Is There Any Effect of Change in Pre-Wash and Post-Wash Semen Parameters on the Success of Intrauterine Insemination?
Abstract
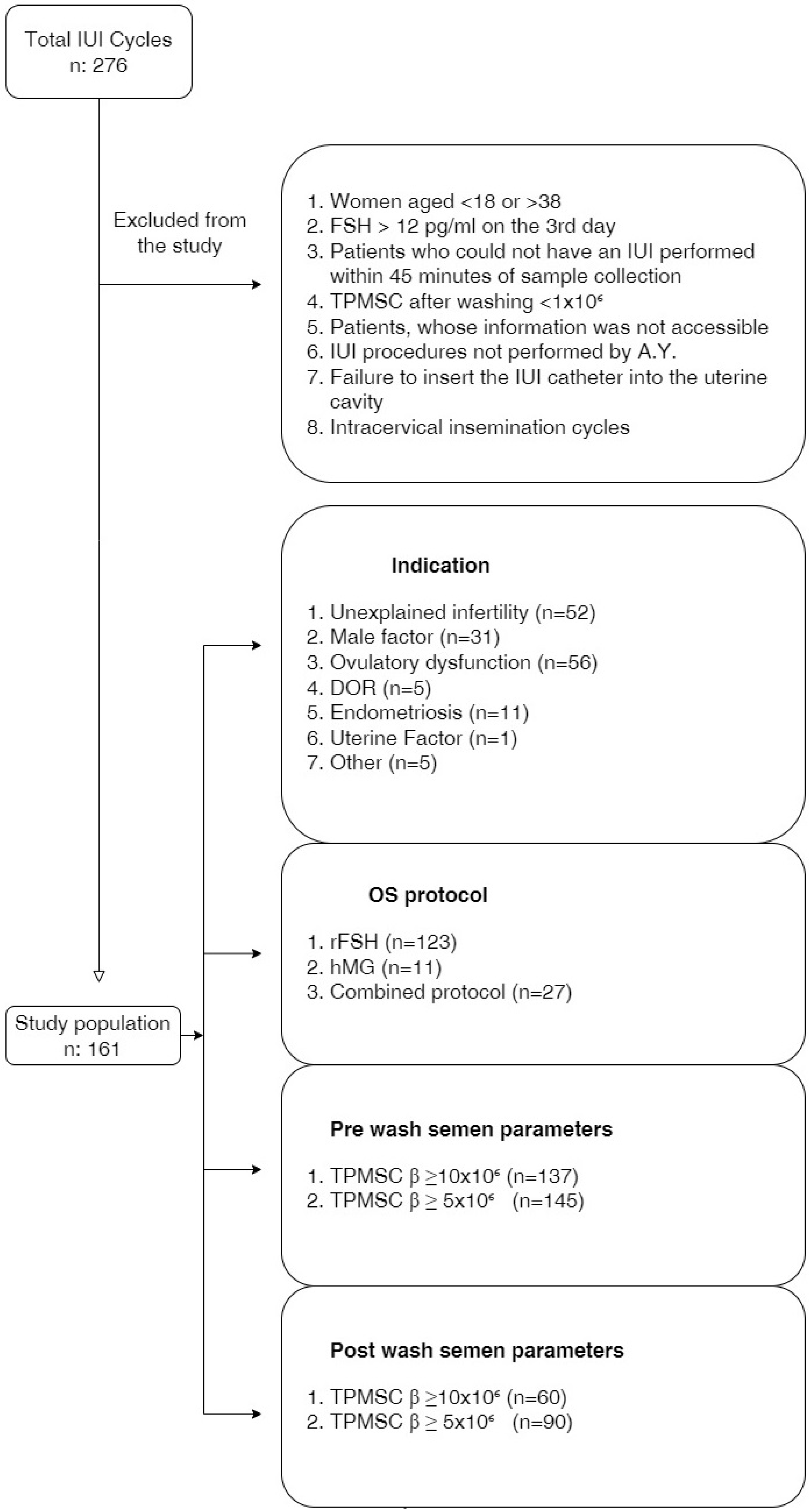
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Ovarian Stimulation and IUI Protocol
2.3. Semen Analysis
2.4. Insemination
2.5. Patient Demographics and Outcome Assessment
2.6. Statistical Analyses
3. Results
3.1. Demographic Data
3.2. Comparison of the Pre-Wash and Post-Wash Semen Parameters Regarding the CPR
3.3. Comparison of the Pre-Wash and Post-Wash Semen Parameters Regarding the LBR
3.4. Comparison of the Reproductive Phases of Couples and Ovarian Stimulation Protocols in Terms of CPR and LBR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vayena, E.; Rowe, P.; Griffin, P. Current Practices and Controversies in Assisted Reproduction. In Report of a Meeting on Medical Ethical and Social Aspets of Assisted Reproduction Held at WHO Headquarters; WHO: Geneva Switzerland, 2002. [Google Scholar]
- Mazzilli, R.; Cimadomo, D.; Vaiarelli, A.; Capalbo, A.; Dovere, L.; Alviggi, E.; Dusi, L.; Foresta, C.; Lombardo, F.; Lenzi, A.; et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: Observational longitudinal cohort study of 1219 consecutive cycles. Fertil. Steril. 2017, 108, 961–972.e3. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: Assessment and Treatment for People with Fertility Problems; Royal College of Obstetricians & Gynaecologists: London, UK, 2013. [Google Scholar]
- Feng, J.; Wang, J.; Zhang, Y.; Zhang, Y.; Jia, L.; Zhang, D.; Zhang, J.; Han, Y.; Luo, S. The Efficacy of Complementary and Alternative Medicine in the Treatment of Female Infertility. Evid.-Based Complement. Altern. Med. 2021, 2021, 6634309. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, S.M.; Cohlen, B.J.; Hughes, E.; Te Velde, E.; Heineman, M.J. Intra-uterine insemination for unexplained subfertility. Cochrane Database Syst. Rev. 2006, 18, CD001838. [Google Scholar]
- Ombelet, W.; Campo, R.; Bosmans, E.; Nijs, M. Intrauterine insemination (IUI) as a first-line treatment in developing countries and methodological aspects that might influence IUI success. Hum. Reprod. 2008, 23 (Suppl. 1), 64–72. [Google Scholar] [CrossRef]
- Ombelet, W.; Dhont, N.; Thijssen, A.; Bosmans, E.; Kruger, T. Semen quality and prediction of IUI success in male subfertility: A systematic review. Reprod. Biomed. Online 2014, 28, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Erdem, M.; Erdem, A.; Mutlu, M.F.; Ozisik, S.; Yildiz, S.; Guler, I.; Karakaya, C. The impact of sperm morphology on the outcome of intrauterine insemination cycles with gonadotropins in unexplained and male subfertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, S.K.; Kim, H.; Lee, J.R.; Jee, B.C.; Kim, S.H. Predictive value of sperm motility before and after preparation for the pregnancy outcomes of intrauterine insemination. Clin. Exp. Reprod. Med. 2021, 48, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, L.; Kos, S.; Beijer, C.; Brinkman, J.W.; van der Horst, F.A.; van den Hoven, L.; Kieslinger, D.C.; Vrouwerff, N.J.v.T.