nordin, S. The association of ABO and Rhesus blood type with the risks of developing SARS-CoV-2 infection: A meta-analysis. Trop. Biomed. 2022, 39, 126–134. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zhang, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship Between the ABO Blood Group and the Coronavirus Disease 2019 (COVID-19) Susceptibility. Clin. Infect. Dis. 2021, 73, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Dai, X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur. J. Prev. Cardiol. 2020, 27, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; Asselta, R.; et al. Genome wide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Abdulla, S.A.; Elawamy, H.A.; Mohamed, N.A.; Abduallah, E.H.; Amshahar, H.A.; Abuzaeid, N.K.; Eisa, M.A.; Osman, M.E.M.; Konozy, E.H.E. Association of ABO blood types and clinical variables with COVID-19 infection severity in Libya. SAGE Open Med. 2023, 11, 20503121231187736. [Google Scholar] [CrossRef]
- Bari, A.; Ch, A.; Hareem, S.; Bano, I.; Rashid, J.; Sadiq, M. Association of Blood Groups with the Severity and Outcome of COVID-19 Infection in Children. J. Coll. Physicians Surg. Pak. 2021, 30, S57–S59. [Google Scholar] [CrossRef]
- Marraccini, C.; Merolle, L.; Schiroli, D.; Razzoli, A.; Gavioli, G.; Iotti, B.; Baricchi, R.; Ottone, M.; Mancuso, P.; Rossi, P.G. A cohort study on the biochemical and haematological parameters of Italian blood donors as possible risk factors of COVID-19 infection and severe disease in the pre- and post-Omicron period. PLoS ONE 2023, 18, e0294272. [Google Scholar] [CrossRef]
- Franchini, M.; Cruciani, M.; Mengoli, C.; Marano, G.; Candura, F.; Lopez, N.; Pati, I.; Pupella, S.; De Angelis, V. ABO blood group and COVID-19: An updated systematic literature review and meta-analysis. Blood Transfus. 2021, 19, 317–326. [Google Scholar] [CrossRef]
- Soriano, J.B.; Peláez, A.; Busquets, X.; Rodrigo-García, M.; Pérez-Urría, E.; Alonso, T.; Girón, R.; Valenzuela, C.; Marcos, C.; García-Castillo, E.; et al. ABO blood group as a determinant of COVID-19 and Long COVID: An observational, longitudinal, large study. PLoS ONE 2023, 18, e0286769. [Google Scholar] [CrossRef]
- Kousathanas, A.; Pairo-Castineira, E.; Rawlik, K.; Stuckey, A.; Odhams, C.A.; Walker, S.; Russell, C.D.; Malinauskas, T.; Wu, Y.; Millar, J.; et al. Whole-genome sequencing reveals host factors underlying critical COVID-19. Nature 2022, 607, 97–103. [Google Scholar] [CrossRef]
- Reilly, J.P.; Meyer, N.J.; Shashaty, M.G.; Feng, R.; Lanken, P.N.; Gallop, R.; Kaplan, S.; Herlim, M.; Oz, N.L.; Hiciano, I.; et al. ABO blood type A is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest 2014, 145, 753–761. [Google Scholar] [CrossRef]
- Valenti, L.; Villa, S.; Baselli, G.; Temporiti, R.; Bandera, A.; Scudeller, L.; Prati, D. Association of ABO blood group and secretor phenotype with severe COVID-19. Transfusion 2020, 60, 3067–3070. [Google Scholar] [CrossRef]
- Mankelow, T.J.; Singleton, B.K.; Moura, P.L.; Stevens-Hernandez, C.J.; Cogan, N.M.; Gyorffy, G.; Kupzig, S.; Nichols, L.; Asby, C.; Pooley, J.; et al. Blood group type A secretors are associated with a higher risk of COVID-19 cardiovascular disease complications. EJHaem 2021, 2, 175–187. [Google Scholar] [CrossRef]
