Current Management and Future Directions for Pulmonary Arterial Hypertension Associated with Congenital Heart Disease
Abstract
:1. Introduction
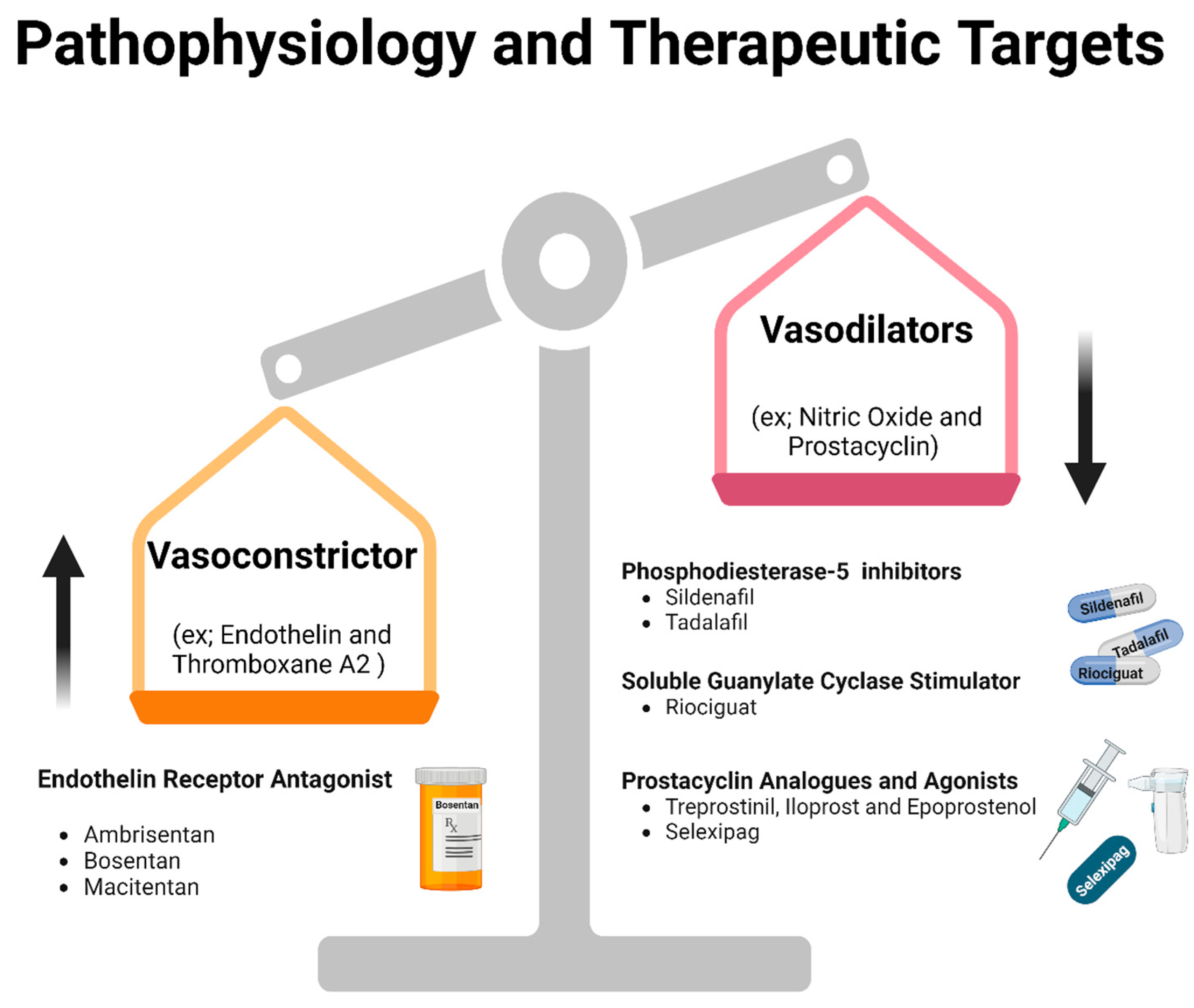
2. Pathophysiology
3. Current Management
3.1. Eisenmenger Syndrome
3.1.1. Endothelin-1 Pathway and ENDOTHELIN RECEPTOR ANTAGONISTS
3.1.2. Phosphodiesterase-5 Inhibitors
3.1.3. Soluble Guanylate Cyclase Stimulator
3.1.4. Prostacyclin Pathway
3.1.5. Combination Therapy
3.2. PAH Associated with Prevalent Left-to-Right Shunts
3.3. PAH Associated with Small/Coincidental Defects
3.4. Postoperative PAH
4. Future Directions and Ongoing Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.N.; Ivy, D.D.; Hauck, A.; Karamlou, T.; Truong, U.; Coleman, R.D.; Sandoval, J.P.; Del Cerro Marin, M.J.; Eghtesady, P.; Tillman, K.; et al. Pulmonary Hypertension in Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circ. Heart Fail. 2023, 16, e00080. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Dimopoulos, K.; Okonko, D.; Li, W.; Babu-Narayan, S.V.; Broberg, C.S.; Johansson, B.; Bouzas, B.; Mullen, M.J.; Poole-Wilson, P.A.; et al. Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation 2005, 112, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Engelfriet, P.M.; Duffels, M.G.; Moller, T.; Boersma, E.; Tijssen, J.G.; Thaulow, E.; Gatzoulis, M.A.; Mulder, B.J. Pulmonary arterial hypertension in adults born with a heart septal defect: The Euro Heart Survey on adult congenital heart disease. Heart 2007, 93, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; Ivy, D.D.; Foreman, A.J.; McGoon, M.D.; Rosenzweig, E.B. Four- and seven-year outcomes of patients with congenital heart disease-associated pulmonary arterial hypertension (from the REVEAL Registry). Am. J. Cardiol. 2014, 113, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Brida, M.; Nashat, H.; Gatzoulis, M.A. Pulmonary arterial hypertension: Closing the gap in congenital heart disease. Curr. Opin. Pulm. Med. 2020, 26, 422–428. [Google Scholar] [CrossRef]
- Nashat, H.; Brida, M.; Price, L.S.; McCabe, C.; Alonso-Gonzalez, R.; Wort, S.J.; Kempny, A.; Dimopoulos, K.; Gatzoulis, M.J. Pulmonary Arterial Hypertension Complicating Congenital Heart Disease: Advances in Therapy. Semin. Respir. Crit. Care Med. 2017, 38, 636–650. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Krasuski, R.A. Pulmonary Hypertension in Adults with Congenital Heart Disease. Cardiol. Clin. 2022, 40, 55–67. [Google Scholar] [CrossRef]
- Kaemmerer, H.; Gorenflo, M.; Huscher, D.; Pittrow, D.; Apitz, C.; Baumgartner, H.; Berger, F.; Bruch, L.; Brunnemer, E.; Budts, W.; et al. Pulmonary Hypertension in Adults with Congenital Heart Disease: Real-World Data from the International COMPERA-CHD Registry. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef]
- Manes, A.; Palazzini, M.; Leci, E.; Bacchi Reggiani, M.L.; Branzi, A.; Galie, N. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: A comparison between clinical subgroups. Eur. Heart J. 2014, 35, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Yoshibayashi, M.; Nishioka, K.; Nakao, K.; Saito, Y.; Matsumura, M.; Ueda, T.; Temma, S.; Shirakami, G.; Imura, H.; Mikawa, H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects. Evidence for increased production of endothelin in pulmonary circulation. Circulation 1991, 84, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Vizza, C.D.; Romeo, E.; Badagliacca, R.; Santoro, G.; Poscia, R.; Sarubbi, B.; Mancone, M.; Argiento, P.; Ferrante, F.; et al. Long term effects of bosentan treatment in adult patients with pulmonary arterial hypertension related to congenital heart disease (Eisenmenger physiology): Safety, tolerability, clinical, and haemodynamic effect. Heart 2007, 93, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Beghetti, M.; Gatzoulis, M.A.; Granton, J.; Berger, R.M.; Lauer, A.; Chiossi, E.; Landzberg, M.; Bosentan