Secukinumab for the Treatment of Axial Spondyloarthritis: Long-Term Real-Life Data from Five Italian Referral Centers
Abstract
1. Introduction
2. Materials and Methods
3. Results
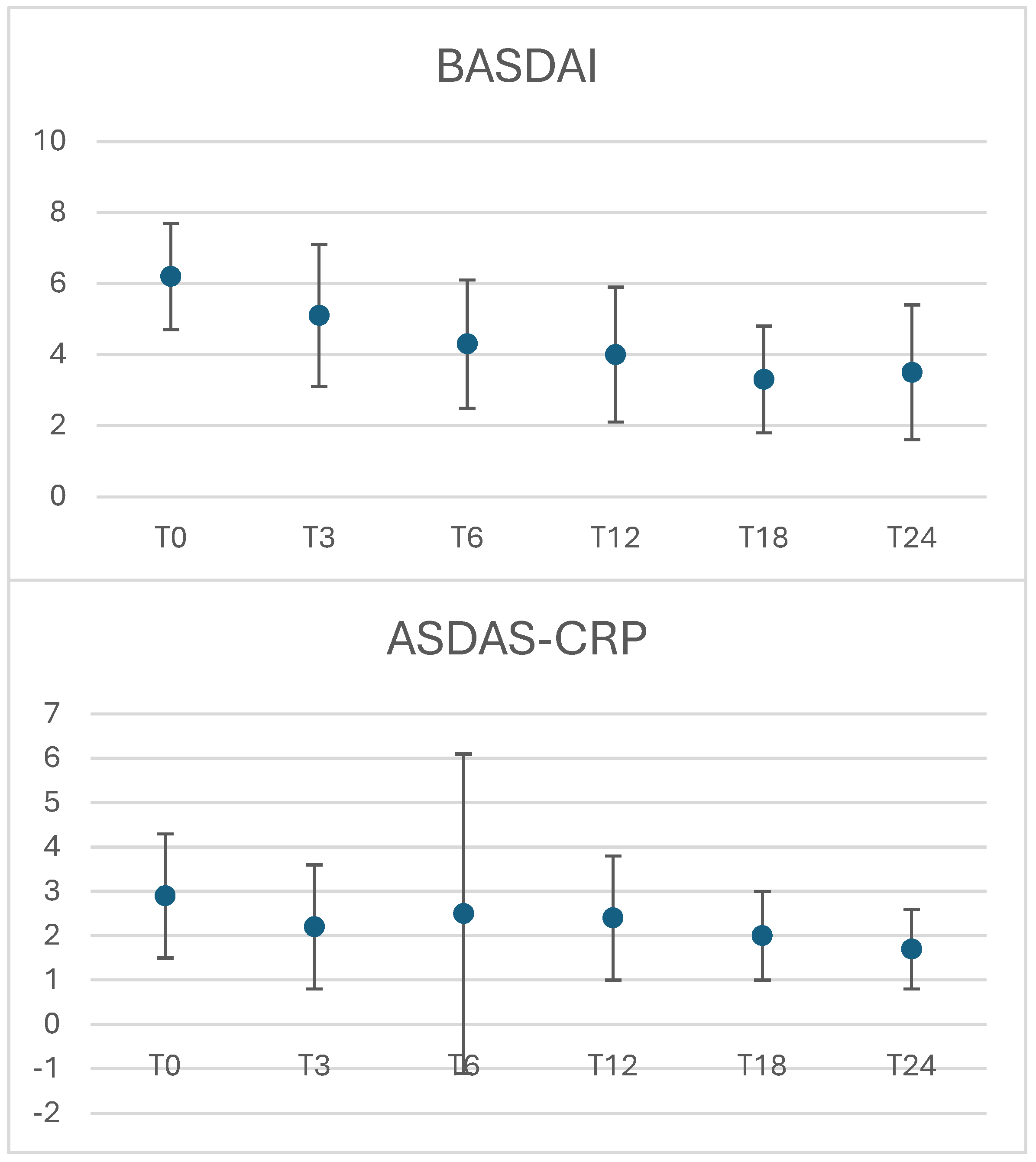
3.1. Clinical Response
3.2. Safety
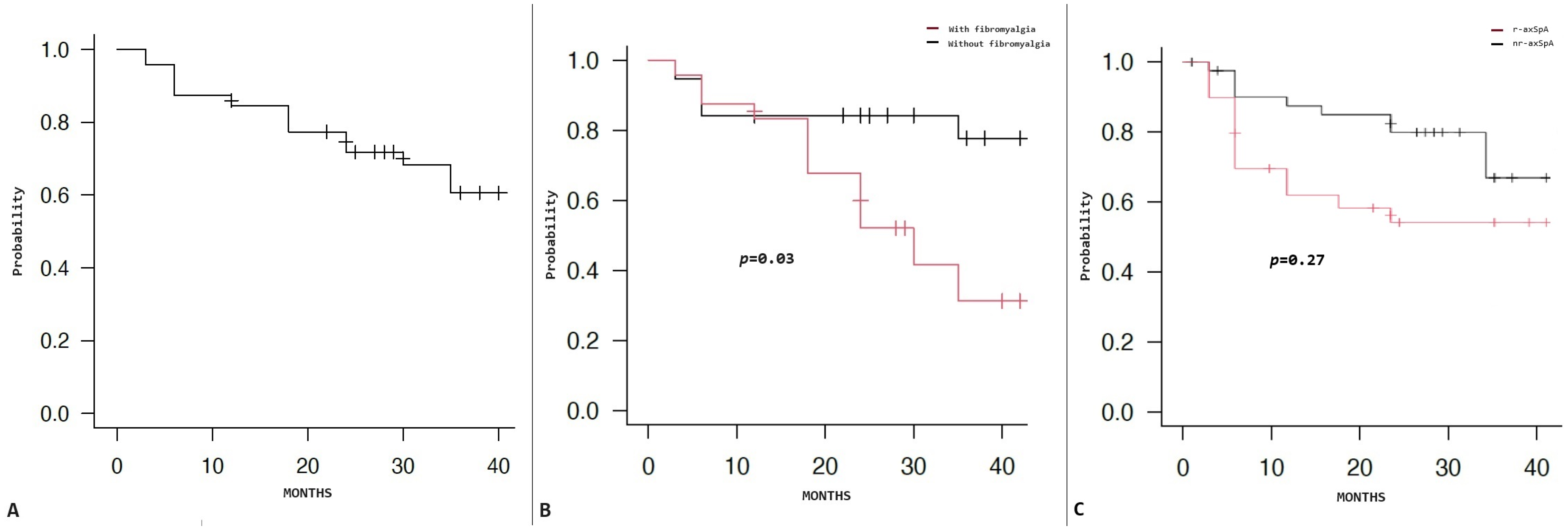
3.3. Retention Rate
3.4. Linear Regression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.; Zhao, S.S.; Nikiphorou, E. The association between comorbidities and disease activity in spondyloarthritis—A narrative review. Best. Pract. Res. Clin. Rheumatol. 2023, 37, 101857. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef]
- Chyuan, I.-T.; Chen, J.-Y. Role of Interleukin- (IL-) 17 in the Pathogenesis and Targeted Therapies in Spondyloarthropathies. Mediat. Inflamm. 2018, 2018, 2403935. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Atul, D. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef]
- Pavelka, K.; Kivitz, A.; Dokoupilova, E.; Blanco, R.; Maradiaga, M.; Tahir, H.; Pricop, L.; Andersson, M.; Readie, A.; Porter, B. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: A randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res. Ther. 2017, 19, 285. [Google Scholar] [CrossRef]
- Kivitz, A.J.; Wagner, U.; Dokoupilova, E.; Supronik, J.; Martin, R.; Talloczy, Z.; Richards, B.R.; Porter, B. Efficacy and Safety of Secukinumab 150 mg with and Without Loading Regimen in Ankylosing Spondylitis: 104-week Results from MEASURE 4 Study. Rheumatol. Ther. 2018, 5, 447–462. [Google Scholar] [CrossRef]
- Gentileschi, S.; Rigante, D.; Sota, J.; Lopalco, G.; Giannotta, M.G.; Emmi, G.; Di Scala, G.; Iannone, F.; Fabiani, C.; Feediani, B.; et al. Long-Term Effectiveness of Secukinumab in Patients with Axial Spondyloarthritis. Mediat. Inflamm. 2020, 2020, 6983272. [Google Scholar] [CrossRef]
- Ramonda, R.; Lorenzin, M.; Chimenti, M.S.; D’Angelo, S.; Marchesoni, A.; Salvarani, C.; Lubrano, E.; Costa, L.; Dal Basco, Y.; Fracassi, E.; et al. Effectiveness and safety of secukinumab in axial spondyloarthritis: A 24-month prospective, multicenter real-life study. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221090310. [Google Scholar] [CrossRef]
