Determination of Quality Indicators for Microvascular Grafts in Cranio-Maxillofacial Surgery—A Retrospective Analysis of 251 Free Flaps
Abstract
1. Introduction
2. Materials and Methods
- Patient data (name, age, date of birth, gender, height, weight, and BMI).
- Preoperative (pre-existing conditions that affect the vascular system, etiology of the defect, localization of the defect, and blood parameters).
- Surgery (date, type of graft, resection, ischemia time, duration of surgery, surgical technique, graft, and complications during anastomosis).
- Inpatient stay (complications and length of stay).
- Postoperative course (adjuvant therapy, complications, and blood parameters).
Statistical Analysis
3. Results
- Higher BMI (median = 24.5; SD = 4.68) as a positively influencing variable (p= 0.009; OR = 0.896 95%CI [0.8249; 0.973]);
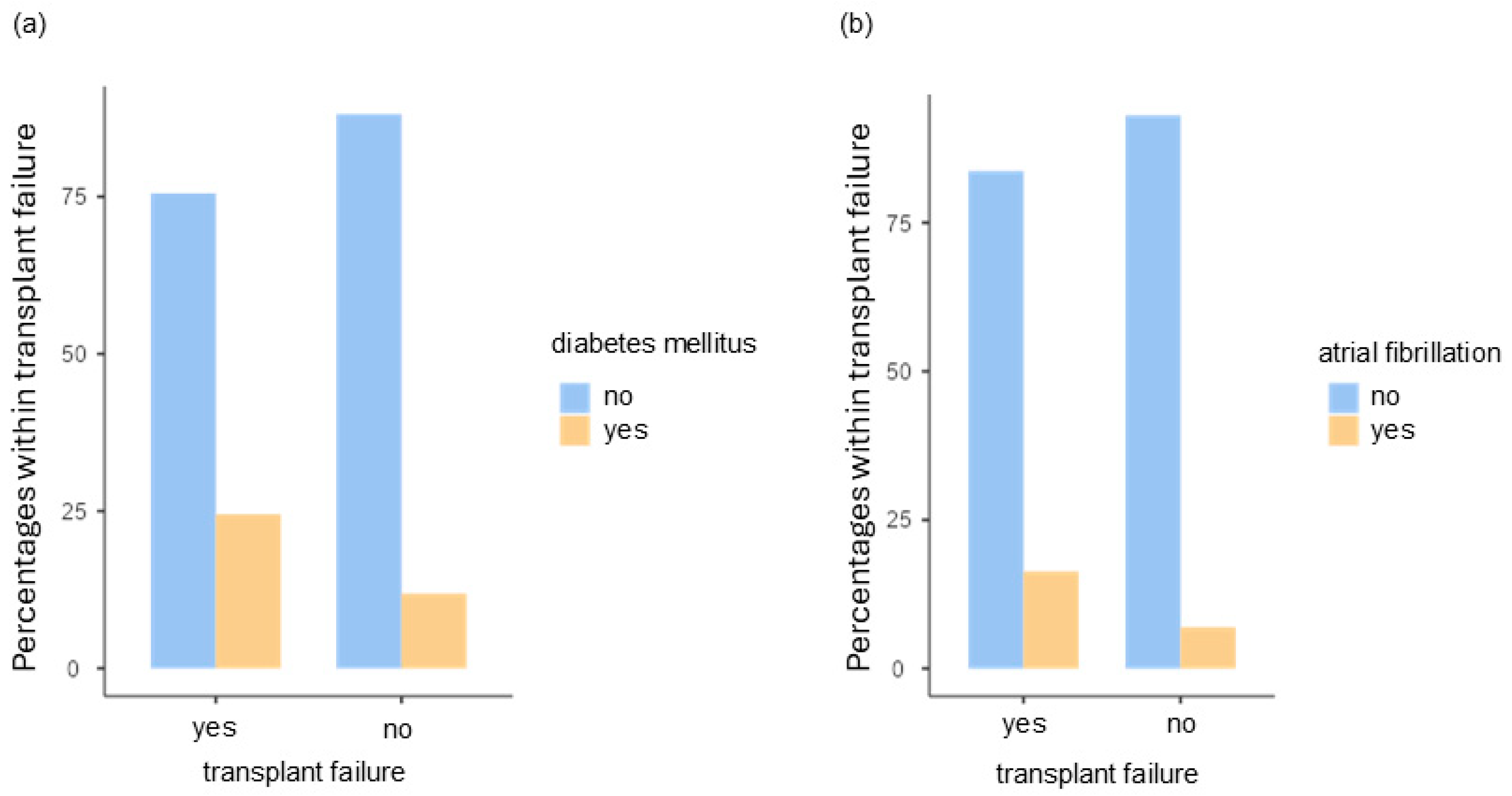
- The presence of diabetes mellitus as a negative predictive value (p = 0.002; OR = 4.234, 95%CI [1.6858; 10.634]);
- Long-term medication by means of platelet aggregation inhibition as a positive influencing factor (p = 0.041, OR 0.277 95%CI [0.0809; 0.947]);
- Extension of the operation time increases the probability of graft failure (p = 0.011; OR = 1.003, 95% CI [1.0006; 1.005]) (s. Table 7).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reuther, J.F.; Steinau, H.U.; Wagner, R. Reconstruction of large defects in the oropharynx with a revascularized intestinal graft: An experimental and clinical report. Plast. Reconstr. Surg. 1984, 73, 345–358. [Google Scholar] [CrossRef]
- Yang, G.F.; Chen, P.J.; Gao, Y.Z.; Liu, X.Y.; Li, J.; Jiang, S.X.; He, S.P. Forearm free skin flap transplantation: A report of 56 cases. 1981. Br. J. Plast. Surg. 1997, 50, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.J.; Atia, A.; Yu, P.; Su, Y.-X. The Anterolateral Thigh Flap in Head and Neck Reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2024, 36, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Tidke, S.S.; Waknis, P.P.; Setiya, S.; Jain, K.M.; Gupta, D.; Sakhariya, S. Donor Site Morbidity in Patients Undergoing Maxillofacial Reconstruction Using Free Fibula Flap versus Deep Circumflex Artery Flap—A Systematic Review. J. Maxillofac. Oral Surg. 2024, 23, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.A. Fibula Free Flap. Plast. Reconstr. Surg. 1989, 84, 71–79. [Google Scholar] [CrossRef]
- McGregor, S.; Zaraska, K.; Lynn, M.; Turkdogan, S.; Tran, K.L.; Prisman, E. Donor site morbidity after scapula free flap surgery of head and neck reconstruction: A systematic review and meta-analysis. Head Neck 2024. [Google Scholar] [CrossRef] [PubMed]
- Swartz, W.M.; Banis, J.C.; Newton, E.D.; Ramasastry, S.S.; Jones, N.F.; Acland, R. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast. Reconstr. Surg. 1986, 77, 530–545. [Google Scholar] [CrossRef]
- Wei, L.; Li, L.; Lv, X.; Yang, G. Factors influencing early postsurgical mobilization following Vascularized Iliac Crest Flap for jaw defect reconstruction. Curr. Probl. Surg. 2024, 61, 101519. [Google Scholar] [CrossRef]
- Bitter, K. Bone transplants from the Iliac crest to the maxillo-facial region by the microsurgical technique. J. Maxillofac. Surg. 1980, 8, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Corbitt, C.; Skoracki, R.J.; Yu, P.; Hanasono, M.M. Free flap failure in head and neck reconstruction. Head Neck 2014, 36, 1440–1445. [Google Scholar] [CrossRef]