-V.; Wolthuis, A.; Hendriks, J.C.; et al. Predictive value of sperm morphology and progressively motile sperm count for pregnancy outcomes in intrauterine insemination. Fertil. Steril. 2016, 105, 1462–1468. [Google Scholar] [CrossRef]
- Ainsworth, A.J.; Barnard, E.P.; Baumgarten, S.C.; Weaver, A.L.; Khan, Z. Intrauterine insemination cycles: Prediction of success and thresholds for poor prognosis and futile care. J. Assist. Reprod. Genet. 2020, 37, 2435–2442. [Google Scholar] [CrossRef]
- Kohn, T.P.; Kohn, J.R.; Ramasamy, R. Effect of Sperm Morphology on Pregnancy Success via Intrauterine Insemination: A Systematic Review and Meta-Analysis. J. Urol. 2018, 199, 812–822. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Sigman, M.; Collura, B.; De Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. Fertil. Steril. 2021, 115, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. Intrauterine Insemination: Current Place in Infertility Management. Eur. Med. J. 2018, 3, 58–66. [Google Scholar] [CrossRef]
- Wiser, A.; Ghetler, Y.; Gonen, O.; Piura, E.; Berkovits, A.; Itskovich, A.; Rom, T.; Shulman, A. Re-evaluation of post-wash sperm is a helpful tool in the decision to perform in vitro fertilisation or intracytoplasmic sperm injection. Andrologia 2012, 44, 73–77. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Inter-Action; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Boomsma, C.M.; Cohlen, B.J.; Farquhar, C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst. Rev. 2019, 10, CD004507. [Google Scholar] [CrossRef]
- Luco, S.M.; Agbo, C.; Behr, B.; Dahan, M.H. The evaluation of pre and post processing semen analysis parameters at the time of intrauterine insemination in couples diagnosed with male factor infertility and pregnancy rates based on stimulation agent. A retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 159–162. [Google Scholar] [CrossRef]
- Afolabi, B.M.; Ezedinachi, E.N.; Arikpo, I.; Ogunwale, A.; Ganiyu, D.F.; Abu, R.; Ajibade, A. Knowledge, non-use, use and source of information on contraceptive methods among women in various stages of reproductive age in rural Lagos, Southwest Nigeria. Open Access J. Contracept. 2015, 6, 65–75. [Google Scholar] [CrossRef]
- Lu, X.M.; Liu, Y.B.; Zhang, D.D.; Cao, X.; Zhang, T.C.; Liu, M.; Shi, H.J.; Dong, X.; Liu, S.Y. Effect of advanced paternal age on reproductive outcomes in IVF cycles of non-male-factor infertility: A retrospective cohort study. Asian J. Androl. 2023, 25, 245–251. [Google Scholar] [CrossRef]