Randomized Trial of Endothelin Antagonist Therapy-5 (BREATHE-5) Investigators. Bosentan therapy in patients with Eisenmenger syndrome: A multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006, 114, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, M.A.; Beghetti, M.; Galie, N.; Granton, J.; Berger, R.M.; Lauer, A.; Chiossi, E.; Landzberg, M.; BREATHE-5 Invest. Longer-term bosentan therapy improves functional capacity in Eisenmenger syndrome: Results of the BREATHE-5 open-label extension study. Int. J. Cardiol. 2008, 127, 27–32. [Google Scholar] [CrossRef]
- Seyfarth, H.-J.; Favreau, N.; Tennert, C.; Ruffert, C.; Halank, M.; Wirtz, H.; Mössner, J.; Rosendahl, J.; Kovacs, P.; Wittenburg, H. Genetic susceptibility to hepatoxicity due to bosentan treatment in pulmonary hypertension. Ann. Hepatol. 2014, 13, 803–809. [Google Scholar] [CrossRef]
- Galie, N.; Rubin, L.; Hoeper, M.; Jansa, P.; Al-Hiti, H.; Meyer, G.; Chiossi, E.; Kusic-Pajic, A.; Simonneau, G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): A double-blind, randomised controlled trial. Lancet 2008, 371, 2093–2100. [Google Scholar] [CrossRef]
- Simonneau, G.; Galie, N.; Jansa, P.; Meyer, G.M.; Al-Hiti, H.; Kusic-Pajic, A.; Lemarie, J.C.; Hoeper, M.M.; Rubin, L.J. Long-term results from the EARLY study of bosentan in WHO functional class II pulmonary arterial hypertension patients. Int. J. Cardiol. 2014, 172, 332–339. [Google Scholar] [CrossRef]
- Diller, G.P.; Dimopoulos, K.; Kaya, M.G.; Harries, C.; Uebing, A.; Li, W.; Koltsida, E.; Gibbs, J.S.; Gatzoulis, M.A. Long-term safety, tolerability and efficacy of bosentan in adults with pulmonary arterial hypertension associated with congenital heart disease. Heart 2007, 93, 974–976. [Google Scholar] [CrossRef]
- Gatzoulis, M.A.; Landzberg, M.; Beghetti, M.; Berger, R.M.; Efficace, M.; Gesang, S.; He, J.; Papadakis, K.; Pulido, T.; Galie, N.; et al. Evaluation of Macitentan in Patients with Eisenmenger Syndrome. Circulation 2019, 139, 51–63. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1494–1563. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Tripp, J. Phosphodiesterase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chamsi-Pasha, H. Sildenafil (viagra) and the heart. J. Fam. Community Med. 2001, 8, 63–66. [Google Scholar] [CrossRef]
- Barnes, H.; Brown, Z.; Burns, A.; Williams, T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst. Rev. 2019, 1, CD012621. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Sharma, M.; Ramakrishnan, S.; Yusuf, J.; Gupta, M.D.; Bhamri, N.; Trehan, V.; Tyagi, S. Phosphodiesterase-5 inhibitor in Eisenmenger syndrome: A preliminary observational study. Circulation 2006, 114, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Nathani, S.; Yusuf, J.; Shrimal, D.; Tyagi, S. Clinical efficacy of phosphodiesterase-5 inhibitor tadalafil in Eisenmenger syndrome—A randomized, placebo-controlled, double-blind crossover study. Congenit. Heart Dis. 2011, 6, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Khaybullina, D.; Patel, A.; Zerilli, T. Riociguat (adempas): A novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. P T 2014, 39, 749–758. [Google Scholar] [PubMed]
- Hoeper, M.M.; Al-Hiti, H.; Benza, R.L.; Chang, S.A.; Corris, P.A.; Gibbs, J.S.R.; Grunig, E.; Jansa, P.; Klinger, J.R.; Langleben, D.; et al. Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): A multicentre, open-label, randomised controlled trial. Lancet Respir. Med. 2021, 9, 573–584. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Ahmetaj-Shala, B.; Kirkby, N.S.; Wright, W.R.; Mackenzie, L.S.; Reed, D.M.; Mohamed, N. Role of prostacyclin in pulmonary hypertension. Glob. Cardiol. Sci. Pract. 2014, 2014, 382–393. [Google Scholar] [CrossRef]
