Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure: Current Evidence and Future Opportunities
Abstract
1. Introduction
2. Catheter Ablation in Patients with Atrial Fibrillation and Heart Failure with Reduced Ejection Fraction
2.1. Effect on Left Ventricular Ejection Fraction in HFrEF
2.2. Effect on Quality of Life in HFrEF
2.3. Effect on Functional Capacity in HFrEF
2.4. Prognostic Impact of Catheter Ablation in HFrEF
2.5. Complication Risks and Atrial Fibrillation Recurrence
2.6. Catheter Ablation for Advanced Heart Failure
3. Atrial Fibrillation and Heart Failure with Preserved Ejection Fraction
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.; Buxton, A.E.; Camm, A.J.; et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008, 358, 2667–2677. [Google Scholar] [CrossRef]
- Nohria, A.; Lewis, E.; Stevenson, L.W. Medical management of advanced heart failure. JAMA 2002, 287, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Hamatani, Y.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; Hasegawa, K.; Abe, M.; Lip, G.Y.H.; Akao, M. Clinical Characteristics and Outcomes in Extreme Elderly (Age ≥ 85 Years) Japanese Patients with Atrial Fibrillation: The Fushimi AF Registry. Chest 2016, 149, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.K.; Mann, D.L. Improving outcomes in heart failure: It’s not unusual beyond usual care. Circulation 2002, 105, 2810–2812. [Google Scholar] [CrossRef] [PubMed]
- Santhanakrishnan, R.; Wang, N.; Larson, M.G.; Magnani, J.W.; McManus, D.D.; Lubitz, S.A.; Ellinor, P.T.; Cheng, S.; Vasan, R.S.; Lee, D.S.; et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation 2016, 133, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kajimoto, K.; Asai, K.; Mizuno, M.; Minami, Y.; Nagashima, M.; Murai, K.; Muanakata, R.; Yumino, D.; Meguro, T.; et al. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: Rationale, design, and preliminary data. Am. Heart J. 2010, 159, 949–955.e941. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, U.; Dahlström, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure with Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, S.; Yokoshiki, H.; Kinugawa, S.; Tsuchihashi-Makaya, M.; Yokota, T.; Takeshita, A.; Tsutsui, H. Effects of atrial fibrillation on long-term outcomes in patients hospitalized for heart failure in Japan: A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ. J. 2009, 73, 2084–2090. [Google Scholar] [CrossRef]
- Son, M.K.; Park, J.J.; Lim, N.K.; Kim, W.H.; Choi, D.J. Impact of atrial fibrillation in patients with heart failure and reduced, mid-range or preserved ejection fraction. Heart 2020, 106, 1160–1168. [Google Scholar] [CrossRef]