- Deodhar, A.; Mcinnes, I.; Baraliakos, X.; Reich, K.; Gottlieb, A.B.; Lebwohl, M.; Schreiber, S.; Bao, W.; Marfo, K.; Richards, H.; et al. FRI0272 Secukinumab Demonstrates a Consistent Safety Profile in Patients with Psoriasis, Psoriatic Arthritis and Ankylosing Spondylitis Over Long Term: Updated Pooled Safety Analyses. Ann. Rheum. Dis. 2020, 79, 722. [Google Scholar] [CrossRef]
- Favalli, E.G.; Marchesoni, A.; Balduzzi, S.; Montecucco, C.; Lomater, C.; Crepaldi, G.; Talamini, S.; Bazzani, C.; Fusaro, E.; Priora, M.; et al. FRI0273 Effectiveness and Retention Rate of Secukinumab for Psoriatic Arthritis and Axial Spondyloarthritis: Real-Life Data from The Italian Lorhen Registry. Ann. Rheum. Dis. 2020, 79, 722–723. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russel, A.S.; Russel, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; Van den Bosch, F.E.; Boteva, B.; Bremander, B.; et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef]
- Mauro, D.; Gandolfo, S.; Tirri, E.; Schett, G.; Maksymowych, W.P.; Ciccia, F. The bone marrow side of axial spondyloarthritis. Nat. Rev. Rheumatol. 2023, 19, 519–532. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; David, P.; Macleod, T.; Watad, A. Predominant ligament-centric soft-tissue involvement differentiates axial psoriatic arthritis from ankylosing spondylitis. Nat. Rev. Rheumatol. 2023, 19, 818–827. [Google Scholar] [CrossRef]
- Sivera, F.; Núñez-Monje, V.; Campos-Fernández, C.; Balaguer-Trull, I.; Robustillo-Villarino, M.; Aguilar-Zamora, M.; Garijo-Bufort, M.; López-Gómez, J.M.; Peña-González, C.; de la Morena, I.; et al. Real-world experience with secukinumab in the entire axial spondyloarthritis spectrum. Front. Med. 2023, 10, 1156557. [Google Scholar] [CrossRef] [PubMed]