- Ruan, C.H.; Dixon, R.A.; Willerson, J.T.; Ruan, K.H. Prostacyclin therapy for pulmonary arterial hypertension. Tex. Heart Inst. J. 2010, 37, 391–399. [Google Scholar]
- Ivy, D.D. Prostacyclin in the intensive care setting. Pediatr. Crit. Care Med. 2010, 11, S41–S45. [Google Scholar] [CrossRef]
- Gomberg-Maitland, M.; Tapson, V.F.; Benza, R.L.; McLaughlin, V.V.; Krichman, A.; Widlitz, A.C.; Barst, R.J. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2005, 172, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Voswinckel, R.; Reichenberger, F.; Gall, H.; Schmehl, T.; Gessler, T.; Schermuly, R.T.; Grimminger, F.; Rubin, L.J.; Seeger, W.; Ghofrani, H.A.; et al. Metered dose inhaler delivery of treprostinil for the treatment of pulmonary hypertension. Pulm. Pharmacol. Ther. 2009, 22, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Kaestle, S.; Yin, J.; Hentschel, T.; Pries, A.R.; Kuppe, H.; Kuebler, W.M. Inhaled nitric oxide versus aerosolized iloprost for the treatment of pulmonary hypertension with left heart disease. Crit. Care Med. 2009, 37, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Yilmaz, H.; Ghofrani, H.A.; Woyda, K.; Pullamsetti, S.; Schulz, A.; Gessler, T.; Dumitrascu, R.; Weissmann, N.; Grimminger, F.; et al. Inhaled iloprost reverses vascular remodeling in chronic experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2005, 172, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.B.; Kerstein, D.; Barst, R.J. Long-term prostacyclin for pulmonary hypertension with associated congenital heart defects. Circulation 1999, 99, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Skoro-Sajer, N.; Gerges, C.; Balint, O.H.; Kohalmi, D.; Kaldararova, M.; Simkova, I.; Jakowitsch, J.; Gabriel, H.; Baumgartner, H.; Gerges, M.; et al. Subcutaneous treprostinil in congenital heart disease-related pulmonary arterial hypertension. Heart 2018, 104, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Chon, M.K.; Cho, K.I.; Cha, K.S.; Seo, J.S.; Kim, D.S. Effects of long-term iloprost treatment on right ventricular function in patients with Eisenmenger syndrome. J. Cardiol. 2017, 69, 741–746. [Google Scholar] [CrossRef]
- van Dissel, A.C.; Post, M.C.; Sieswerda, G.; Vliegen, H.W.; van Dijk, A.P.J.; Mulder, B.J.; Bouma, B.J. Selexipag for pulmonary arterial hypertension in a wide range of adult congenital heart disease. Int. J. Cardiol. Congenit. Heart Dis. 2021, 4, 100144. [Google Scholar] [CrossRef]
- Demerouti, E.A.; Karyofyllis, P.; Apostolopoulou, S.C. Use of the Prostacyclin Receptor Agonist Selexipag in Patients with Pulmonary Arterial Hypertension Associated with Eisenmenger Syndrome. Can. J. Cardiol. 2021, 37, 1286–1288. [Google Scholar] [CrossRef]
- Van Dissel, A.; Post, M.; Sieswerda, G.T.; Vliegen, H.W.; Van Dijk, A.P.; Duijnhouwer, A.L.; Mulder, B.J.; Bouma, B.J. Early experience with selexipag for the treatment of adults with pulmonary arterial hypertension associated with congenital heart disease. Eur. Heart J. 2020, 41, ehaa946.2191. [Google Scholar] [CrossRef]
- Sitbon, O.; Cottin, V.; Canuet, M.; Clerson, P.; Gressin, V.; Perchenet, L.; Bertoletti, L.; Bouvaist, H.; Picard, F.; Prevot, G.; et al. Initial combination therapy of macitentan and tadalafil in pulmonary arterial hypertension. Eur. Respir. J. 2020, 56, 2000673. [Google Scholar] [CrossRef] [PubMed]
- Luna-Lopez, R.; Segura de la Cal, T.; Sarnago Cebada, F.; Martin de Miguel, I.; Hinojosa, W.; Cruz-Utrilla, A.; Velazquez, M.T.; Delgado, J.F.; Mendoza, A.; Arribas Ynsaurriaga, F.; et al. Triple vasodilator therapy in pulmonary arterial hypertension associated with congenital heart disease. Heart 2023. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, R. Pulmonary arterial hypertension associated with congenital heart disease: Classification and pathophysiology. J. Congenit. Cardiol. 2020, 4, 16. [Google Scholar] [CrossRef]