- Crijns, H.J.; Van den Berg, M.P.; Van Gelder, I.C.; Van Veldhuisen, D.J. Management of atrial fibrillation in the setting of heart failure. Eur. Heart J. 1997, 18 (Suppl. C), 45–49. [Google Scholar] [CrossRef]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Gorenek, B.; Halvorsen, S.; Kudaiberdieva, G.; Bueno, H.; Van Gelder, I.C.; Lettino, M.; Marin, F.; Masip, J.; Mueller, C.; Okutucu, S.; et al. Atrial fibrillation in acute heart failure: A position statement from the Acute Cardiovascular Care Association and European Heart Rhythm Association of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 348–357. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; Cunningham, J.W.; Pedro Ferreira, J.; Zannad, F.; Packer, M.; Fonarow, G.C.; McMurray, J.J.V.; Solomon, S.D. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef]
- Khan, M.N.; Jaïs, P.; Cummings, J.; Di Biase, L.; Sanders, P.; Martin, D.O.; Kautzner, J.; Hao, S.; Themistoclakis, S.; Fanelli, R.; et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008, 359, 1778–1785. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Connelly, D.T.; Hawkins, N.M.; Steedman, T.; Payne, J.; Shaw, M.; Denvir, M.; Bhagra, S.; Small, S.; Martin, W.; et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: A randomised controlled trial. Heart 2011, 97, 740–747. [Google Scholar] [CrossRef]
- Jones, D.G.; Haldar, S.K.; Hussain, W.; Sharma, R.; Francis, D.P.; Rahman-Haley, S.L.; McDonagh, T.A.; Underwood, S.R.; Markides, V.; Wong, T. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 2013, 61, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J.; Berriman, T.J.; Diab, I.; Kamdar, R.; Richmond, L.; Baker, V.; Goromonzi, F.; Sawhney, V.; Duncan, E.; Page, S.P.; et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ. Arrhythm. Electrophysiol. 2014, 7, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Mohanty, P.; Mohanty, S.; Santangeli, P.; Trivedi, C.; Lakkireddy, D.; Reddy, M.; Jais, P.; Themistoclakis, S.; Dello Russo, A.; et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation 2016, 133, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Kuck, K.H.; Merkely, B.; Zahn, R.; Arentz, T.; Seidl, K.; Schlüter, M.; Tilz, R.R.; Piorkowski, C.; Gellér, L.; Kleemann, T.; et al. Catheter Ablation Versus Best Medical Therapy in Patients with Persistent Atrial Fibrillation and Congestive Heart Failure: The Randomized AMICA Trial. Circ. Arrhythm. Electrophysiol. 2019, 12, e007731. [Google Scholar] [CrossRef]
- Parkash, R.; Wells, G.A.; Rouleau, J.; Talajic, M.; Essebag, V.; Skanes, A.; Wilton, S.B.; Verma, A.; Healey, J.S.; Sterns, L.; et al. Randomized Ablation-Based Rhythm-Control Versus Rate-Control Trial in Patients with Heart Failure and Atrial Fibrillation: Results from the RAFT-AF trial. Circulation 2022, 145, 1693–1704. [Google Scholar] [CrossRef]
- Rector, T.S. Patient’s self-assessment of their congestive heart failure: Content, reliability, validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Fail. 1987, 3, 198–209. [Google Scholar]