- Sweeny, L.; Topf, M.; Wax, M.K.; Rosenthal, E.L.; Greene, B.J.; Heffelfinger, R.; Krein, H.; Luginbuhl, A.; Petrisor, D.; Troob, S.H.; et al. Shift in the timing of microvascular free tissue transfer failures in head and neck reconstruction. Laryngoscope 2020, 130, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Creff, G.; Mazoue, V.; Coudert, P.; Crouy Chanel, O.d.; Jegoux, F. Risk factors associated with early and late free flap complications in head and neck osseous reconstruction. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.A.; Disa, J.J.; Cordeiro, P.G.; Hu, Q.Y. A review of 716 consecutive free flaps for oncologic surgical defects: Refinement in donor-site selection and technique. Plast. Reconstr. Surg. 1998, 102, 722–732; discussion 733–734. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Devine, J.; Magennis, P.; Sillifant, P.; Rogers, S.; Vaughan, E. Factors that influence the outcome of salvage in free tissue transfer. Br. J. Oral Maxillofac. Surg. 2003, 41, 16–20. [Google Scholar] [CrossRef]
- Novakovic, D.; Patel, R.S.; Goldstein, D.P.; Gullane, P.J. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009, 1, 33. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.B.; Sweeny, L.; Ravipati, P.; Heffelfinger, R.; Krein, H.; Luginbuhl, A.; Goldman, R.; Curry, J. Factors Associated with Free Flap Failures in Head and Neck Reconstruction. Otolaryngol.–Head Neck Surg. 2019, 161, 598–604. [Google Scholar] [CrossRef]
- Frederick, J.W.; Sweeny, L.; Carroll, W.R.; Peters, G.E.; Rosenthal, E.L. Outcomes in head and neck reconstruction by surgical site and donor site. Laryngoscope 2013, 123, 1612–1617. [Google Scholar] [CrossRef]
- Fujioka, M. Factors Predicting Total Free Flap Loss after Microsurgical Reconstruction Following the Radical Ablation of Head and Neck Cancers. ISRN Plast. Surg. 2013, 2013, 1–5. [Google Scholar] [CrossRef][Green Version]
- Stevens, M.N.; Freeman, M.H.; Shinn, J.R.; Kloosterman, N.; Carr, S.; Mannion, K.; Rohde, S.L. Preoperative Predictors of Free Flap Failure. Otolaryngol.–Head Neck Surg. 2023, 168, 180–187. [Google Scholar] [CrossRef]
- Othman, S.; Robinson, E.; Kamdar, D.; Pereira, L.; Miles, B.; Kasabian, A.; Ricci, J.A.; Knobel, D. Microvascular Free-Flap Head and Neck Reconstruction: The Utility of the Modified Frailty Five-Item Index. J. Reconstr. Microsurg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.M.; Riley, C.A.; Fitzpatrick, J.C.; Hasney, C.P.; Moore, B.A.; McCoul, E.D. Postoperative anticoagulation after free flap reconstruction for head and neck cancer: A systematic review. Laryngoscope 2018, 128, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Forner, D.; Williams, B.A.; Makki, F.M.; Trites, J.R.; Taylor, S.M.; Hart, R.D. Late free flap failure in head and neck reconstruction: A systematic review. Ear Nose Throat J. 2018, 97, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Tasch, C.; Pattiss, A.; Maier, S.; Lanthaler, M.; Pierer, G. Free Flap Outcome in Irradiated Recipient Sites: A Systematic Review and Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4216. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chin, R.Y.; Eslick, G.D.; Sritharan, N.; Paramaesvaran, S. Outcomes of microvascular free flap reconstruction for mandibular osteoradionecrosis: A systematic review. J. Craniomaxillofac. Surg. 2015, 43, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, K. Multivariate Analysemethoden: Eine Anwendungsorientierte Einführung, 10th ed.; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 3540004912. [Google Scholar]
- Hemmerich, W. StatistikGuru: Poweranalyse und Stichprobenberechnung für Regression. 2019. Available online: https://statistikguru.de/rechner/poweranalyse-regression.html (accessed on 2 September 2024).
