Comparison between Sugammadex and Neostigmine after Video-Assisted Thoracoscopic Surgery–Thymectomy in Patients with Myasthenia Gravis: A Single-Center Retrospective Exploratory Analysis
Abstract
1. Introduction
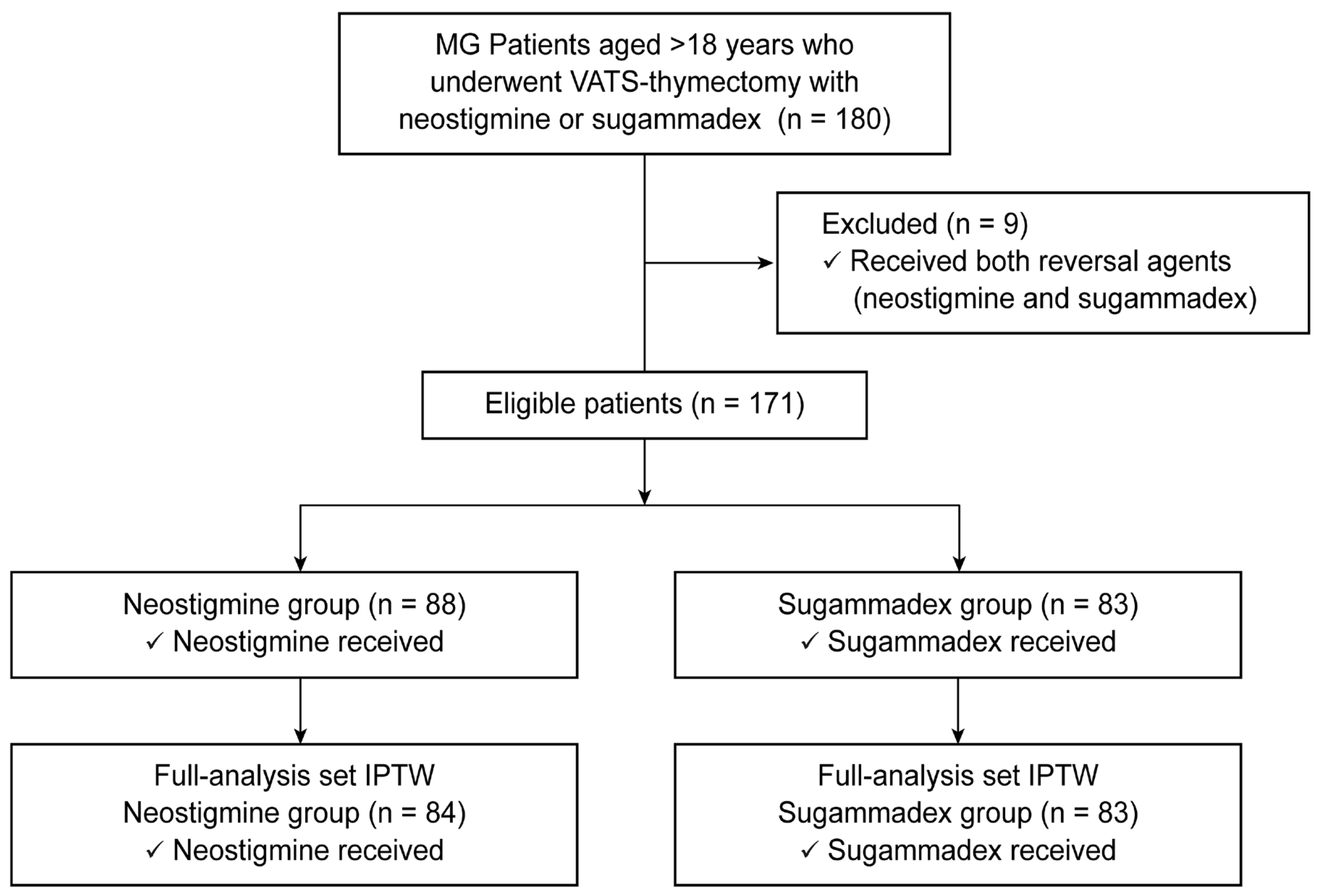
2. Materials and Methods
2.1. Study Population
2.2. Intraoperative Management
2.3. Neuromuscular Blockade
2.4. Variables and Outcomes
2.5. Statistical Analyses
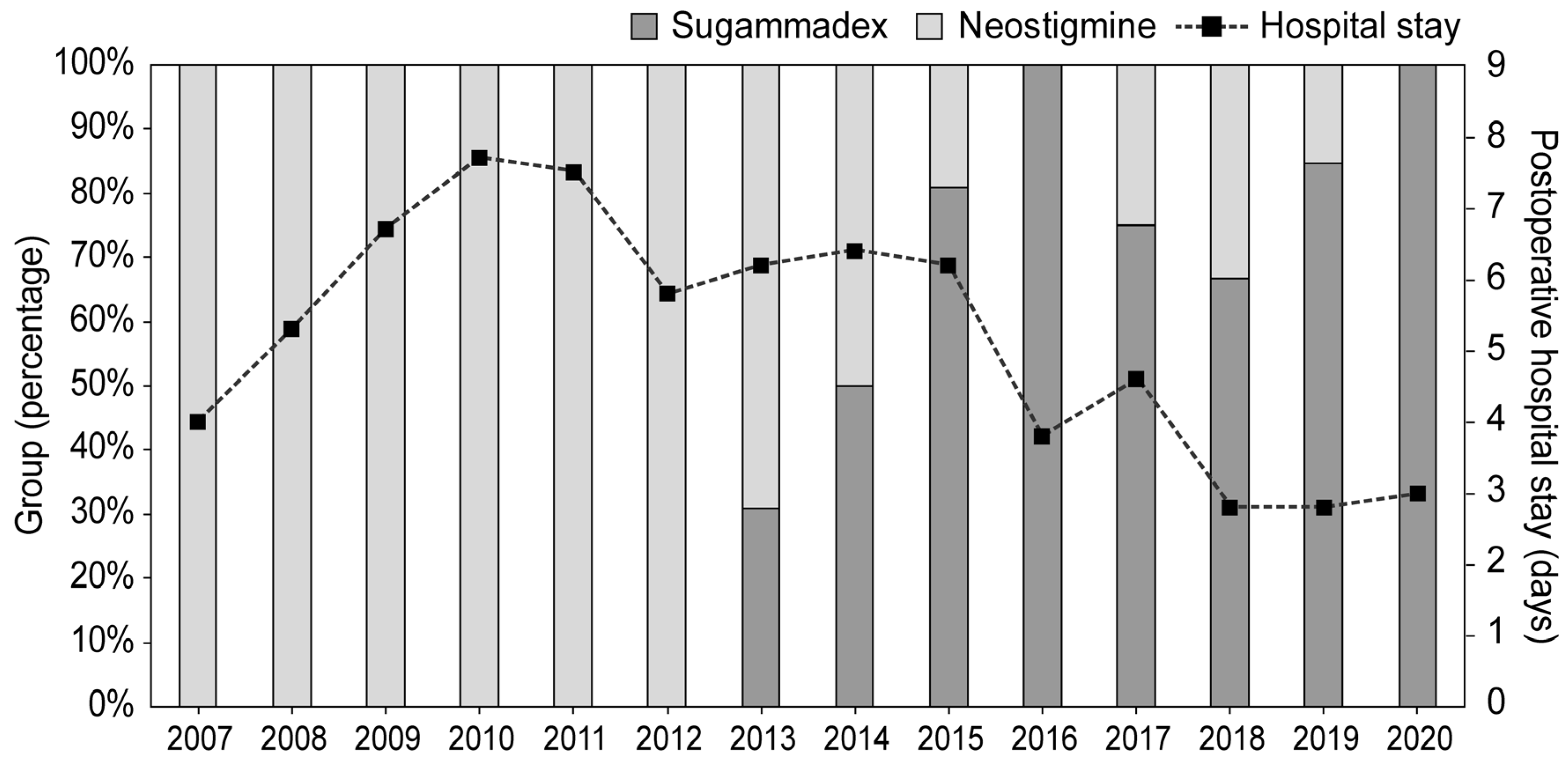
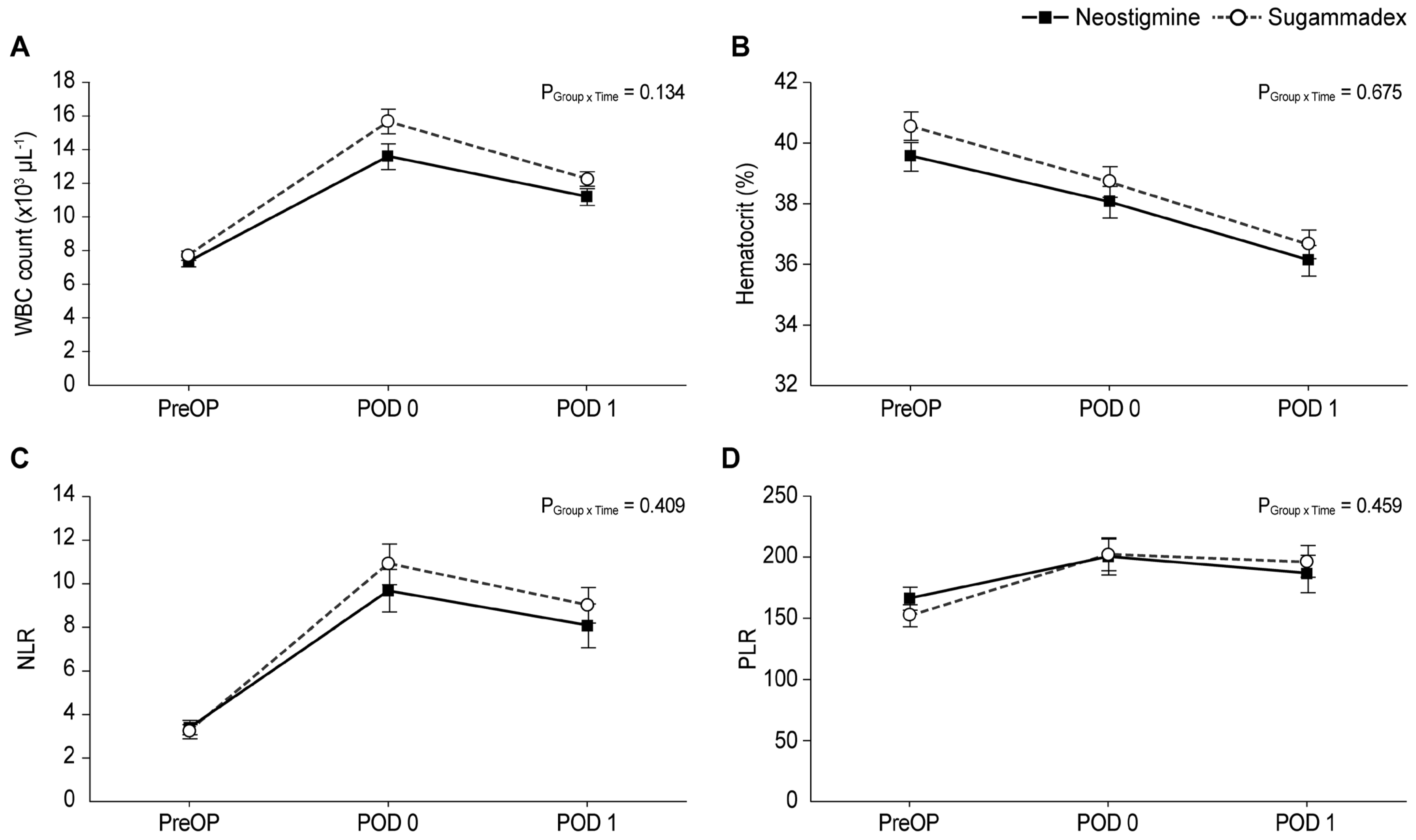
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sungur Ulke, Z.; Yavru, A.; Camci, E.; Ozkan, B.; Toker, A.; Senturk, M. Rocuronium and sugammadex in patients with myasthenia gravis undergoing thymectomy. Acta Anaesthesiol. Scand. 2013, 57, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.; Slinger, P.; Roscoe, A.; McRae, K.; Van Rensburg, A. A randomised controlled trial comparing the glidescope® and the macintosh laryngoscope for double-lumen endobronchial intubation. Anaesthesia 2013, 68, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- van den Bersselaar, L.R.; Gubbels, M.; Riazi, S.; Heytens, L.; Jungbluth, H.; Voermans, N.C.; Snoeck, M.M.J. Mapping the current evidence on the anesthetic management of adult patients with neuromuscular disorders-a scoping review. Can. J. Anaesth. 2022, 69, 756–773. [Google Scholar] [CrossRef]
- Dillon, F.X. Anesthesia issues in the perioperative management of myasthenia gravis. Semin. Neurol. 2004, 24, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Bom, A.; Bradley, M.; Cameron, K.; Clark, J.K.; Van Egmond, J.; Feilden, H.; MacLean, E.J.; Muir, A.W.; Palin, R.; Rees, D.C.; et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew. Chem. Int. Ed. Engl. 2002, 41, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Epemolu, O.; Bom, A.; Hope, F.; Mason, R. Reversal of neuromuscular blockade and simultaneous increase in plasma rocuronium concentration after the intravenous infusion of the novel reversal agent org 25,969. Anesthesiology 2003, 99, 632–637, discussion 636A. [Google Scholar] [CrossRef]