- Bilbao, A.; Escobar, A.; García-Perez, L.; Navarro, G.; Quirós, R. The Minnesota living with heart failure questionnaire: Comparison of different factor structures. Health Qual. Life Outcomes 2016, 14, 23. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.H.; Klein, G.; Natale, A.; Packer, D.; et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Sparks, P.B.; Morton, J.B.; Kistler, P.M.; Vohra, J.K.; Medi, C.; Rosso, R.; Teh, A.; Halloran, K.; Kalman, J.M. Low risk of major complications associated with pulmonary vein antral isolation for atrial fibrillation: Results of 500 consecutive ablation procedures in patients with low prevalence of structural heart disease from a single center. J. Cardiovasc. Electrophysiol. 2011, 22, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Brugada, J.; Hindricks, G.; Maggioni, A.; Tavazzi, L.; Vardas, P.; Anselme, F.; Inama, G.; Jais, P.; Kalarus, Z.; et al. ESC-EURObservational Research Programme: The Atrial Fibrillation Ablation Pilot Study, conducted by the European Heart Rhythm Association. Europace 2012, 14, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Murakawa, Y.; Nogami, A.; Hirao, K.; Shoda, M.; Aonuma, K.; Ikeguchi, S.; Iwa, T.; Ushinohama, H.; Uno, K.; Okishige, K.; et al. A brief report on the nationwide survey of catheter ablation in Japan/the Japanese Catheter Ablation Registry (JCAR). J. Arrhythm. 2012, 28, 122–126. [Google Scholar] [CrossRef]
- Deshmukh, A.; Patel, N.J.; Pant, S.; Shah, N.; Chothani, A.; Mehta, K.; Grover, P.; Singh, V.; Vallurupalli, S.; Savani, G.T.; et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93,801 procedures. Circulation 2013, 128, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Brugada, J.; Hindricks, G.; Maggioni, A.P.; Tavazzi, L.; Vardas, P.; Laroche, C.; Anselme, F.; Inama, G.; Jais, P.; et al. The atrial fibrillation ablation pilot study: A European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur. Heart J. 2014, 35, 1466–1478. [Google Scholar] [CrossRef]
- Arbelo, E.; Brugada, J.; Blomström-Lundqvist, C.; Laroche, C.; Kautzner, J.; Pokushalov, E.; Raatikainen, P.; Efremidis, M.; Hindricks, G.; Barrera, A.; et al. Contemporary management of patients undergoing atrial fibrillation ablation: In-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur. Heart J. 2017, 38, 1303–1316. [Google Scholar] [CrossRef]
- De Greef, Y.; Ströker, E.; Schwagten, B.; Kupics, K.; De Cocker, J.; Chierchia, G.B.; de Asmundis, C.; Stockman, D.; Buysschaert, I. Complications of pulmonary vein isolation in atrial fibrillation: Predictors and comparison between four different ablation techniques: Results from the MIddelheim PVI-registry. Europace 2018, 20, 1279–1286. [Google Scholar] [CrossRef]
- Steinbeck, G.; Sinner, M.F.; Lutz, M.; Müller-Nurasyid, M.; Kääb, S.; Reinecke, H. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: A nationwide in-hospital analysis of administrative data for Germany in 2014. Eur. Heart J. 2018, 39, 4020–4029. [Google Scholar] [CrossRef]
- Tripathi, B.; Arora, S.; Kumar, V.; Abdelrahman, M.; Lahewala, S.; Dave, M.; Shah, M.; Tan, B.; Savani, S.; Badheka, A.; et al. Temporal trends of in-hospital complications associated with catheter ablation of atrial fibrillation in the United States: An update from Nationwide Inpatient Sample database (2011–2014). J. Cardiovasc. Electrophysiol. 2018, 29, 715–724. [Google Scholar] [CrossRef]