- Magwira, C.A.; Nndwamato, N.P.; Selabe, G.; Seheri, M.L. Lewis a-b- histo-blood group antigen phenotype is predictive of severe COVID-19 in the black South African population group. Glycobiology 2023, 34, cwad090. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, C.; Sækmose, S.; Larsen, R.; Brodersen, T.; Didriksen, M.; Hjalgrim, H.; Banasik, K.; Nielsen, K.R.; Bruun, M.T.; Dowsett, J.; et al. A large cohort study of the effects of Lewis, ABO, 13 other blood groups, and secretor status on COVID-19 susceptibility, severity, and long COVID-19. Transfusion 2023, 63, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Matzhold, E.M.; Berghold, A.; Bemelmans, M.K.B.; Banfi, C.; Stelzl, E.; Kessler, H.H.; Steinmetz, I.; Krause, R.; Wurzer, H.; Schlenke, P.; et al. Lewis and ABO histo-blood types and the secretor status of patients hospitalized with COVID-19 implicate a role for ABO antibodies in susceptibility to infection with SARS-CoV-2. Transfusion 2021, 61, 2736–2745. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Maggipinto, G.; Minon, J.M. COVID-19 and ABO blood group: Another viewpoint. Br. J. Haematol. 2020, 190, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Bloch, E.M.; Pirenne, F.; Al-Riyami, A.Z.; Crowe, E.; Dau, L.; Land, K.; Townsend, M.; Jecko, T.; Rahimi-Levene, N.; et al. ABO blood group and COVID-19: A review on behalf of the ISBT COVID-19 Working Group. Vox Sang. 2021, 116, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.J.; Gjerding, L.A.M.; Exsteen, M.B.; Benfield, T.; Larsen, R.; Clausen, F.B.; Rieneck, K.; Krog, G.R.; Eriksson, F.; Dziegiel, M.H. Reduced susceptibility to COVID-19 associated with ABO blood group and pre-existing anti-A and anti-B antibodies. Immunobiology 2023, 228, 152399. [Google Scholar] [CrossRef] [PubMed]
- Khder Mustafa, S.; Zrar Omar, S.; Kamal Ahmad, K.; Basil Khudhur, L. The association of ABO blood group distribution and clinical characteristics in patients with SARS-CoV-2. J. Infect. Dev. Ctries. 2023, 17, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Damiani, A.S.; Zizza, A.; Banchelli, F.; Gigante, M.; De Feo, M.L.; Ostuni, A.; Marinelli, V.; Quagnano, S.; Negro, P.; Di Renzo, N.; et al. Association between ABO blood groups and SARS-CoV-2 infection in blood donors of Puglia region. Ann. Hematol. 2023, 102, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Guillon, P.; Clément, M.; Sébille, V.; Rivain, J.-G.; Chou, C.-F.; Ruvoën-Clouet, N.; Le Pendu, J. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology 2008, 18, 1085–1093. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, M.; Leache, L.; Librero, J.; Jericó, C.; Germán, M.E.; García-Erce, J.A. ABO blood group and risk of COVID-19 infection and complications: A systematic review and meta-analysis. Transfusion 2022, 62, 493–505. [Google Scholar] [CrossRef]
- Tamayo-Velasco, Á.; Peñarrubia-Ponce, M.J.; Álvarez, F.J.; de la Fuente, I.; Pérez-González, S.; Andaluz-Ojeda, D. ABO Blood System and COVID-19 Susceptibility: Anti-A and Anti-B Antibodies Are the Key Points. Front. Med. 2022, 9, 882477. [Google Scholar] [CrossRef] [PubMed]
- Lilova, Z.; Hassan, F.; Riaz, M.; Ironside, J.; Ken-Dror, G.; Han, T.; Sharma, P. Blood group and ischemic stroke, myocardial infarction, and peripheral vascular disease: A meta-analysis of over 145,000 cases and 2,000,000 controls. J. Stroke Cerebrovasc. Dis. 2023, 32, 107215. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Bayoumi, N.; Vickers, M.A.; Clark, P. ABO(H) blood groups and vascular disease: A systematic review and meta-analysis. J. Thromb. Haemost. 2008, 6, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Chasman, D.I.; Kellogg, M.; Zee, R.Y.L.; Rifai, N.; Badola, S.; Miletich, J.P.; Ridker, P.M. Novel association of ABO histo-blood group antigen with soluble ICAM-1: Results of a genome-wide association study of 6578 women. PLoS Genet. 2008, 4, e1000118. [Google Scholar] [CrossRef] [PubMed]