- Hristovska, A.M.; Duch, P.; Allingstrup, M.; Afshari, A. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia 2018, 73, 631–641. [Google Scholar] [CrossRef]
- Petrun, A.M.; Mekis, D.; Kamenik, M. Successful use of rocuronium and sugammadex in a patient with myasthenia. Eur. J. Anaesthesiol. 2010, 27, 917–918. [Google Scholar] [CrossRef]
- Unterbuchner, C.; Fink, H.; Blobner, M. The use of sugammadex in a patient with myasthenia gravis. Anaesthesia 2010, 65, 302–305. [Google Scholar] [CrossRef]
- de Boer, H.D.; van Egmond, J.; Driessen, J.J.; Booij, L.H. Sugammadex in patients with myasthenia gravis. Anaesthesia 2010, 65, 653. [Google Scholar] [CrossRef]
- Komasawa, N.; Noma, H.; Sugi, T.; Sukenaga, N.; Kakiuchi, H. Effective reversal of muscle relaxation by rocuronium using sugammadex in a patient with myasthenia gravis undergoing laparoscopic cholecystectomy. Masui Jpn. J. Anesthesiol. 2011, 60, 476–479. [Google Scholar]
- Rudzka-Nowak, A.; Piechota, M. Anaesthetic management of a patient with myasthenia gravis for abdominal surgery using sugammadex. Arch. Med. Sci. 2011, 7, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Diemunsch, P.; Boet, S. Use of rocuronium and sugammadex for caesarean delivery in a patient with myasthenia gravis. Int. J. Obstet. Anesth. 2012, 21, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Casarotti, P.; Mendola, C.; Cammarota, G.; Della Corte, F. High-dose rocuronium for rapid-sequence induction and reversal with sugammadex in two myasthenic patients. Acta Anaesthesiol. Scand. 2014, 58, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.D.; Shields, M.O.; Booij, L.H. Reversal of neuromuscular blockade with sugammadex in patients with myasthenia gravis: A case series of 21 patients and review of the literature. Eur. J. Anaesthesiol. 2014, 31, 715–721. [Google Scholar] [CrossRef]