- Fink, T.; Metzner, A.; Willems, S.; Eckardt, L.; Ince, H.; Brachmann, J.; Spitzer, S.G.; Deneke, T.; Schmitt, C.; Hochadel, M.; et al. Procedural success, safety and patients satisfaction after second ablation of atrial fibrillation in the elderly: Results from the German Ablation Registry. Clin. Res. Cardiol. 2019, 108, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, N.; Széplaki, G.; Herczeg, S.; Tahin, T.; Salló, Z.; Nagy, V.K.; Osztheimer, I.; Özcan, E.E.; Merkely, B.; Gellér, L. Repeat procedure is a new independent predictor of complications of atrial fibrillation ablation. Europace 2019, 21, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Bänsch, D.; Ernst, S.; Schaumann, A.; Hachiya, H.; Chen, M.; Chun, J.; Falk, P.; Khanedani, A.; Antz, M.; et al. Complete isolation of left atrium surrounding the pulmonary veins: New insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation 2004, 110, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Date, T.; Kanzaki, Y.; Inada, K.; Matsuo, S.; Shibayama, K.; Miyanaga, S.; Miyazaki, H.; Sugimoto, K.; Mochizuki, S. Segmental pulmonary vein antrum isolation using the “large-size” lasso catheter in patients with atrial fibrillation. Circ. J. 2007, 71, 753–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumagai, K. Catheter ablation of atrial fibrillation—State of the Art. Circ. J. 2011, 75, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Medi, C.; Sparks, P.B.; Morton, J.B.; Kistler, P.M.; Halloran, K.; Rosso, R.; Vohra, J.K.; Kumar, S.; Kalman, J.M. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: Results from long-term follow-up. J. Cardiovasc. Electrophysiol. 2011, 22, 137–141. [Google Scholar] [CrossRef]
- Weerasooriya, R.; Khairy, P.; Litalien, J.; Macle, L.; Hocini, M.; Sacher, F.; Lellouche, N.; Knecht, S.; Wright, M.; Nault, I.; et al. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J. Am. Coll. Cardiol. 2011, 57, 160–166. [Google Scholar] [CrossRef]
- Sawhney, N.; Anousheh, R.; Chen, W.C.; Narayan, S.; Feld, G.K. Five-year outcomes after segmental pulmonary vein isolation for paroxysmal atrial fibrillation. Am. J. Cardiol. 2009, 104, 366–372. [Google Scholar] [CrossRef]
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; Neven, K.; Köktürk, B.; Konstantinidou, M.; Metzner, A.; et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: Lessons from a 5-year follow-up. Circulation 2010, 122, 2368–2377. [Google Scholar] [CrossRef]
- Nogami, A.; Kurita, T.; Abe, H.; Ando, K.; Ishikawa, T.; Imai, K.; Usui, A.; Okishige, K.; Kusano, K.; Kumagai, K.; et al. JCS/JHRS 2019 Guideline on Non-Pharmacotherapy of Cardiac Arrhythmias. Circ. J. 2021, 85, 1104–1244. [Google Scholar] [CrossRef]
- Teh, A.W.; Kistler, P.M.; Lee, G.; Medi, C.; Heck, P.M.; Spence, S.J.; Sparks, P.B.; Morton, J.B.; Kalman, J.M. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J. Cardiovasc. Electrophysiol. 2012, 23, 232–238. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Corleto, A.; Biondi-Zoccai, G.; Anselmino, M.; Ferraris, F.; di Biase, L.; Natale, A.; Hunter, R.J.; Schilling, R.J.; Miyazaki, S.; et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: A meta-analysis. Int. J. Cardiol. 