- Wacker, J.; Joye, R.; Genecand, L.; Lador, F.; Beghetti, M. Pulmonary vascular disease as a complication of pediatric congenital heart diseases. Transl. Pediatr. 2023, 12, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, K.; Peset, A.; Gatzoulis, M.A. Evaluating operability in adults with congenital heart disease and the role of pretreatment with targeted pulmonary arterial hypertension therapy. Int. J. Cardiol. 2008, 129, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Haworth, S.G.; Hislop, A.A. Treatment and survival in children with pulmonary arterial hypertension: The UK Pulmonary Hypertension Service for Children 2001–2006. Heart 2009, 95, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.A.; Barst, R.J.; Haworth, S.G.; Rabinovitch, M.; Al Dabbagh, M.; Del Cerro, M.J.; Ivy, D.; Kashour, T.; Kumar, K.; Harikrishnan, S.; et al. Repair of congenital heart disease with associated pulmonary hypertension in children: What are the minimal investigative procedures? Consensus statement from the Congenital Heart Disease and Pediatric Task Forces, Pulmonary Vascular Research Institute (PVRI). Pulm. Circ. 2014, 4, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Opotowsky, A.R.; Hess, E.; Maron, B.A.; Brittain, E.L.; Baron, A.E.; Maddox, T.M.; Alshawabkeh, L.I.; Wertheim, B.M.; Xu, M.; Assad, T.R.; et al. Thermodilution vs Estimated Fick Cardiac Output Measurement in Clinical Practice: An Analysis of Mortality From the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) Program and Vanderbilt University. JAMA Cardiol. 2017, 2, 1090–1099. [Google Scholar] [CrossRef]
- Egidy Assenza, G.; Krieger, E.V.; Baumgartner, H.; Cupido, B.; Dimopoulos, K.; Louis, C.; Lubert, A.M.; Stout, K.K.; Valente, A.M.; Zeppenfeld, K.; et al. AHA/ACC vs ESC Guidelines for Management of Adults with Congenital Heart Disease: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2021, 78, 1904–1918. [Google Scholar] [CrossRef]
- Mantegazza, V.; Apostolo, A.; Hager, A. Cardiopulmonary Exercise Testing in Adult Congenital Heart Disease. Ann. Am. Thorac. Soc. 2017, 14, S93–S101. [Google Scholar] [CrossRef]
- Hansmann, G.; Koestenberger, M.; Alastalo, T.P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transpl. 2019, 38, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Liew, N.; Rashid, Z.; Tulloh, R. Strategies for the Management of Pulmonary Arterial Hypertension in Patients with Congenital Heart Disease. J. Congenit. Heart Dis. 2020, 4 (Suppl. 1), 21. Available online: https://jcongenitalcardiology.biomedcentral.com/articles/10.1186/s40949-020-00052-w (accessed on 7 November 2023). [CrossRef]
- Bradley, E.A.; Ammash, N.; Martinez, S.C.; Chin, K.; Hebson, C.; Singh, H.S.; Aboulhosn, J.; Grewal, J.; Billadello, J.; Chakinala, M.M.; et al. “Treat-to-close”: Non-repairable ASD-PAH in the adult: Results from the North American ASD-PAH (NAAP) Multicenter Registry. Int. J. Cardiol. 2019, 291, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Akagi, T.; Sakamoto, I.; Kanazawa, H.; Nakazawa, G.; Murakami, T.; Yao, A.; Nanasato, M.; Saji, M.; Hirokami, M.; et al. Efficacy of treat-and-repair strategy for atrial septal defect with pulmonary arterial hypertension. Heart 2022, 108, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Brida, M.; Gatzoulis, M.A. Pulmonary arterial hypertension in adult congenital heart disease. Heart 2018, 104, 1568–1574. [Google Scholar] [CrossRef]
- Savale, L.; Manes, A. Pulmonary arterial hypertension populations of special interest: Portopulmonary hypertension and pulmonary arterial hypertension associated with congenital heart disease. Eur. Heart J. Suppl. 2019, 21, K37–K45. [Google Scholar] [CrossRef]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Gomez Sanchez, M.A.; Krishna Kumar, R.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D34–D41. [Google Scholar] [CrossRef]