- Backhaus, K. Multivariate Analysemethoden: Eine Anwendungsorientierte Einführung, 11th ed.; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 9783540278702. [Google Scholar]
- Khan, U.; Hathi, K.; MacKay, C.; Corsten, M. The Complications of Osseous Reconstruction in the Head and Neck: A Systematic Review and Meta-analysis. Otolaryngol.-Head Neck Surg. 2024, 171, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, J.-F.; Zhang, X.; Wang, H.-M. Intraoperative factors associated with free flap failure in the head and neck region: A four-year retrospective study of 216 patients and review of the literature. Int. J. Oral Maxillofac. Surg. 2019, 48, 447–451. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Singh, N.; Deune, E.G.; Silverman, R.; Tufaro, A.P. Recipient vessel analysis for microvascular reconstruction of the head and neck. Ann. Plast. Surg. 2004, 52, 148–155; discussion 156–157. [Google Scholar] [CrossRef]
- Kroll, S.S.; Schusterman, M.A.; Reece, G.P.; Miller, M.J.; Evans, G.R.; Robb, G.L.; Baldwin, B.J. Choice of flap and incidence of free flap success. Plast. Reconstr. Surg. 1996, 98, 459–463. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, Y.C.; Jeon, D.N.; Jeong, W.S.; Koh, K.S.; Oh, T.S.; Eom, J.S.; Kim, E.K.; Hong, J.P.; Suh, H.P. Impact of Recipient Vein Selection on Venous Patency and Free Flap Survival in 652 Head and Neck Reconstructions. J. Reconstr. Microsurg. 2020, 36, 73–81. [Google Scholar] [CrossRef]
- Ishimaru, M.; Ono, S.; Suzuki, S.; Matsui, H.; Fushimi, K.; Yasunaga, H. Risk Factors for Free Flap Failure in 2,846 Patients with Head and Neck Cancer: A National Database Study in Japan. J. Oral Maxillofac. Surg. 2016, 74, 1265–1270. [Google Scholar] [CrossRef]
- Kovatch, K.J.; Hanks, J.E.; Stevens, J.R.; Stucken, C.L. Current practices in microvascular reconstruction in otolaryngology-head and neck surgery. Laryngoscope 2019, 129, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Wei, F.-C. Microsurgical free flap in head and neck reconstruction. Head Neck 2010, 32, 1236–1245. [Google Scholar] [CrossRef]
- Lese, I.; Biedermann, R.; Constantinescu, M.; Grobbelaar, A.O.; Olariu, R. Predicting risk factors that lead to free flap failure and vascular compromise: A single unit experience with 565 free tissue transfers. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.P.; Shabani, S.; Mhaskar, R.; McMullen, C.; Padhya, T.A.; Mifsud, M.J. Diabetes mellitus in major head and neck cancer surgery: Systematic review and meta-analysis. Head Neck 2020, 42, 3031–3040. [Google Scholar] [CrossRef]
- Nanayakkara, N.; Curtis, A.J.; Heritier, S.; Gadowski, A.M.; Pavkov, M.E.; Kenealy, T.; Owens, D.R.; Thomas, R.L.; Song, S.; Wong, J.; et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: Systematic review and meta-analyses. Diabetologia 2021, 64, 275–287. [Google Scholar] [CrossRef]