- Pendu, J.L.; Breiman, A.; Rocher, J.; Dion, M.; Ruvoën-Clouet, N. ABO Blood Types and COVID-19: Spurious, Anecdotal, or Truly Important Relationships? A Reasoned Review of Available Data. Viruses 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Velasco, Á.; Ponce, M.J.P.; Álvarez, F.J.; Gonzalo-Benito, H.; de la Fuente, I.; Pérez-González, S.; Rico, L.; García, M.T.J.; Rodríguez, A.S.; Villaizan, M.H.; et al. Can the Cytokine Profile According to ABO Blood Groups Be Related to Worse Outcome in COVID-19 Patients? Yes, They Can. Front. Immunol. 2021, 12, 726283. [Google Scholar] [CrossRef] [PubMed]
- Hoiland, R.L.; Fergusson, N.A.; Mitra, A.R.; Griesdale, D.E.G.; Devine, D.V.; Stukas, S.; Cooper, J.; Thiara, S.; Foster, D.; Chen, L.Y.C.; et al. The association of ABO blood group with indices of disease severity and multiorgan dysfunction in COVID-19. Blood Adv. 2020, 4, 4981–4989. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Arthur, C.M.; Jan, H.-M.; Garcia-Beltran, W.F.; Patel, K.R.; Rathgeber, M.F.; Verkerke, H.P.; Cheedarla, N.; Jajosky, R.P.; Paul, A.; et al. Blood group A enhances SARS-CoV-2 infection. Blood 2023, 142, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.C.; Melo, C.G.F.; Oliveira, J.L. The influence of ABO blood groups on COVID-19 susceptibility and severity: A molecular hypothesis based on carbohydrate-carbohydrate interactions. Med. Hypotheses 2020, 144, 110155. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 2023, 29, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated with Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.; Bordino, V.; Cornio, A.R.; Meddis, D.; Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Bert, F.; Zotti, C.M. Does ABO blood group influence antibody response to SARS-CoV-2 vaccination? Vox Sang. 2022, 117, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.D.; McMillan, D.; Levi, M.L. COVID-19 mRNA Vaccination, ABO Blood Type and the Severity of Self-Reported Reactogenicity in a Large Healthcare System: A Brief Report of a Cross-Sectional Study. Cureus 2021, 13, e20810. [Google Scholar] [CrossRef] [PubMed]
- Alessa, M.Y.; Aledili, F.J.; Alnasser, A.A.; Aldharman, S.S.; Al Dehailan, A.M.; Abuseer, H.O.; Saleh, A.A.A.; Alsalem, H.A.; Alsadiq, H.M.; Alsultan, A.S. The Side Effects of COVID-19 Vaccines and Its Association with ABO Blood Type Among the General Surgeons in Saudi Arabia. Cureus 2022, 14, e23628. [Google Scholar] [CrossRef] [PubMed]
- Almalki, O.S.; Santali, E.Y.; Alhothali, A.A.; Ewis, A.A.; Shady, A.; Fathelrahman, A.I.; Abdelwahab, S.F. The role of blood groups, vaccine type and gender in predicting the severity of side effects among university students receiving COVID-19 vaccines. BMC Infect. Dis. 2023, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Deleers, M.; Breiman, A.; Daubie, V.; Maggetto, C.; Barreau, I.; Besse, T.; Clémenceau, B.; Ruvoën-Clouet, N.; Fils, J.-F.; Maillart, E.; et al. COVID-19 and blood groups: ABO antibody levels may also matter. Int. J. Infect. Dis. 2021, 104, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Wacklin, P.; Mäkivuokko, H.; Alakulppi, N.; Nikkilä, J.; Tenkanen, H.; Räbinä, J.; Partanen, J.; Aranko, K.; Mättö, J. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS ONE 2011, 6, e20113. [Google Scholar] [CrossRef] [PubMed]