- Soyer-Calıskan, C.; Hatirnaz, K.; Celik, S.; Başbuğ, A.; Hatirnaz, E.; Hatirnaz, Ş.; Dahan, M.H. A Comparison of Triple and Double Sperm Washing for Density Gradient Preparation in Intrauterine Insemination Cycles when Overnight Incubation of Specimens Occurred: A Retrospective Cohort. Clin. Exp. Obstet. Gynecol. 2022, 49, 250. [Google Scholar] [CrossRef]
- Mathes, M.; Kastrick, E.; Sayles, H.; Gustin, S. How low is too low? Postwash total motile sperm count effect on pregnancy outcomes in intrauterine insemination. Hum. Ferti. 2023, 26, 1108–1113. [Google Scholar] [CrossRef]
- Findeklee, S.; Radosa, J.C.; Radosa, M.P.; Hammadeh, M.E. Correlation between total sperm count and sperm motility and pregnancy rate in couples undergoing intrauterine insemination. Sci. Rep. 2020, 10, 7555. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Sjaarda, L.A.; Peterson, C.M.; Hotaling, J.M.; Mills, J.L.; Mendola, P.; Carrell, D.T.; Johnstone, E.; Chen, Z.; Perkins, N.J.; et al. Longitudinal semen parameter assessments and live birth: Variability and implications for treatment strategies. Fertil. Steril. 2022, 118, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Zippl, A.L.; Wachter, A.; Rockenschaub, P.; Toth, B.; Seeber, B. Predicting success of intrauterine insemination using a clinically based scoring system. Arch. Gynecol. Obstet. 2022, 306, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Çil, N.; Kabukçu, C.; Çabuş, Ü.; Turan, T.; Mete, G.A. Retrospective comparison of the semen preparation techniques for intrauterine insemination: Swim-up versus density gradient method. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102321. [Google Scholar] [CrossRef]
- Gordon, C.E.; Hammer, K.C.; James, K.; Lanes, A.; Vagios, S.; Starosta, A.; Hornstein, M.; Souter, I. Optimizing pregnancy outcomes in intrauterine insemination cycles by stratifying pre-wash total motile count and patient-specific factors: A patient counseling tool. J. Assist. Reprod. Genet. 2022, 39, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Z.; Wang, H.; Yang, L.; Ma, C.; Sun, L. Prewash and postwash total progressively motile sperm counts have poor predictive value for clinical pregnancy after intrauterine insemination. Int. J. Gynaecol. Obstet. 2021, 153, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Stanhiser, J.; Mersereau, J.E.; Dock, D.; Boylan, C.; Caprell, H.; Coward, R.M.; Berger, D.S.; Fritz, M. Sperm morphology from the actual inseminated sample does not predict clinical pregnancy following intrauterine insemination. FS Rep. 2020, 2, 16–21. [Google Scholar] [CrossRef]
- Geraldo Orrego, J.; Mackenna Iñiguez, A.; Schwarze Meza, J.E.; Ortega Parraguez, V.; Carrasco Rojas, J.; Palma Ceppi, C. Impact of sperm morphology on pregnancy rates after intrauterine insemination. Rev. Int. Androl. 2023, 21, 100326. [Google Scholar] [CrossRef]