- James, S.; Gallagher, R.; Dunbabin, J.; Perry, L. Prevalence of vascular complications and factors predictive of their development in young adults with type 1 diabetes: Systematic literature review. BMC Res. Notes 2014, 7, 593. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Kantar, R.S.; Rifkin, W.J.; David, J.A.; Cammarata, M.J.; Diaz-Siso, J.R.; Levine, J.P.; Golas, A.R.; Ceradini, D.J. Diabetes is not associated with increased rates of free flap failure: Analysis of outcomes in 6030 patients from the ACS-NSQIP database. Microsurgery 2019, 39, 14–23. [Google Scholar] [CrossRef]
- Ye, W.; Guo, K.S.; Gallant, J.-N.; Stevens, M.N.; Weiss, V.L.; Bendfeldt, G.A.; O’Brien, M.T.; Rosenthal, E.L.; Netterville, J.L.; Mannion, K.; et al. Impact of comorbidities on immediate post-operative complications in oral cavity free flap patients. Am. J. Otolaryngol. 2024, 45, 104068. [Google Scholar] [CrossRef]
- Prasada, S.; Desai, M.Y.; Saad, M.; Smilowitz, N.R.; Faulx, M.; Menon, V.; Moudgil, R.; Chaudhury, P.; Hussein, A.A.; Taigen, T.; et al. Preoperative Atrial Fibrillation and Cardiovascular Outcomes After Noncardiac Surgery. J. Am. Coll. Cardiol. 2022, 79, 2471–2485. [Google Scholar] [CrossRef]
- Curtis, A.B.; Korada, S.K.C. Should Atrial Fibrillation Be Included in Preoperative Risk Assessment for Noncardiac Surgery? J. Am. Coll. Cardiol. 2022, 79, 2486–2488. [Google Scholar] [CrossRef] [PubMed]
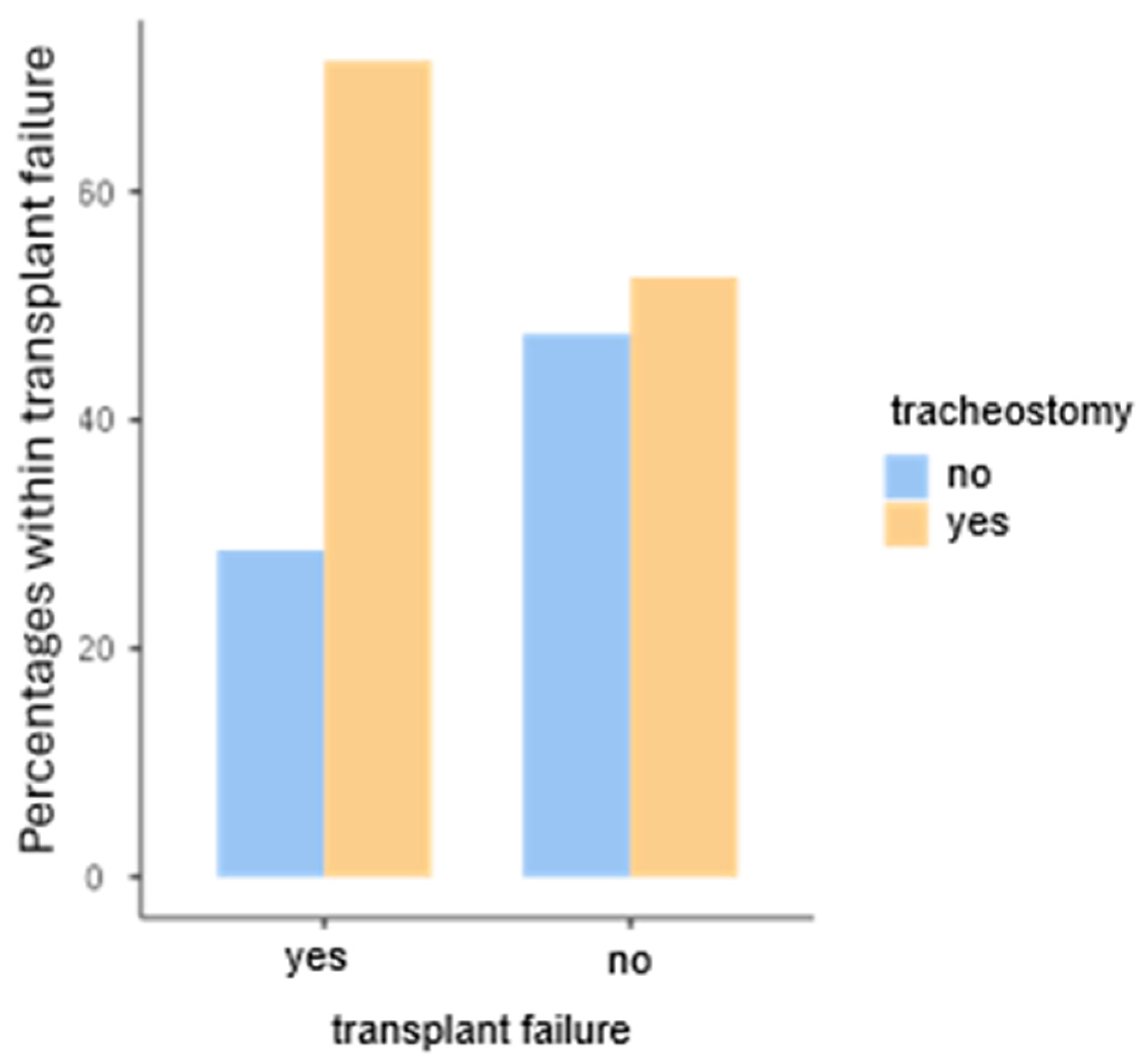
- Poisson, M.; Longis, J.; Schlund, M.; Pere, M.; Michel, G.; Delagranda, A.; Mouawad, F.; Piot, B.; Bertin, H. Postoperative morbidity of free flaps in head and neck cancer reconstruction: A report regarding 215 cases. Clin. Oral Investig. 2019, 23, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Corner, A.; Diba, A.; Hankins, M. Development of a tracheostomy scoring system to guide airway management after major head and neck surgery. Int. J. Oral Maxillofac. Surg. 2009, 38, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Coyle, M.J.; Shrimpton, A.; Perkins, C.; Fasanmade, A.; Godden, D. First do no harm: Should routine tracheostomy after oral and maxillofacial oncological operations be abandoned? Br. J. Oral Maxillofac. Surg. 2012, 50, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.B.; Patel, A.; Del Corral, G.A.; Sexton, K.W.; Ehrenfeld, J.M.; Guillamondegui, O.D.; Shack, R.B. Preoperative anemia predicts thrombosis and free flap failure in microvascular reconstruction. Ann. Plast. Surg. 2012, 69, 364–367. [Google Scholar] [CrossRef]
- Yu, S.; Wei, K.; Zhou, D.; Lin, Q.; Li, T. Predictive factors of postoperative complications related to free flap reconstruction in head and neck cancer patients admitted to intensive care unit: A retrospective cohort study. BMC Anesthesiol. 2024, 24, 258. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Roh, S.-G.; Lee, N.-H.; Yang, K.-M. Is obesity a predisposing factor for free flap failure and complications? Comparison between breast and nonbreast reconstruction: Systematic review and meta-analysis. Medicine 2016, 95, e4072. [Google Scholar] [CrossRef]