- García-Dorta, A.; León-Suarez, P.; Peña, S.; Hernández-Díaz, M.; Rodríguez-Lozano, C.; González-Dávila , E.; Hernández-Hernández, M.V.; Díaz-González, F. Association of Gender, Diagnosis, and Obesity with Retention Rate of Secukinumab in Spondyloarthropathies: Results Form a Multicenter Real-World Study. Front. Med. 2022, 8, 815881. [Google Scholar] [CrossRef]
- Christiansen, S.N.; Horskjær Rasmussen, S.; Pons, M.; Michelsen, B.; Glintborg, B.; Gudbjornsson, B.; Gondal, G.; Vencovsky, J.; Loft, A.G.; Rotar, Z.; et al. Patient-reported outcomes in axial spondyloarthritis and psoriatic arthritis patients treated with secukinumab for 24 months in daily clinical practice. Semin. Arthritis Rheum. 2024, 65, 152388. [Google Scholar] [CrossRef]
- Egeberg, A.; Rosenø, N.A.L.; Thein, D.; Lørup, E.H.; Nielsen, M.L.; Nymand, L.; Kristensen, L.E.; Thyssen, J.P.; Thomsen, S.F.; Cordtz, R.L.; et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—A nationwide cohort study from the DANBIO and DERMBIO registries. Semin. Arthritis Rheum. 2022, 53, 151979. [Google Scholar] [CrossRef]
- Moltó, A.; Etcheto, A.; Gossec, L.; Boudersa, N.; Claudepierre, P.; Roux, N.; Lemeunier, L.; Martin, A.; Sparsa, L.; Coquerelle, P.; et al. Evaluation of the impact of concomitant fibromyalgia on TNF alpha blockers’ effectiveness in axial spondyloarthritis: Results of a prospective, multicentre study. Ann. Rheum. Dis. 2018, 77, 533–540. [Google Scholar] [CrossRef]
- Larid, G.; Baudens, G.; Tiemdjo-Djimaffo, G.; Coquerelle, P.; Goeb, V.; Guyot, M.H.; Marguerie, L.; Maury, F.; Veillard, E.; Houvenagel, E.; et al. Retention rate of subcutaneous TNF inhibitors in axial spondyloarthritis in a multicentre study from the RIC-FRANCE network. Sci. Rep. 2024, 14, 1374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Demographic Features | Mean ± SD |
|---|---|
| Age (Years) | 53.9 ± 12.7 |
| Disease Duration | 14.5 ± 10.0 |
| Male/Female | 30/41 |
| Clinical Features | N. patients (%) |
| HLA-B27 | 27 (38) |
| Gut Involvement | 1 (1.4) |
| Ocular Involvement | 1 (1.4) |
| Peripheral Involvement | 46 (64.8) |
| r-ax-SpA | 29 (40.8) |
| Dactylitis | 2 (2.9) |