- Fuentes Ávila, A.; Blasco Sanz, R.; Cortés Alaguero, C. Effect of Sperm Morphology in Intrauterine Insemination: Analysis of 115 Cycles and Literature Review. Obstet. Gynecol. Surv. 2021, 76, 170–174. [Google Scholar] [CrossRef]
- Pino, V.; Sanz, A.; Valdés, N.; Crosby, J.; Mackenna, A. The efects of aging on semen parameters and sperm DNA fragmenta-tion. JBRA Assist. Reprod. 2020, 24, 82–86. [Google Scholar]
- Huniadi, A.; Bimbo-Szuhai, E.; Botea, M.; Zaha, I.; Beiusanu, C.; Pallag, A.; Stefan, L.; Bodog, A.; Șandor, M.; Grierosu, C. Fertility Predictors in Intrauterine Insemination (IUI). J. Pers. Med. 2023, 13, 395. [Google Scholar] [CrossRef]
- Starosta, A.; Gordon, C.E.; Hornstein, M.D. Predictive factors for intrauterine insemination outcomes: A review. Fertil. Res. Pract. 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, M.; Harlow, B.L.; Hornstein, M.D. Influence of age, diagnosis, and cycle number on pregnancy rates with gonad-otropininduced controlled ovarian hyperstimulation and intrauterine insemination. Fertil. Steril. 1999, 72, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Osaikhuwuomwan, J.; Osemwenkha, A.; Iribhogbe, O.; Aziken, M.; Orhue, A. The effect of female age on the outcome of intrauterine insemination treatment in a public hospital-assisted reproduction technology unit. Niger. J. Clin. Pract. 2018, 21, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, K.; Isa, A.; Habous, M.; Almannie, R.; Abu-Rafea, B.; Binsaleh, S. Postwash total motile sperm count: Should it be included as a standard male infertility work up. Can. J. Urol. 2017, 24, 8847–8852. [Google Scholar]
- Wang, X.; Zhang, Y.; Sun, H.-L.; Wang, L.-T.; Li, X.-F.; Wang, F.; Wang, Y.-L.; Li, Q.-C. Factors Affecting Artificial Insemination Pregnancy Outcome. Int. J. Gen. Med. 2021, 14, 3961–3969. [Google Scholar] [CrossRef]
- Cohlen, B.; Bijkerk, A.; Van der Poel, S.; Ombelet, W. IUI: Review and systematic assessment of the evidence that supports global recommendations. Hum. Reprod. Update 2018, 24, 300–319. [Google Scholar] [CrossRef]
| n (%) | Mean ± SD | |
|---|---|---|
| Mean age of women (years) | 161 | 28.15 ± 4.1 |
| FSH levels (mIU/mL) | 161 | 6.2 ± 1.96 |
| LH levels (mIU/mL) | 161 | 8.9 ± 6.87 |
| Estradiol levels (pg/mL) | 160 | 63.4 ± 61.72 |
| Prolactin levels (ng/mL) | 159 | 18.1 ± 8.2 |
| TSH levels (mIU/mL) | 161 | 2.2 ± 1.1 |
| Mean age of men (years) | 161 | 32 ± 5.2 |
| Number of cycles | 161 | 1.3 ± 0.49 |
| Gravida | 161 | 0.2 ± 0.5 |
| Parity | 161 | 0.1 ± 0.3 |
| Infertility type | ||
| Primary | 150 (93.2) | |
| Secondary | 11 (6.8) | |
| Indication | ||
| Unexplained infertility | 52 (32.3) | |
| Male factor | 31 (19.3) | |
| Ovulatory dysfunction | 56 (34.8) | |
| DOR | 5 (3.1) | |
| Endometriosis | 11 (6.8) | |
| Uterine factor | 1 (0.6) | |
| Other | 5 (3.1) | |
| OS protocol | ||
| rFSH | 123 (76.4) | |
| hMG | 11 (6.8) | |
| Combined protocol | 27 (16.8) | |
| Clinical pregnancy rate | 24 (15) | |