- Asaad, M.; Yao, C.; Kambhampati, P.; Mitchell, D.; Liu, J.; Lewis, C.M.; Yu, P.; Hanasono, M.M.; Chang, E.I. Impact of Body Mass Index on Surgical Outcomes in Oncologic Microvascular Head and Neck Reconstruction. Ann. Surg. Oncol. 2022, 29, 5109–5121. [Google Scholar] [CrossRef]
- Crippen, M.M.; Brady, J.S.; Mozeika, A.M.; Eloy, J.A.; Baredes, S.; Park, R.C.W. Impact of Body Mass Index on Operative Outcomes in Head and Neck Free Flap Surgery. Otolaryngol.–Head Neck Surg. 2018, 159, 817–823. [Google Scholar] [CrossRef]
- Hyun, D.-J.; Joo, Y.-H.; Kim, M.-S. Impact of pre-operative body mass index in head and neck cancer patients undergoing microvascular reconstruction. J. Laryngol. Otol. 2017, 131, 972–976. [Google Scholar] [CrossRef]
- Shum, J.; Markiewicz, M.R.; Park, E.; Bui, T.; Lubek, J.; Bell, R.B.; Dierks, E.J. Low prealbumin level is a risk factor for microvascular free flap failure. J. Oral Maxillofac. Surg. 2014, 72, 169–177. [Google Scholar] [CrossRef]
- Vandersteen, C.; Dassonville, O.; Chamorey, E.; Poissonnet, G.; Nao, E.E.M.; Pierre, C.S.; Leyssale, A.; Peyrade, F.; Falewee, M.N.; Sudaka, A.; et al. Impact of patient comorbidities on head and neck microvascular reconstruction. A report on 423 cases. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Abouyared, M.; Katz, A.P.; Ein, L.; Ketner, J.; Sargi, Z.; Nicolli, E.; Leibowitz, J.M. Controversies in free tissue transfer for head and neck cancer: A review of the literature. Head Neck 2019, 41, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Cannady, S.B.; Hatten, K.; Wax, M.K. Postoperative Controversies in the Management of Free Flap Surgery in the Head and Neck. Facial Plast. Surg. Clin. N. Am. 2016, 24, 309–314. [Google Scholar] [CrossRef]
- Senchenkov, A.; Lemaine, V.; Tran, N.V. Management of perioperative microvascular thrombotic complications—The use of multiagent anticoagulation algorithm in 395 consecutive free flaps. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 1293–1303. [Google Scholar] [CrossRef]
- Mishu, M.D.; Zolper, E.G.; Dekker, P.K.; Fleury, C.M.; Bekeny, J.C.; Fan, K.L.; Attinger, C.E.; Evans, K.K. Should Antiplatelet Therapy Be Withheld Perioperatively? The First Study Examining Outcomes in Patients Receiving Dual Antiplatelet Therapy in the Lower Extremity Free Flap Population. Plast. Reconstr. Surg. 2022, 149, 95e–103e. [Google Scholar] [CrossRef]
- Tarabishy, S.P.; Inglesby, D.; Tapp, M.; Del Corral, G.; Herrera, F.A. Thrombocytosis is associated with complications after microvascular surgery: An NSQIP data analysis. Microsurgery 2020, 40, 288–297. [Google Scholar] [CrossRef]
- Irawati, N.; Every, J.; Dawson, R.; Leinkram, D.; Elliott, M.; Ch’ng, S.; Low, H.; Palme, C.E.; Clark, J.; Wykes, J. Effect of operative time on complications associated with free flap reconstruction of the head and neck. Clin. Otolaryngol. 2023, 48, 175–181. [Google Scholar] [CrossRef]
- Brady, J.S.; Desai, S.V.; Crippen, M.M.; Eloy, J.A.; Gubenko, Y.; Baredes, S.; Park, R.C.W. Association of Anesthesia Duration with Complications After Microvascular Reconstruction of the Head and Neck. JAMA Facial Plast. Surg. 2018, 20, 188–195. [Google Scholar] [CrossRef]