| Enthesitis | 36 (52.9) |
| Fibromyalgia | 24 (33.8) |
| Hypercholesterolemia | 11 (19.6) |
| Arterial Hypertension | 15 (27.3) |
| Hyperuricemia | 2 (3.6) |
| Hypertriglyceridemia | 4 (7.1) |
| Cardiovascular Comorbidities | |
| 3 (5.4) 1 (1.8) 2 (3.6) 1 (1.8) |
| Therapeutic Features | N (%) |
| Previous cDMARD Exposure | 40 (57.1) |
| Previous bDMARD Exposure | 52 (75.4) |
| Concomitant cDMARDs | 17 (23.9) |
| Secukinumab, 150 mg/4 w | 53 (74.6) |
| Secukinumab, 300 mg/4 w | 18 (25.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentileschi, S.; Cannistrà, C.; Gaggiano, C.; Damiani, A.; Carli, L.; Benucci, M.; Cantini, F.; Niccoli, L.; Vitale, A.; Baldi, C.; et al. Secukinumab for the Treatment of Axial Spondyloarthritis: Long-Term Real-Life Data from Five Italian Referral Centers. J. Pers. Med. 2024, 14, 1105. https://doi.org/10.3390/jpm14111105
Gentileschi S, Cannistrà C, Gaggiano C, Damiani A, Carli L, Benucci M, Cantini F, Niccoli L, Vitale A, Baldi C, et al. Secukinumab for the Treatment of Axial Spondyloarthritis: Long-Term Real-Life Data from Five Italian Referral Centers. Journal of Personalized Medicine. 2024; 14(11):1105. https://doi.org/10.3390/jpm14111105
Chicago/Turabian StyleGentileschi, Stefano, Carlo Cannistrà, Carla Gaggiano, Arianna Damiani, Linda Carli, Maurizio Benucci, Fabrizio Cantini, Laura Niccoli, Antonio Vitale, Caterina Baldi, and et al. 2024. "Secukinumab for the Treatment of Axial Spondyloarthritis: Long-Term Real-Life Data from Five Italian Referral Centers" Journal of Personalized Medicine 14, no. 11: 1105. https://doi.org/10.3390/jpm14111105
APA StyleGentileschi, S., Cannistrà, C., Gaggiano, C., Damiani, A., Carli, L., Benucci, M., Cantini, F., Niccoli, L., Vitale, A., Baldi, C., Delle Sedie, A., Cantarini, L., Mosca, M., Frediani, B., & Guiducci, S. (2024). Secukinumab for the Treatment of Axial Spondyloarthritis: Long-Term Real-Life Data from Five Italian Referral Centers. Journal of Personalized Medicine, 14(11), 1105. https://doi.org/10.3390/jpm14111105