- Dabbous, A.S.; Nehme, P.W.; Abou Leila, A.M. Anesthetic management of aortic valve replacement in a myasthenia gravis patient, the era of a new reversal. Middle East J. Anaesthesiol. 2016, 23, 491–494. [Google Scholar]
- Shah, D.; Dharmarajah, A. Use of sugammadex in an octagenerian with myaesthenia gravis undergoing emergency laporotomy. J. Clin. Anesth. 2017, 37, 109–110. [Google Scholar] [CrossRef]
- Vymazal, T.; Krecmerova, M.; Bicek, V.; Lischke, R. Feasibility of full and rapid neuromuscular blockade recovery with sugammadex in myasthenia gravis patients undergoing surgery—A series of 117 cases. Ther. Clin. Risk Manag. 2015, 11, 1593–1596. [Google Scholar] [CrossRef]
- Mouri, H.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Effect of sugammadex on postoperative myasthenic crisis in myasthenia gravis patients: Propensity score analysis of a japanese nationwide database. Anesth. Analg. 2020, 130, 367–373. [Google Scholar] [CrossRef]
- Tsukada, S.; Shimizu, S.; Fushimi, K. Rocuronium reversed with sugammadex for thymectomy in myasthenia gravis: A retrospective analysis of complications from japan. Eur. J. Anaesthesiol. 2021, 38, 850–855. [Google Scholar] [CrossRef]
- Kim, N.; Lee, S.H.; Choi, K.W.; Lee, H.; Oh, Y.J. Effects of positive end-expiratory pressure on pulmonary oxygenation and biventricular function during one-lung ventilation: A randomized crossover study. J. Clin. Med. 2019, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Oh, Y.J.; Kim, M.; Song, S.H.; Kim, N. Effects of iloprost on oxygenation during one-lung ventilation in patients with low diffusing capacity for carbon monoxide: A randomized controlled study. J. Clin. Med. 2022, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Buder, T.; Claudius, C.; Skovgaard, L.T.; Eriksson, L.I.; Mirakhur, R.K.; Viby-Mogensen, J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents ii: The stockholm revision. Acta Anaesthesiol. Scand. 