- Kim, B.D.; Ver Halen, J.P.; Grant, D.W.; Kim, J.Y.S. Anesthesia duration as an independent risk factor for postoperative complications in free flap surgery: A review of 1,305 surgical cases. J. Reconstr. Microsurg. 2014, 30, 217–226. [Google Scholar] [CrossRef]
- Bahethi, R.R.; Gold, B.S.; Seckler, S.G.; Kinberg, E.; Stepan, K.O.; Gray, M.L.; DeMaria, S.; Miles, B.A. Efficiency of microvascular free flap reconstructive surgery: An observational study. Am. J. Otolaryngol. 2020, 41, 102692. [Google Scholar] [CrossRef] [PubMed]
- Sawaf, T.; Renslo, B.; Virgen, C.; Farrokhian, N.; Yu, K.M.; Gessert, T.G.; Jackson, C.; O’Neill, K.; Sperry, B.; Kakarala, K. Team Consistency in Reducing Operative Time in Head and Neck Surgery with Microvascular Free Flap Reconstruction. Laryngoscope 2023, 133, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Jones, B.; Luu, M.; Li, R.; Glaser, S.; Massarelli, E.; Freeman, M.; Gernon, T.; Maghami, E.; Kang, R.; et al. Factors predictive of 90-day mortality after surgical resection for oral cavity cancer: Development of a recursive partitioning analysis for risk stratification. Head Neck 2021, 43, 2731–2739. [Google Scholar] [CrossRef]
- Gaubatz, M.E.; Bukatko, A.R.; Simpson, M.C.; Polednik, K.M.; Adjei Boakye, E.; Varvares, M.A.; Osazuwa-Peters, N. Racial and socioeconomic disparities associated with 90-day mortality among patients with head and neck cancer in the United States. Oral Oncol. 2019, 89, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lodders, J.N.; Parmar, S.; Stienen, N.L.M.; Martin, T.J.; Karagozoglu, K.H.; Heymans, M.W.; Nandra, B.; Forouzanfar, T. Incidence and types of complications after ablative oral cancer surgery with primary microvascular free flap reconstruction. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e744–e750. [Google Scholar] [CrossRef]
| Parameters | n, MD ± SD | |
|---|---|---|
| Gender | Female | 115 (45.8%) |
| Male | 136 (54.2%) | |
| Age | Female | 63.0 ± 17.0 years |
| Male | 59.4 ± 14.8 years | |
| Height | Female | 165.0 ±6.9 |
| Male | 178.0 ± 7.91 | |
| Weight | Female | 64.4 ± 12.8 |
| Male | 79.9 ± 16.9 | |
| BMI | Female | 23.9 ± 4.6 |
| Male | 25.2 ± 47 | |
| ASA Classification | ||
| I | 32 (12.8%) | |
| II | 123 (49.2%) | |
| III | 89 (35.6%) | |
| IV | 6 (2.4%) | |
| Nicotine abuse | ||
| NA | 44 (17.5%) | |
| Never | 87 (34.7%) | |
| Former smoker | 42 (16.7%) | |
| Active smoker | 78 (31.1%) | |
| PY | 19.4 ± 22.7 (0–104 PY) | |
| Alcohol abuse | ||
| NA | 68 (27.1%) | |
| Never | 109 (43.4%) | |
| Rarely | 2 (0.8%) | |
| Occasionally | 13 (5.2%) | |
| Daily | 40 (15.9%) | |
| Sober | 19 (7.6%) |
| Pre-Existing Conditions | n, Percentage (5%) | |
|---|---|---|
| Hypercholesterolemia | Yes | 26 (10.4%) |
| No | 225 (89.6%) | |
| Diabetes mellitus | Yes | 36 (14.3%) |
| No | 215 (85.7%) | |
| Hypertension | Yes | 120 (47.8%) |
| No | 131 (52.2%) | |
| Coronary heart disease | Yes | 24 (9.6%) |
| No | 227 (90.4%) | |
| Atrial fibrillation, cardiac arrhythmia | Yes | 22 (8.8%) |