2013, 167, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Berruezo, A.; Tamborero, D.; Mont, L.; Benito, B.; Tolosana, J.M.; Sitges, M.; Vidal, B.; Arriagada, G.; Méndez, F.; Matiello, M.; et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 2007, 28, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Nedios, S.; Kosiuk, J.; Koutalas, E.; Kornej, J.; Sommer, P.; Arya, A.; Richter, S.; Rolf, S.; Husser, D.; Hindricks, G.; et al. Comparison of left atrial dimensions in CT and echocardiography as predictors of long-term success after catheter ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2015, 43, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.; Ferreira, A.M.; Oliveira, S.; Santos, P.G.; Durazzo, A.; Carmo, P.; Santos, K.R.; Cavaco, D.; Parreira, L.; Morgado, F.; et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. Int. J. Cardiol. 2015, 184, 56–61. [Google Scholar] [CrossRef]
- Patel, D.; Mohanty, P.; Di Biase, L.; Shaheen, M.; Lewis, W.R.; Quan, K.; Cummings, J.E.; Wang, P.; Al-Ahmad, A.; Venkatraman, P.; et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: The impact of continuous positive airway pressure. Circ. Arrhythm. Electrophysiol. 2010, 3, 445–451. [Google Scholar] [CrossRef]
- Bisbal, F.; Guiu, E.; Calvo, N.; Marin, D.; Berruezo, A.; Arbelo, E.; Ortiz-Pérez, J.; de Caralt, T.M.; Tolosana, J.M.; Borràs, R.; et al. Left atrial sphericity: A new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2013, 24, 752–759. [Google Scholar] [CrossRef]
- Bieging, E.T.; Morris, A.; Wilson, B.D.; McGann, C.J.; Marrouche, N.F.; Cates, J. Left atrial shape predicts recurrence after atrial fibrillation catheter ablation. J. Cardiovasc. Electrophysiol. 2018, 29, 966–972. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ. Heart Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Winkle, R.A.; Jarman, J.W.; Mead, R.H.; Engel, G.; Kong, M.H.; Fleming, W.; Patrawala, R.A. Predicting atrial fibrillation ablation outcome: The CAAP-AF score. Heart Rhythm. 2016, 13, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Hindricks, G.; Arya, A.; Sommer, P.; Husser, D.; Bollmann, A. The APPLE Score—A Novel Score for the Prediction of Rhythm Outcomes after Repeat Catheter Ablation of Atrial Fibrillation. PLoS ONE 2017, 12, e0169933. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Hindricks, G.; Shoemaker, M.B.; Husser, D.; Arya, A.; Sommer, P.; Rolf, S.; Saavedra, P.; Kanagasundram, A.; Patrick Whalen, S.; et al. The APPLE score: A novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin. Res. Cardiol. 2015, 104, 871–876. [Google Scholar] [CrossRef]
- Canpolat, U.; Aytemir, K.; Yorgun, H.; Şahiner, L.; Kaya, E.B.; Oto, A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: Promising results from the Turkish Cryoablation Registry. Int. J. Cardiol. 2013, 169, 201–206. [Google Scholar] [CrossRef]
- Wójcik, M.; Berkowitsch, A.; Greiss, H.; Zaltsberg, S.; Pajitnev, D.; Deubner, N.; Hamm, C.W.; Pitschner, H.F.; Kuniss, M.; Neumann, T. Repeated catheter ablation of atrial fibrillation: How to predict outcome? Circ. J. 2013, 77, 2271–2279. [Google Scholar] [CrossRef]