2007, 51, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, G.; Meacci, E.; Cusumano, G.; Cesario, A.; Chiappetta, M.; Dall’armi, V.; Evoli, A.; Costa, R.; Lococo, F.; Primieri, P.; et al. Thymectomy in myasthenia gravis: Proposal for a predictive score of postoperative myasthenic crisis. Eur. J. Cardiothorac. Surg. 2014, 45, e76–e88, discussion e88. [Google Scholar] [CrossRef]
- Xue, L.; Wang, L.; Dong, J.; Yuan, Y.; Fan, H.; Zhang, Y.; Wang, Q.; Ding, J. Risk factors of myasthenic crisis after thymectomy for thymoma patients with myasthenia gravis. Eur. J. Cardiothorac. Surg. 2017, 52, 692–697. [Google Scholar] [CrossRef]
- Xu, S.; Ross, C.; Raebel, M.A.; Shetterly, S.; Blanchette, C.; Smith, D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010, 13, 273–277. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (iptw) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Lumley, T.; Scott, A.J. Two-sample rank tests under complex sampling. Biometrika 2013, 100, 831–842. [Google Scholar] [CrossRef]
- Lee, S.; Zhou, J.; Leung, K.S.K.; Wai, A.K.C.; Jeevaratnam, K.; King, E.; Liu, T.; Wong, W.T.; Chang, C.; Wong, I.C.K.; et al. Comparison of sodium-glucose cotransporter-2 inhibitor and dipeptidyl peptidase-4 inhibitor on the risks of new-onset atrial fibrillation, stroke and mortality in diabetic patients: A propensity score-matched study in hong kong. Cardiovasc. Drugs Ther. 2023, 37, 561–569. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.M.; Kwak, B.J.; Park, Y.; Jun, E.; Song, K.B.; Hwang, D.W.; Kim, S.C.; Lee, J.H. Clinical outcomes between a minimally invasive and open extended cholecystectomy for t2 gallbladder cancer: A propensity score matching analysis. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 538–544. [Google Scholar] [CrossRef]