| No | 229 (90.4%) | |
| Arteriosclerosis | Yes | 23 (9.2%) |
| No | 228 (90.8%) | |
| Post-thromboembolic events (e.g., LAE) | Yes | 34 (13.5%) |
| No | 217 (86.5%) |
| Parameters | n, Percentage (%) | |
|---|---|---|
| Underlying disease | MONJ | 3 (1.2%) |
| Osteomyelitis | 3 (1.2%) | |
| Osteoradionecrosis | 17 (6.8%) | |
| SCC | 172 (68.5%) | |
| Reconstruction | 52 (20.7%) | |
| Others | 4 (1.6%) | |
| Site of resection | ||
| Lip | 2 (0.8%) | |
| Floor of mouth | 6 (2.4%) | |
| Upper Jaw | 33 (13.1%) | |
| Lower Jaw | 67 (26.7%) | |
| Cranium | 16 (6.4%) | |
| Cheek | 21 (8.4%) | |
| Tongue | 27 (10.8%) | |
| Overlapping | 78 (31.1%) | |
| Localization of the defect | ||
| Left | 87 (34.7%) | |
| Left-accentuated, crossing the midline | 9 (3.6%) | |
| Overlapping | 9 (3.6%) | |
| Mid | 36 (14.3%) | |
| Right | 102 (40.6%) | |
| Right-accentuated, crossing the midline | 8 (3.2%) | |
| T-Stadium (n = 172) | ||
| NA | 81 (32.3%) | |
| Tx | 14 (5.6%) | |
| pT1 | 41 (16.3%) | |
| pT2 | 38 (15.1%) | |
| pT3 | 32 (12.7%) | |
| pT4a | 43 (17.1%) | |
| pT4b | 2 (0.8%) |
| Parameters | n, Percentage (%) | |
|---|---|---|
| Flap design | ||
| Fascio-cutaneous | 157 (62.5%) | |
| Musculo-cutaneous | 33 (13.1%) | |
| Osteo-musculocutaneous | 61 (24.3%) | |
| Arterial anastomosis | End-to-end | 248 (98.8%) |
| End-to-Side | 3 (1.2%) | |
| Venous anastomosis | End-to-end | 202 (80.5%) |
| End-to-Side | 49 (19.5%) | |
| Percutaneous gastric tube | Yes | 129 (51.4%) |
| No | 122 (48.6%) | |
| Duration (n = 102) | 54.9 ± 52.9 (2–120) days | |
| Neck dissection | ||
| Right side | None | 128 (51.0%) |
| Lymphnode-Extirpation | 3 (1.2%) | |
| Level I–III | 94 (37.5%) | |
| Level I–V | 26 (10.4%) | |
| Left side | None | 123 (49.0%) |
| Lymphnode-Extirpation | 4 (1.6%) | |
| Level I–III | 92 (36.7%) | |
| Level I–V | 32 (12.7%) | |
| Duration of surgery | 551 ± 170 min | |
| Duration invasive ventilation | 37.4 ± 33.3 | |
| Length of stay | 37.4 ± 33.3 days | |
| Adjuvant therapy | ||
| None | 150 (59.8%) | |
| Radiation | 55 (21.0% | |
| Radio-Chemotherapy | 46 (18.3%) |
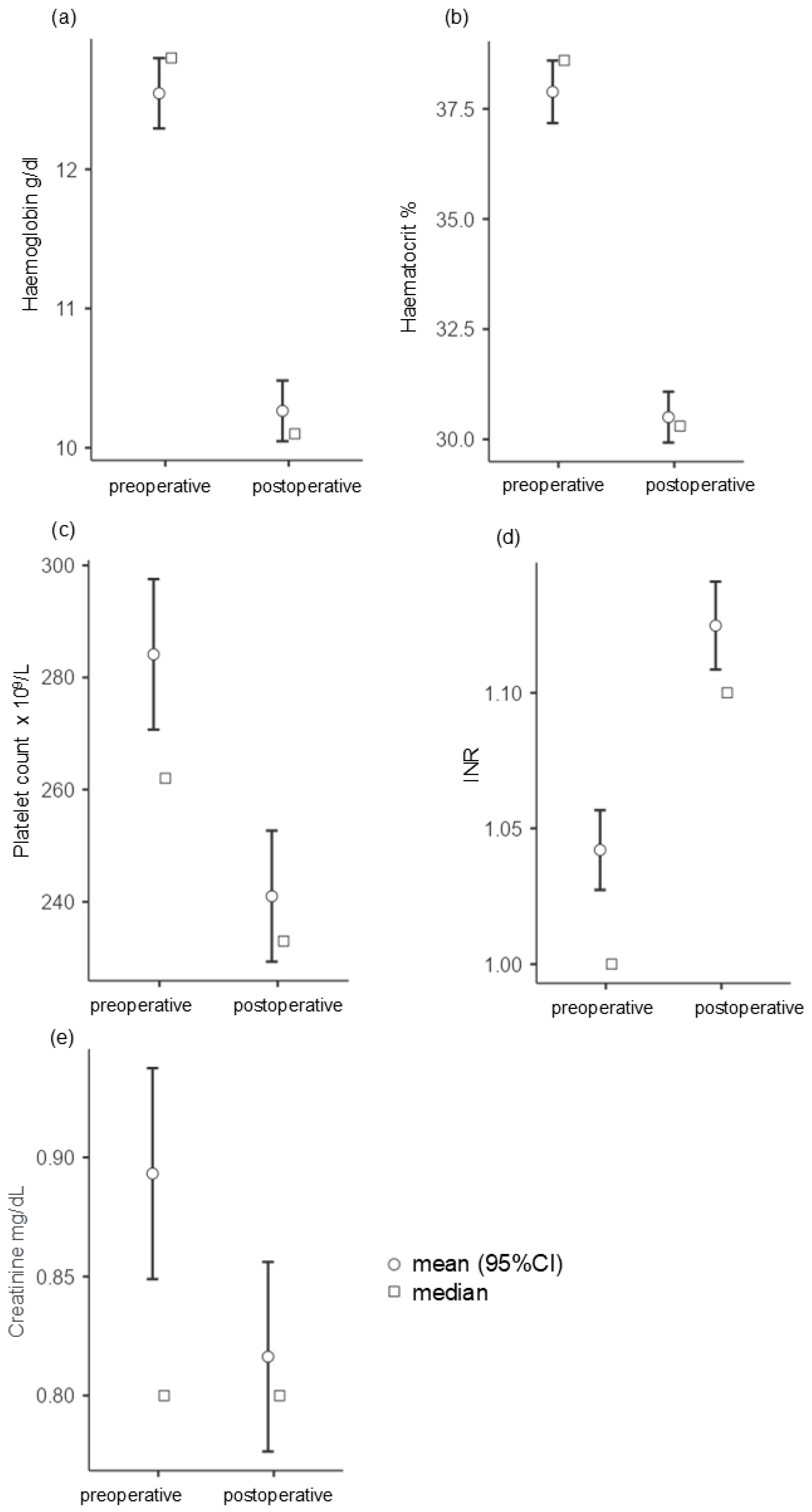
| Blood Values | MD ± SD | |
|---|---|---|
| Hemoglobin | Preoperative | 12.5 ± 2.04 |
| Postoperative | 10.3 ± 1.76 | |
| Hematocrit | Preoperative | 37.9 ± 5.73 |
| Postoperative | 30.5 ± 4.65 | |
| INR | Preoperative | 1.04 ± 0.114 |
| Postoperative | 1.12 ± 0.127 | |
| Platelet count | Preoperative | 284 ± 109 |
| Postoperative | 241 ± 94.3 | |
| Creatinine | Preoperative | 0.895 ± 0.357 |
| Postoperative | 0.816 ± 0.321 |
| Complications | n, Percentage (%) | |
|---|---|---|
| Thrombosis of the pedicle | Yes | 78 (31.1%) |
| No | 173 (68.9%) | |
| Complications of the flap | ||
| None | 174 (69.3%) | |
| Ischaemia | 36 (14.3%) | |
| Venous stasis | 41 (16.3%) | |
| Other local complications | None | 168 (66.9%) |
| Bleeding | 15 (6%) | |
| Dehiscence | 38 (15.1%) | |
| Necrosis | 18 (7.2%) | |
| Other | 1 (0.4%) | |
| Flap revision without explantation | Yes | 50 (19.9%) |