- Arshad, H.B.; Duarte, V.E. Evaluation and Management of Pulmonary Arterial Hypertension in Congenital Heart Disease. Methodist. Debakey Cardiovasc. J. 2021, 17, 145–151. [Google Scholar] [CrossRef]
- Beghetti, M.; Channick, R.N.; Chin, K.M.; Di Scala, L.; Gaine, S.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; McLaughlin, V.V.; Preiss, R.; et al. Selexipag treatment for pulmonary arterial hypertension associated with congenital heart disease after defect correction: Insights from the randomised controlled GRIPHON study. Eur. J. Heart Fail. 2019, 21, 352–359. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Galie, N.; Grimminger, F.; Grunig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Feldman, J.; McLaughlin, V.V.; Rischard, F.; Lange, T.J.; White, R.J.; Peacock, A.J.; Gerhardt, F.; Ebrahimi, R.; Brooks, G.; et al. Selonsertib in adults with pulmonary arterial hypertension (ARROW): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2022, 10, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; McKie, M.A.; Law, M.; Roussakis, A.A.; Harbaum, L.; Church, C.; Coghlan, J.G.; Condliffe, R.; Howard, L.S.; Kiely, D.G.; et al. Positioning imatinib for pulmonary arterial hypertension: A phase I/II design comprising dose finding and single-arm efficacy. Pulm. Circ. 2021, 11, 20458940211052823. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Barst, R.J.; Bourge, R.C.; Feldman, J.; Frost, A.E.; Galie, N.; Gomez-Sanchez, M.A.; Grimminger, F.; Grunig, E.; Hassoun, P.M.; et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: Results of the randomized IMPRES study. Circulation 2013, 127, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- A Study of AV-101 (Dry Powder Inhaled Imatinib) in Patients with Pulmonary Arterial Hypertension (PAH) (IMPAHCT). Available online: https://clinicaltrials.gov/study/NCT05036135?cond=Pulmonary%20Hypertension&intr=Imatinib&rank=10#collaborators-and-investigators (accessed on 7 November 2023).
- Frantz, R.P.; Benza, R.L.; Channick, R.N.; Chin, K.; Howard, L.S.; McLaughlin, V.V.; Sitbon, O.; Zamanian, R.T.; Hemnes, A.R.; Cravets, M.; et al. TORREY, a Phase 2 study to evaluate the efficacy and safety of inhaled seralutinib for the treatment of pulmonary arterial hypertension. Pulm. Circ. 2021, 11, 20458940211057071. [Google Scholar] [CrossRef] [PubMed]
- Pullamsetti, S.S.; Sitapara, R.; Osterhout, R.; Weiss, A.; Carter, L.L.; Zisman, L.S.; Schermuly, R.T. Pharmacology and Rationale for Seralutinib in the Treatment of Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2023, 24, 12653. [Google Scholar] [CrossRef] [PubMed]
- Galkin, A.; Sitapara, R.; Clemons, B.; Garcia, E.; Kennedy, M.; Guimond, D.; Carter, L.L.; Douthitt, A.; Osterhout, R.; Gandjeva, A.; et al. Inhaled seralutinib exhibits potent efficacy in models of pulmonary arterial hypertension. Eur. Respir. J. 2022, 60, 2102356. [Google Scholar] [CrossRef] [PubMed]
- Ravi Sitapara, D.S.; Salter-Cid, L.; Zisman, L. In Vivo Efficacy of a Novel, Inhaled Pdgfra/b Inhibitor, Gb002, in The Rat Monocrotaline and Pneumonectomy Model of Pulmonary Arterial Hypertension. Available online: https://www.ahajournals.org/doi/abs/10.1161/circ.140.suppl_1.12947 (accessed on 7 November 2023).
- Richard Aranda, M. Open-label Extension Study of GB002 in Adult Subjects with Pulmonary Arterial Hypertension (PAH). Available online: https://clinicaltrials.gov/study/NCT04816604?cond=Pulmonary%20Hypertension&intr=Seralutinib&rank=2#collaborators-and-investigators (accessed on 7 November 2023).
- Richard Aranda, M. Efficacy and Safety of Seralutinib in Adult Subjects with PAH (PROSERA). Available online: https://clinicaltrials.gov/study/NCT05934526?cond=Pulmonary%20Hypertension&intr=Seralutinib&rank=1#collaborators-and-investigators (accessed on 7 November 2023).