- Mäkivuokko, H.; Lahtinen, S.J.; Wacklin, P.; Tuovinen, E.; Tenkanen, H.; Nikkilä, J.; Björklund, M.; Aranko, K.; Ouwehand, A.C.; Mättö, J. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol. 2012, 12, 94. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Canani, R.B.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The Dynamic Interplay between the Gut Microbiota and Autoimmune Diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; He, N.; Huang, Y.B.; Song, F.J.; Chen, K.X. ABO blood groups and risk of cancer: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 4643–4650. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V.; Nariya, S.; Bhakta, N.R.; Beigelman, A.; Castro, M.; Dyer, A.-M.; Israel, E.; Kraft, M.; Martin, R.J.; et al. National Heart, Lung and Blood Institute’s “AsthmaNet”. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J. Allergy Clin. Immunol. 2017, 140, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum. Microb. J. 2020, 17, 100073. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Guo, L.; Ma, T.; Sun, Y.; Song, A.; Wang, W.; Gu, X.; Wu, W.; Xie, X.; Zhang, L.; et al. Association of ABO blood group with respiratory disease hospitalization and severe outcomes: A retrospective cohort study in blood donors. Int. J. Infect. Dis. 2022, 122, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Nguyen, N.Y.; Dunstan, S.J.; Baillie, J.K. The role of host genetics in susceptibility to influenza: A systematic review. PLoS ONE 2012, 7, e33180. [Google Scholar] [CrossRef]
- Raza, M.W.; Blackwell, C.C.; Molyneaux, P.; James, V.S.; Ogilvie, M.M.; Inglis, J.M.; Weir, D.M. Association between secretor status and respiratory viral illness. BMJ 1991, 303, 815–818. [Google Scholar] [CrossRef][Green Version]
- Mackenzie, J.S.; Fimmel, P.J. The effect of ABO blood groups on the incidence of epidemic influenza and on the response to live attenuated and detergent split influenza virus vaccines. J. Hyg. 1978, 80, 21–30. [Google Scholar] [CrossRef]
| FUT1 | FUT2 (Secretor Gene) | FUT3 (Lewis Gene) | |
|---|---|---|---|
| Other gene names | H gene | B12QTL1, SE, Se2, SEC2, sej | Le gene |
| Size (kb) | ~4.00 | 9.98 | 2.37 |
| Chromosomal location | 19q13.3 | 19q13.33 | 19p13.3 |
| Encoded enzymes | α-1,2-fucosyltransferase 1 (α2FucT1) | α-1,2-fucosyltransferase 2 (α2FucT2) | α1-4-fucosyltransferase (FucT) |
| Functions | Regulation of the expression of the H antigen mainly on erythrocyte membranes | Regulation of the expression of the H antigen mainly in epithelial cells and in bodily fluids such as saliva | Synthesis of the Lea and Leb antigens |
| Key reviews | [11,13,14] | [11,14,15] | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferous, S.; Siafakas, N.; Boufidou, F.; Patrinos, G.P.; Tsakris, A.; Anastassopoulou, C. Investigating ABO Blood Groups and Secretor Status in Relation to SARS-CoV-2 Infection and COVID-19 Severity. J. Pers. Med. 2024, 14, 346. https://doi.org/10.3390/jpm14040346
Ferous S, Siafakas N, Boufidou F, Patrinos GP, Tsakris A, Anastassopoulou C. Investigating ABO Blood Groups and Secretor Status in Relation to SARS-CoV-2 Infection and COVID-19 Severity. Journal of Personalized Medicine. 2024; 14(4):346. https://doi.org/10.3390/jpm14040346
Chicago/Turabian StyleFerous, Stefanos, Nikolaos Siafakas, Fotini Boufidou, George P. Patrinos, Athanasios Tsakris, and Cleo Anastassopoulou. 2024. "Investigating ABO Blood Groups and Secretor Status in Relation to SARS-CoV-2 Infection and COVID-19 Severity" Journal of Personalized Medicine 14, no. 4: 346. https://doi.org/10.3390/jpm14040346
APA StyleFerous, S., Siafakas, N., Boufidou, F., Patrinos, G. P., Tsakris, A., & Anastassopoulou, C. (2024). Investigating ABO Blood Groups and Secretor Status in Relation to SARS-CoV-2 Infection and COVID-19 Severity. Journal of Personalized Medicine, 14(4), 346. https://doi.org/10.3390/jpm14040346