- Blichfeldt-Lauridsen, L.; Hansen, B.D. Anesthesia and myasthenia gravis. Acta Anaesthesiol. Scand. 2012, 56, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.A.; Murphy, G.S. Anesthetic consideration for neuromuscular diseases. Curr. Opin. Anaesthesiol. 2017, 30, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, J.; Gaszyński, T.; Gaszyński, W. Neuromuscular block reversal with sugammadex in a morbidly obese patient with myasthenia gravis. Anaesthesiol. Intensive Ther. 2012, 44, 28–30. [Google Scholar] [PubMed]
- Kiss, G.; Lacour, A.; d’Hollander, A. Fade of train-of-four ratio despite administration of more than 12 mg kg−1 sugammadex in a myasthenia gravis patient receiving rocuronium. Br. J. Anaesth. 2013, 110, 854–855. [Google Scholar] [CrossRef]
- Sugi, Y.; Nitahara, K.; Shiroshita, T.; Higa, K. Restoration of train-of-four ratio with neostigmine after insufficient recovery with sugammadex in a patient with myasthenia gravis. A&A Case Rep. 2013, 1, 43–45. [Google Scholar]
- Kim, R.K.; Kim, S.Y. Rapid return of spontaneous respiration after general anesthesia with sugammadex in a patient with myasthenia gravis. J. Lifestyle Med. 2016, 6, 43–46. [Google Scholar] [CrossRef][Green Version]
- Fernandes, H.D.S.; Ximenes, J.L.S.; Nunes, D.I.; Ashmawi, H.A.; Vieira, J.E. Failure of reversion of neuromuscular block with sugammadex in patient with myasthenia gravis: Case report and brief review of literature. BMC Anesthesiol. 2019, 19, 160. [Google Scholar] [CrossRef]
- Gross, D.J.; Zangbar, B.; Muthu, N.; Chang, E.H.; Badami, A.; Stein, L.; Gruessner, R.; Poston, R. Saving the split: The benefits of vats thymectomy. J. Thorac. Dis. 2019, 11, 1428–1432. [Google Scholar] [CrossRef]
- Gilhus, N.E. Myasthenia gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef]
| Variables | Original Dataset before IPTW | Pseudo Dataset after IPTW | ||||||
|---|---|---|---|---|---|---|---|---|
| Neostigmine (n = 88) | Sugammadex (n = 83) | p-Value | SMD | Neostigmine (n = 84) | Sugammadex (n = 83) | p-Value | SMD | |
| Age, year | 44 (30, 53) | 44 (30, 54) | 0.733 | 0.037 | 40 (28, 52) | 46 (31, 54) | 0.427 | 0.143 |