- Yoshida, T.; Matsuura, K.; Goya, S.; Ma, D.; Shimada, K.; Kitpipatkun, P.; Namiki, R.; Uemura, A.; Suzuki, K.; Tanaka, R. Metformin prevents the development of monocrotaline-induced pulmonary hypertension by decreasing serum levels of big endothelin-1. Exp. Ther. Med. 2020, 20, 149. [Google Scholar] [CrossRef]
- Abdelazeem, H.; Tu, L.; Thuillet, R.; Ottaviani, M.; Boulfrad, A.; Beck, T.; Senbel, A.; Mani, S.; Castier, Y.; Guyard, A.; et al. AMPK activation by metformin protects against pulmonary hypertension in rats and relaxes isolated human pulmonary artery. Eur. J. Pharmacol. 2023, 946, 175579. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Y.; Su, X.; Zhu, Y.; Liu, L.; Pan, Y.; Zhu, B.; Yang, L.; Gao, L.; Li, M. Activation of AMPK inhibits PDGF-induced pulmonary arterial smooth muscle cells proliferation and its potential mechanisms. Pharmacol. Res. 2016, 107, 117–124. [Google Scholar] [CrossRef]
- Liao, S.; Li, D.; Hui, Z.; McLachlan, C.S.; Zhang, Y. Metformin added to bosentan therapy in patients with pulmonary arterial hypertension associated with congenital heart defects: A pilot study. ERJ Open. Res. 2018, 4, 00060–2018. [Google Scholar] [CrossRef]
- Peter, J.; Leary, M. Repurposing a Histamine Antagonist to Benefit Patients with Pulmonary Hypertension (REHAB-PH). Available online: https://clinicaltrials.gov/study/NCT03554291?cond=Pulmonary%20Hypertension&intr=famotidine&rank=1#collaborators-and-investigators (accessed on 7 November 2023).
- Zeng, Z.; Shen, L.; Li, X.X.; Luo, T.; Wei, X.; Zhang, J.W.; Cao, S.P.; Huang, X.B.; Fukushima, Y.; Bin, J.P.; et al. Disruption of histamine H receptor slows heart failure progression through reducing myocardial apoptosis and fibrosis. Clin. Sci. 2014, 127, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Leary, P.J.; Barr, R.G.; Bluemke, D.A.; Bristow, M.R.; Kronmal, R.A.; Lima, J.A.; Ralph, D.D.; Ventetuolo, C.E.; Kawut, S.M. H2 receptor antagonists and right ventricular morphology: The MESA right ventricle study. Ann. Am. Thorac. Soc. 2014, 11, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.P.; Baird, G.L.; Atalay, M.K.; Agarwal, S.; Arcuri, D.; Klinger, J.R.; Mullin, C.J.; Morreo, H.; Normandin, B.; Shiva, S.; et al. Experimental design of the Effects of Dehydroepiandrosterone in Pulmonary Hypertension (EDIPHY) trial. Pulm. Circ. 2021, 11, 2045894021989554. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.K.; Alzoubi, A.; Gupte, R.; Chettimada, S.; Watanabe, M.; Kahn, A.G.; Okada, T.; McMurtry, I.F.; Gupte, S.A. Increased reactive oxygen species, metabolic maladaptation, and autophagy contribute to pulmonary arterial hypertension-induced ventricular hypertrophy and diastolic heart failure. Hypertension 2014, 64, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Ventetuolo, C.E. Effects of DHEA in Pulmonary Hypertension (EDIPHY). Available online: https://clinicaltrials.gov/study/NCT03648385?cond=Pulmonary%20Hypertension&intr=DHEA%20&rank=1#collaborators-and-investigators (accessed on 7 November 2023).
- Lazarus, H.M.; Denning, J.; Wring, S.; Palacios, M.; Hoffman, S.; Crizer, K.; Kamau-Kelley, W.; Symonds, W.; Feldman, J. A trial design to maximize knowledge of the effects of rodatristat ethyl in the treatment of pulmonary arterial hypertension (ELEVATE 2). Pulm. Circ. 2022, 12, e12088. [Google Scholar] [CrossRef]
- Eddahibi, S.; Guignabert, C.; Barlier-Mur, A.M.; Dewachter, L.; Fadel, E.; Dartevelle, P.; Humbert, M.; Simonneau, G.; Hanoun, N.; Saurini, F.; et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension—Critical role for serotonin-induced smooth muscle hyperplasia. Circulation 2006, 113, 1857–1864. [Google Scholar] [CrossRef]
- Barbosa Lopes, A.A. Pentoxifylline as an Adjunct Therapy for Patients with Eisenmenger Syndrome. Available online: https://clinicaltrials.gov/study/NCT05611268?cond=Pulmonary%20Hypertension&intr=Pentoxifylline&rank=1#collaborators-and-investigators (accessed on 7 November 2023).
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grunig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.B.; Ghofrani, H.A.; Escribano Subias, P.; et al. Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur. Respir. J. 2023, 61, 2201347. [Google Scholar] [CrossRef]
- A Study of Sotatercept in Participants with PAH WHO FC III or FC IV at High Risk of Mortality (MK-7962-006/ZENITH) (ZENITH). Available online: https://clinicaltrials.gov/study/NCT04896008?cond=Pulmonary%20Hypertension&intr=Sotatercept%20&rank=6#collaborators-and-investigators (accessed on 7 November 2023).