- Kosiuk, J.; Dinov, B.; Kornej, J.; Acou, W.J.; Schönbauer, R.; Fiedler, L.; Buchta, P.; Myrda, K.; Gąsior, M.; Poloński, L.; et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm. 2015, 12, 2207–2212. [Google Scholar] [CrossRef]
- Mesquita, J.; Ferreira, A.M.; Cavaco, D.; Moscoso Costa, F.; Carmo, P.; Marques, H.; Morgado, F.; Mendes, M.; Adragão, P. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure—ATLAS score. Europace 2018, 20, f428–f435. [Google Scholar] [CrossRef]
- Kornej, J.; Schumacher, K.; Dinov, B.; Kosich, F.; Sommer, P.; Arya, A.; Husser, D.; Bollmann, A.; Lip, G.Y.H.; Hindricks, G. Prediction of electro-anatomical substrate and arrhythmia recurrences using APPLE, DR-FLASH and MB-LATER scores in patients with atrial fibrillation undergoing catheter ablation. Sci. Rep. 2018, 8, 12686. [Google Scholar] [CrossRef]
- Mujović, N.; Marinković, M.; Marković, N.; Shantsila, A.; Lip, G.Y.; Potpara, T.S. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: The MB-LATER clinical score. Sci. Rep. 2017, 7, 40828. [Google Scholar] [CrossRef]
- Sohns, C.; Fox, H.; Marrouche, N.F.; Crijns, H.; Costard-Jaeckle, A.; Bergau, L.; Hindricks, G.; Dagres, N.; Sossalla, S.; Schramm, R.; et al. Catheter Ablation in End-Stage Heart Failure with Atrial Fibrillation. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef]
- Kotecha, D.; Lam, C.S.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yoshikawa, T.; Saito, Y.; Takeishi, Y.; Yamamoto, K.; Ogawa, H.; Anzai, T. Clinical Characteristics, Management, and Outcomes of Japanese Patients Hospitalized for Heart Failure with Preserved Ejection Fraction—A Report From the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) Registry. Circ. J. 2018, 82, 1534–1545. [Google Scholar] [CrossRef]
- Kosmala, W. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: How to Fight Allied Enemies. J. Am. Coll. Cardiol. 2020, 76, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Temma, T.; Nagai, T.; Watanabe, M.; Kamada, R.; Takahashi, Y.; Hagiwara, H.; Koya, T.; Nakao, M.; Omote, K.; Kamiya, K.; et al. Differential Prognostic Impact of Atrial Fibrillation in Hospitalized Heart Failure Patients with Preserved Ejection Fraction According to Coronary Artery Disease Status—Report From the Japanese Nationwide Multicenter Registry. Circ. J. 2020, 84, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.G.; Swedberg, K.; Ducharme, A.; Granger, C.B.; Michelson, E.L.; McMurray, J.J.; Puu, M.; Yusuf, S.; Pfeffer, M.A. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J. Am. Coll. Cardiol. 2006, 47, 1997–2004. [Google Scholar] [CrossRef]
- Linssen, G.C.; Rienstra, M.; Jaarsma, T.; Voors, A.A.; van Gelder, I.C.; Hillege, H.L.; van Veldhuisen, D.J. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur. J. Heart Fail. 2011, 13, 1111–1120. [Google Scholar] [CrossRef]
- Cikes, M.; Claggett, B.; Shah, A.M.; Desai, A.S.; Lewis, E.F.; Shah, S.J.; Anand, I.S.; O’Meara, E.; Rouleau, J.L.; Sweitzer, N.K.; et al. Atrial Fibrillation in Heart Failure with Preserved Ejection Fraction: The TOPCAT Trial. JACC Heart Fail. 2018, 6, 689–697. [Google Scholar] [CrossRef]