| Live birth rate | 23 (14.3) | |
| Abortus | 17 (10.6) | |
| Multiple gestation | 9 (5.6) | |
| OHSS | 0 | |
| Presence of teratozoospermia | 24 (15) |
| CPR (+) Mean/n (%) | CPR (−) Mean/n (%) | p-Value | |
|---|---|---|---|
| Pre-wash | |||
| Sperm count (per mL) α | 71.24 | 82.63 | * 0.28 |
| TPMSC α | 75.74 | 81.88 | * 0.56 |
| TPMSC β ≥ 10 × 10⁶ | 17 (12.4) | 120 (87.6) | ** 0.18 |
| TPMSC β ≥ 5 × 10⁶ | 22 (14.2) | 133 (85.8) | ** 1.0 |
| Percentage of normal morphology | 77.39 | 81.6 | * 0.68 |
| Teratozoospermia β | |||
| (+) | 5 (21.7) | 19 (13.8) | ** 0.344 |
| (−) | 18 (78.3) | 119 (86.2) | |
| Post-wash | |||
| Sperm count (per mL) α | 76.89 | 81.68 | * 0.65 |
| TPMSC α | 78.58 | 81.4 | * 0.79 |
| TPMSC β ≥ 10 × 10⁶ | 7 (11.7) | 53 (88.3) | *** 0.49 |
| TPMSC β ≥ 5 × 10⁶ | 16 (16) | 84 (84) | *** 0.49 |
| Delta values | |||
| Δ sperm count (per mL) α | 73.02 | 82.33 | * 0.38 |
| Δ TPMSC α | 76.48 | 81.75 | * 0.61 |
| LBR (+) Mean/n (%) | LBR (−) Mean/n (%) | p-Value | |
|---|---|---|---|
| Pre-wash | |||
| Sperm count (per mL) α | 64.74 | 82.92 | * 0.28 |
| TPMSC α | 66.15 | 82.75 | * 0.56 |
| TPMSC β ≥ 10 × 10⁶ | 11 (8.03) | 126 (91.97) | ** 0.02 |
| TPMSC β ≥ 5 × 10⁶ | 16 (10.3) | 139 (89.7) | *** 0.64 |
| Percentage of normal morphology | 17 (10.6) | 144 (89.4) | * 0.69 |
| Teratozoospermia β | |||
| (+) | 5 (29.4) | 19 (13.2) | ** 0.14 |
| (−) | 12 (70.6) | 125 (86.8) | |
| Post-wash | |||
| Sperm count (per mL) α | 74.21 | 81.8 | * 0.65 |
| TPMSC α | 75.44 | 81.66 | * 0.79 |
| TPMSC β ≥ 10 × 10⁶ | 55 (91.67) | 5 (8.33) | *** 0.6 |
| TPMSC β ≥ 5 × 10⁶ | 11 (11) | 89 (89) | *** 1 |
| Delta values | |||
| Δ sperm count (per mL) α | 65.97 | 82.77 | * 0.38 |
| Δ TPMSC α | 65.82 | 82.79 | * 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yavuzcan, A.; Yurtçu, E.; Keyif, B.; Osmanlıoğlu, Ş. Is There Any Effect of Change in Pre-Wash and Post-Wash Semen Parameters on the Success of Intrauterine Insemination? J. Pers. Med. 2024, 14, 43. https://doi.org/10.3390/jpm14010043
Yavuzcan A, Yurtçu E, Keyif B, Osmanlıoğlu Ş. Is There Any Effect of Change in Pre-Wash and Post-Wash Semen Parameters on the Success of Intrauterine Insemination? Journal of Personalized Medicine. 2024; 14(1):43. https://doi.org/10.3390/jpm14010043
Chicago/Turabian StyleYavuzcan, Ali, Engin Yurtçu, Betül Keyif, and Şeyma Osmanlıoğlu. 2024. "Is There Any Effect of Change in Pre-Wash and Post-Wash Semen Parameters on the Success of Intrauterine Insemination?" Journal of Personalized Medicine 14, no. 1: 43. https://doi.org/10.3390/jpm14010043
APA StyleYavuzcan, A., Yurtçu, E., Keyif, B., & Osmanlıoğlu, Ş. (2024). Is There Any Effect of Change in Pre-Wash and Post-Wash Semen Parameters on the Success of Intrauterine Insemination? Journal of Personalized Medicine, 14(1), 43. https://doi.org/10.3390/jpm14010043