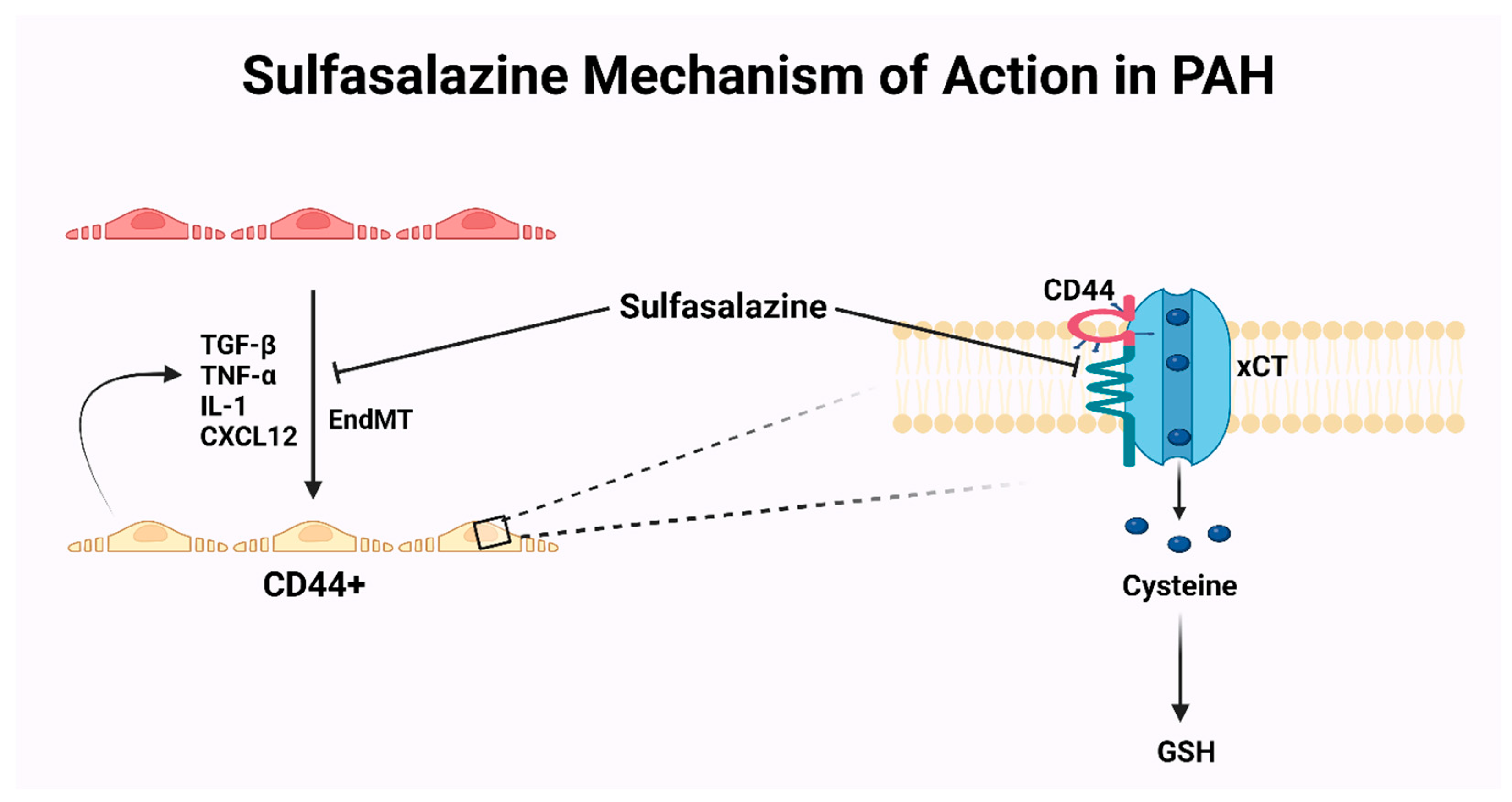
- Isobe, S.; Kataoka, M.; Endo, J.; Moriyama, H.; Okazaki, S.; Tsuchihashi, K.; Katsumata, Y.; Yamamoto, T.; Shirakawa, K.; Yoshida, N.; et al. Endothelial-Mesenchymal Transition Drives Expression of CD44 Variant and xCT in Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2019, 61, 367–379. [Google Scholar] [CrossRef]
- Agrawal, V.; Hemnes, A.R. CD44 and xCT: The Silver Bullet for Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension? Am. J. Respir. Cell Mol. Biol. 2019, 61, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Shen, J. Pilot Study of the Safety and Efficacy of Sulfasalazine in Pulmonary Arterial Hypertension. Available online: https://clinicaltrials.gov/study/NCT04528056?cond=Pulmonary%20Hypertension&intr=Sulfasalazine&rank=1#collaborators-and-investigators (accessed on 7 November 2023).
- Chelladurai, P.; Boucherat, O.; Stenmark, K.; Kracht, M.; Seeger, W.; Bauer, U.M.; Bonnet, S.; Pullamsetti, S.S. Targeting histone acetylation in pulmonary hypertension and right ventricular hypertrophy. Br. J. Pharmacol. 2021, 178, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Van der Feen, D.E.; Kurakula, K.; Tremblay, E.; Boucherat, O.; Bossers, G.P.L.; Szulcek, R.; Bourgeois, A.; Lampron, M.C.; Habbout, K.; Martineau, S.; et al. Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 200, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.; Blais-Lecours, P. Apabetalone for Pulmonary Arterial Hypertension (APPROACH-2). Available online: https://clinicaltrials.gov/study/NCT04915300?cond=Pulmonary%20Hypertension&intr=Apabetalone&rank=2#collaborators-and-investigators (accessed on 7 November 2023).