- Cikes, M.; Planinc, I.; Claggett, B.; Cunningham, J.; Milicic, D.; Sweitzer, N.; Senni, M.; Gori, M.; Linssen, G.; Shah, S.J.; et al. Atrial Fibrillation in Heart Failure with Preserved Ejection Fraction: The PARAGON-HF Trial. JACC Heart Fail. 2022, 10, 336–346. [Google Scholar] [CrossRef]
- Butt, J.H.; Kondo, T.; Jhund, P.S.; Comin-Colet, J.; de Boer, R.A.; Desai, A.S.; Hernandez, A.F.; Inzucchi, S.E.; Janssens, S.P.; Kosiborod, M.N.; et al. Atrial Fibrillation and Dapagliflozin Efficacy in Patients with Preserved or Mildly Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2022, 80, 1705–1717. [Google Scholar] [CrossRef]
- Fukui, A.; Tanino, T.; Yamaguchi, T.; Hirota, K.; Saito, S.; Okada, N.; Akioka, H.; Shinohara, T.; Yufu, K.; Takahashi, N. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J. Cardiovasc. Electrophysiol. 2020, 31, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Rattka, M.; Kühberger, A.; Pott, A.; Stephan, T.; Weinmann, K.; Baumhardt, M.; Aktolga, D.; Teumer, Y.; Bothner, C.; Scharnbeck, D.; et al. Catheter ablation for atrial fibrillation in HFpEF patients-A propensity-score-matched analysis. J. Cardiovasc. Electrophysiol. 2021, 32, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jaswaney, R.; Jani, C.; Zuzek, Z.; Thakkar, S.; Patel, H.P.; Patel, M.; Patel, N.; Tripathi, B.; Lahewala, S.; et al. Catheter Ablation for Atrial Fibrillation in Patients with Concurrent Heart Failure. Am. J. Cardiol. 2020, 137, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure: Results From the CABANA Trial. Circulation 2021, 143, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
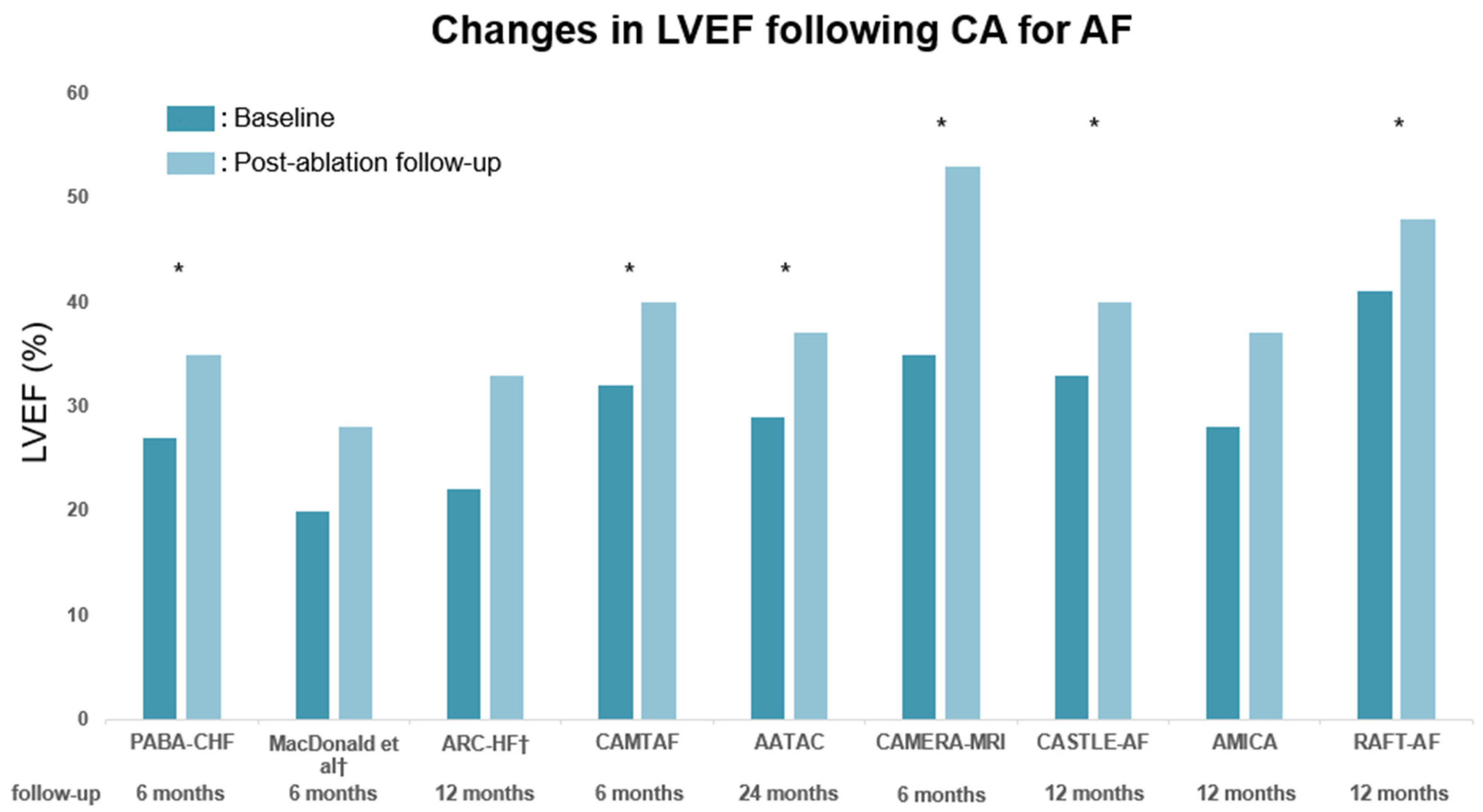
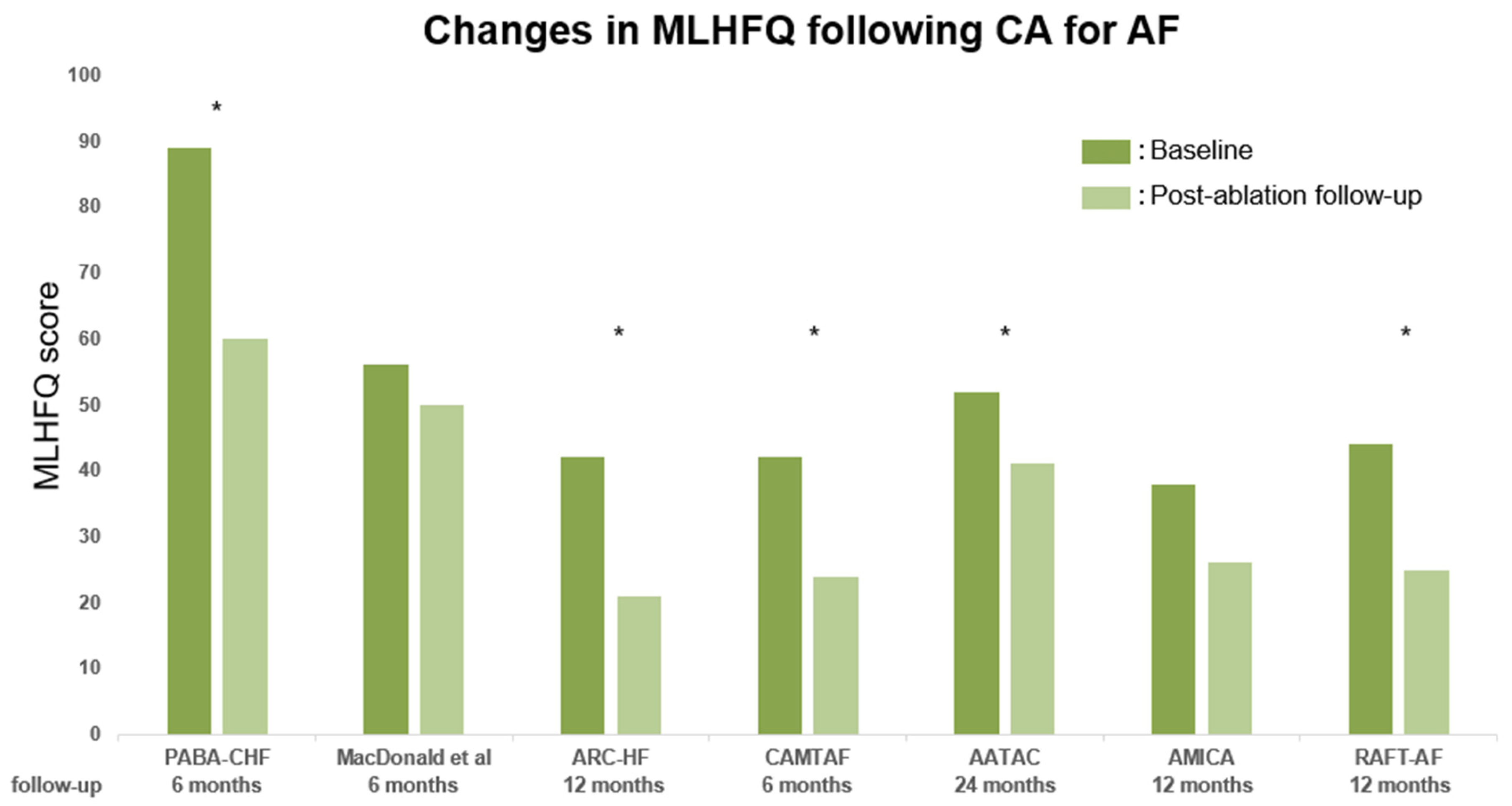


| Trials (RCTs) | Sample Size | Patient Population | Comparison Group | Primary Outcome | Follow-Up (Years) | LVEF (Mean) | Findings |
|---|---|---|---|---|---|---|---|
| (publication year) Ref | (number of PAF) | ||||||
| PABA-CHF (2008) [20] | 81 (42) | Symptomatic AF, NYHA II–III, | AV node ablation + BiV pacing | Composite of LVEF (TTE), | 0.5 | 28 | Beneficial effect of CA on |
| and LVEF ≤ 40% (TTE) | 6MWD, and MLWHQ score | LVEF, 6MWD, QOL | |||||
| MacDonald et al. (2011) [21] | 41 (0) | Persistent AF, NYHA II–IV, | Pharmacological rate control | Change in LVEF (CMR) | 0.5 | 18 | No beneficial effect of CA on |
| and LVEF < 35% (RNVG) | (RNVG) | LVEF (CMR), 6MWD, QOL | |||||
| AF recurrence 50% in CA arm | |||||||
| ARC-HF (2013) [22] | 52 (0) | Persistent AF, NYHA II–IV, | Pharmacological rate control | Change in peak VO2 | 1 | 24 | Beneficial effect of CA on |
| and LVEF ≤ 35% (RNVG) | peak VO2, QOL | ||||||
| CAMTAF (2014) [23] | 50 (0) | Persistent AF, NYHA II–IV, | Pharmacological rate control | Change in LVEF (TTE) | 0.5 | 32 | Beneficial effect of CA on |
| and LVEF < 50% (TTE) | LVEF, peak VO2, QOL | ||||||
| AATAC (2016) [24] | 203 (0) | Persistent AF, NYHA II–IV, | Amiodarone | Recurrence of AF | 2 | 30 | Beneficial effect of CA on |