| No | 201 (80.1%) | |
| Failure of the flap | Yes | 49 (19.5%) |
| No | 202 (80.5%) | |
| General medical complications | None | 122 (48.6) |
| Yes | 51.39% | |
| Ileus | 2 | |
| Peritonitis | 3 | |
| Chylous fistula | 2 | |
| Pneumonia | 14 | |
| Respiratory insufficiency | 3 | |
| Pneumogenic sepsis | 1 | |
| ARDS (Acute Respiratory Distress Syndrome) | 2 | |
| Pneumothorax | 3 | |
| Pulmonary embolism | 2 | |
| Thrombosis (e.g., DVT, deep vein thrombosis) | 4 | |
| Stroke | 2 | |
| Cardiac decompensation | 2 | |
| Myocardial infarction | 5 | |
| Cardiogenic shock | 1 | |
| Hypoxia | 2 | |
| Cardiopulmonary resuscitation | 6 | |
| Multi-organ failure | 1 | |
| Acute renal failure | 9 | |
| Sepsis | 3 | |
| MRSA | 10 | |
| 3MRGN | 1 | |
| Delirium | 24 | |
| Others | 15 | |
| Deceased within 90 days | Yes | 19 (7.6%) |
| No | 232 (92.4%) |
| Model Fit Measures | |||||||
|---|---|---|---|---|---|---|---|
| Overall Model Test | |||||||
| Model | Deviance | AIC | R2McF | R2N | χ2 | df | p |
| 1 | 216 | 230 | 254 | 0.162 | 26.2 | 6 | <0.001 |
| Model Coefficients—Transplant failure yes/no | |||||||
| 95% Confidence Interval | |||||||
| Predictor | Estimate | SE | Z | p | Odds Ratio | Lower | Upper |
| Intercept | −0.658 | 0.775 | −0.848 | 0.396 | 0.518 | 0.113 | 2.37 |
| Age | 0.199 | 0.217 | 0.914 | 0.361 | 1.22 | 0.797 | 1.87 |
| BMI | 0.302 | 0.162 | 1.863 | 0.062 | 1.353 | 0.984 | 1.86 |
| Gender | |||||||
| female–male | 0.15685 | 0.35621 | 0.4403 | 0.660 | 1.170 | 0.5820 | 2.351 |
| Diabetes mellitus: | |||||||
| yes/no | 144.316 | 0.46987 | 30.714 | 0.002 | 4.234 | 16.858 | 10.634 |
|
Inhibition of platelet aggregation: | |||||||
| yes/no | −128.415 | 0.62755 | −20.463 | 0.041 | 0.277 | 0.0809 | 0.947 |
| Time of surgery (min) | 0.00255 | 0.00100 | 25.430 | 0.011 | 1.003 | 10.006 | 1.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moellmann, H.L.; Karnatz, N.; Degirmenci, I.; Rana, M. Determination of Quality Indicators for Microvascular Grafts in Cranio-Maxillofacial Surgery—A Retrospective Analysis of 251 Free Flaps. J. Pers. Med. 2024, 14, 1061. https://doi.org/10.3390/jpm14101061
Moellmann HL, Karnatz N, Degirmenci I, Rana M. Determination of Quality Indicators for Microvascular Grafts in Cranio-Maxillofacial Surgery—A Retrospective Analysis of 251 Free Flaps. Journal of Personalized Medicine. 2024; 14(10):1061. https://doi.org/10.3390/jpm14101061
Chicago/Turabian StyleMoellmann, Henriette Louise, Nadia Karnatz, Ilkan Degirmenci, and Majeed Rana. 2024. "Determination of Quality Indicators for Microvascular Grafts in Cranio-Maxillofacial Surgery—A Retrospective Analysis of 251 Free Flaps" Journal of Personalized Medicine 14, no. 10: 1061. https://doi.org/10.3390/jpm14101061
APA StyleMoellmann, H. L., Karnatz, N., Degirmenci, I., & Rana, M. (2024). Determination of Quality Indicators for Microvascular Grafts in Cranio-Maxillofacial Surgery—A Retrospective Analysis of 251 Free Flaps. Journal of Personalized Medicine, 14(10), 1061. https://doi.org/10.3390/jpm14101061