- Spiekerkoetter, E.; Tian, X.; Cai, J.; Hopper, R.K.; Sudheendra, D.; Li, C.G.; El-Bizri, N.; Sawada, H.; Haghighat, R.; Chan, R.; et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Investig. 2013, 123, 3600–3613. [Google Scholar] [CrossRef] [PubMed]
- Spiekerkoetter, E.; Sung, Y.K.; Sudheendra, D.; Scott, V.; Del Rosario, P.; Bill, M.; Haddad, F.; Long-Boyle, J.; Hedlin, H.; Zamanian, R.T. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1602449. [Google Scholar] [CrossRef]
- Ilgin, S.; Burukoglu, D.; Atli, O.; Sirmagul, B. Effects of everolimus in combination with sildenafil in monocrotaline-induced pulmonary hypertension in rats. Cardiovasc. Toxicol. 2012, 12, 46–55. [Google Scholar] [CrossRef]
- Houssaini, A.; Abid, S.; Mouraret, N.; Wan, F.; Rideau, D.; Saker, M.; Marcos, E.; Tissot, C.M.; Dubois-Rande, J.L.; Amsellem, V.; et al. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 48, 568–577. [Google Scholar] [CrossRef]
- Phase 1/1b Study with Nab-sirolimus for Patients with Severe Pulmonary Arterial Hypertension. Available online: https://clinicaltrials.gov/study/NCT02587325?cond=Pulmonary%20Hypertension&intr=nab-sirolimus&rank=1#publications (accessed on 7 November 2023).


| Medication | Mechanism of Action | Trial | Primary Endpoint |
|---|---|---|---|
| Selonsertib | ASK1(MAP3K5) inhibitor | (ARROW) NCT02234141 | PVR |
| Imatinib | BCR-ABL tyrosine kinase inhibitor | (IMPAHCT) NCT05036135 | PVR 6MWD |
| (PIPAH) NCT04416750 | MTD and PVR | ||
| Seralutinib | Selective tyrosine kinase inhibitor on PDGF Ra/b, CSF1R, and c-kit pathways | (PROSERA) NCT05934526 | 6MWD |
| (TORREY) NCT04456998 | PVR and adverse events | ||
| Pentoxifylline | PDE-i that inhibits TNF and leukotrienes synthesis, increases thrombomodulin. Hemorheological agent | NCT05611268 | Thrombomodulin levels |
| Metformin | AMPK activator | Prospective randomized study | 6MWD Endothelin-1 levels |
| H2 antagonists | Ameliorates RV strain | (REHAB-PH) NCT03554291 | 6MWD |
| DHEA | Protective effects on cardiomyocytes by reducing G6PD-derived NADPH | (EDIPHY) NCT03648385 | RV longitudinal strain |
| Rodatristat | Peripheral tryptophan hydroxylase-1 inhibitor | (ELEVATE2) NCT04712669 | PVR |
| Sotatercept | Decoy receptor for ActRIIA ligands to restore the balance between TGF-b superfamily members | (STELLAR) NCT04576988 | 6MWD and adverse events |
| (PULSAR) NCT03496207 (ZENITH) NCT04896008 | PVR and adverse events Morbidity and mortality | ||
| Sulfasalazine | Interference with CD44v–xCT axis | NCT04528056 | Clinical adverse events |
| Spironolactone | Aldosterone antagonist, decreases remodeling | NCT01712620 | 6MWD |
| Apabetalone | Alters genetic transcription by inhibiting BRD4. Supportive effect on overloaded RV | (APPROACH-2) NCT04915300 | PVR |
| Tacrolimus (FK-506) | Restores BMPR2 signaling | (TransForm PAH) NCT01647945 | safety |
| albumin-bound sirolimus (Rapamycin) | mTOR inhibitor | NCT02587325 | MTD and DLT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.K.; Abbas, M.T.; Kamel, M.A.; Farina, J.M.; Pereyra, M.; Scalia, I.G.; Barry, T.; Chao, C.-J.; Marcotte, F.; Ayoub, C.; et al. Current Management and Future Directions for Pulmonary Arterial Hypertension Associated with Congenital Heart Disease. J. Pers. Med. 2024, 14, 5. https://doi.org/10.3390/jpm14010005
Mahmoud AK, Abbas MT, Kamel MA, Farina JM, Pereyra M, Scalia IG, Barry T, Chao C-J, Marcotte F, Ayoub C, et al. Current Management and Future Directions for Pulmonary Arterial Hypertension Associated with Congenital Heart Disease. Journal of Personalized Medicine. 2024; 14(1):5. https://doi.org/10.3390/jpm14010005
Chicago/Turabian StyleMahmoud, Ahmed K., Mohammed Tiseer Abbas, Moaz A. Kamel, Juan M. Farina, Milagros Pereyra, Isabel G. Scalia, Timothy Barry, Chieh-Ju Chao, Francois Marcotte, Chadi Ayoub, and et al. 2024. "Current Management and Future Directions for Pulmonary Arterial Hypertension Associated with Congenital Heart Disease" Journal of Personalized Medicine 14, no. 1: 5. https://doi.org/10.3390/jpm14010005
APA StyleMahmoud, A. K., Abbas, M. T., Kamel, M. A., Farina, J. M., Pereyra, M., Scalia, I. G., Barry, T., Chao, C.-J., Marcotte, F., Ayoub, C., Scott, R. L., Majdalany, D. S., & Arsanjani, R. (2024). Current Management and Future Directions for Pulmonary Arterial Hypertension Associated with Congenital Heart Disease. Journal of Personalized Medicine, 14(1), 5. https://doi.org/10.3390/jpm14010005