| LVEF < 40% (TTE), and ICD/CRTD | LVEF, 6MWD, QOL, and | ||||||
| prognosis (death + HF) | |||||||
| CAMERA-MRI (2017) [25] | 68 (0) | Persistent AF, idiopathic cardiomyopathy | Pharmacological rate control | Change in LVEF (CMR) | 0.5 | 33 | Beneficial effect of CA on |
| NYHA II–IV, and LVEF ≤ 45% (CMR) | LVEF (especially in patients | ||||||
| with non-LGE in CMR) | |||||||
| CASTLE-AF (2018) [26] | 363 (118) | Symptomatic AF, NYHA II–IV, | Pharmacological treatment | Composite of death and | 5 | 32 | Beneficial effect of CA on |
| LVEF < 35% (TTE), and ICD/CRTD | (rate/rhythm control) | HF hospitalization | (median 3.2) | prognosis (death + HF), | |||
| and LVEF | |||||||
| AMICA (2019) [27] | 140 (0) | Persistent AF, NYHA II–III, | Pharmacological treatment | Change in LVEF (TTE) | 1 | 26 | No beneficial effect of CA on |
| LVEF < 35% (TTE), and ICD/CRTD | (rate/rhythm control) | LVEF, 6MWD, QOL | |||||
| RAFT-AF (2022) [28] | 411 (30) | Symptomatic AF, NYHA II–III, | Pharmacological rate control | Composite of death and | 2 | 41 | Nonsignificant trend for improved |
| HF, elevated NT-proBNP | /AV node ablation + BiV pacing | HF events | prognosis (death + HF) with CA | ||||
| Beneficial effect on LVEF, | |||||||
| 6MWD, QOL |
| Studies | Study Design | Sample Size | Primary Outcome | Follow-Up (Years) | Findings |
|---|---|---|---|---|---|
| (publication year) Ref | |||||
| Fukui et al. | Single-center retrospective | 85 | HF readmission | 2.2 | Significant association between CA |
| (2020) [81] | cohort study | and a lower risk of HF readmission | |||
| Arora et al. | Retrospective cohort study | 56,395 | Death + HF readmission | 1 | No association between CA and |
| (2020) [83] | using a national database | better prognosis | |||
| Rattka et al. | Single-center retrospective | 127 | Death + HF readmission | 1.5 | Significant association between CA |
| (2021) [82] | cohort study | and a lower risk of the primary outcome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, S.; Kitai, T.; Skoularigis, J.; Spiliopoulos, K.; Xanthopoulos, A. Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure: Current Evidence and Future Opportunities. J. Pers. Med. 2023, 13, 1394. https://doi.org/10.3390/jpm13091394
Suzuki S, Kitai T, Skoularigis J, Spiliopoulos K, Xanthopoulos A. Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure: Current Evidence and Future Opportunities. Journal of Personalized Medicine. 2023; 13(9):1394. https://doi.org/10.3390/jpm13091394
Chicago/Turabian StyleSuzuki, Sho, Takeshi Kitai, John Skoularigis, Kyriakos Spiliopoulos, and Andrew Xanthopoulos. 2023. "Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure: Current Evidence and Future Opportunities" Journal of Personalized Medicine 13, no. 9: 1394. https://doi.org/10.3390/jpm13091394
APA StyleSuzuki, S., Kitai, T., Skoularigis, J., Spiliopoulos, K., & Xanthopoulos, A. (2023). Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure: Current Evidence and Future Opportunities. Journal of Personalized Medicine, 13(9), 1394. https://doi.org/10.3390/jpm13091394