| Sex, female | 59 (67) | 57 (69) | 0.820 | 0.035 | 53 (64) | 54 (65) | 0.904 | 0.021 |
| Body mass index, kg/m2 | 22.4 (20.6, 25.6) | 22.5 (21.0, 24.7) | 0.908 | 0.092 | 22.4 (20.4, 25.5) | 23.0 (21.0, 24.7) | 0.773 | 0.049 |
| ASA physical status | <0.001 * | 0.714 | 0.676 | 0.072 | ||||
| II | 69 (78) | 38 (46) | 55 (66) | 52 (62) | ||||
| III | 19 (22) | 45 (54) | 29 (34) | 31 (38) | ||||
| Comorbidities | ||||||||
| Hypertension | 14 (16) | 13 (16) | 0.965 | 0.007 | 13 (15) | 13 (16) | 0.952 | 0.010 |
| Diabetes | 6 (7) | 3 (4) | 0.498 | 0.144 | 5 (6) | 3 (3) | 0.425 | 0.122 |
| Smoking history | 19 (22) | 17 (20) | 0.859 | 0.027 | 17 (21) | 21 (25) | 0.553 | 0.101 |
| Smoking history, PYRs | 0 (0, 0) | 0 (0, 0) | 0.750 | 0.166 | 0 (0, 0) | 0 (0, 0) | 0.599 | 0.001 |
| Preoperative MG history | ||||||||
| Disease duration, months | 6 (2.75, 24) | 8 (3, 27) | 0.492 | 0.133 | 7 (2.5, 24) | 7 (3, 17) | 0.787 | 0.024 |
| Pyridostigmine duration, months | 2 (1, 9.5) | 4 (1, 21) | 0.118 | 0.296 | 2 (1, 13) | 3 (1, 10) | 0.873 | 0.090 |
| Pyridostigmine dose, mg/day | 240 (180, 480) | 240 (180, 360) | 0.020 * | 0.294 | 240 (180, 480) | 240 (180, 360) | 0.605 | 0.001 |
| Ach receptor antibody, nmol/L | 10.4 ± 4.8 | 10.2 ± 5.6 | 0.799 | 0.040 | 10.5 ± 4.6 | 10.1 ± 5.5 | 0.656 | 0.074 |
| Preoperative QMG score | 9 (6, 14) | 9.5 (5, 13) | 0.514 | 0.049 | 9 (5, 13) | 9 (5, 13) | 0.687 | 0.020 |
| MG crisis history | 1 (1) | 6 (7) | 0.058 | 0.308 | 2 (2) | 3 (4) | 0.492 | 0.121 |
| Preoperative pulmonary function test | ||||||||
| FEV1, L | 2.6 ± 0.7 | 2.7 ± 0.7 | 0.312 | 0.161 | 2.7 ± 0.7 | 2.6 ± 0.7 | 0.637 | 0.084 |
| FVC, L | 3.0 (2.6, 3.6) | 3.2 (2.8, 3.8) | 0.100 | 0.288 | 3.1 (2.6, 3.8) | 3.3 (2.7, 3.7) | 0.575 | 0.066 |
| Variables | Original Dataset before IPTW | Pseudo Dataset after IPTW | ||||
|---|---|---|---|---|---|---|
| Neostigmine (n = 88) | Sugammadex (n = 83) | p-Value | Neostigmine (n = 84) | Sugammadex (n = 83) | p-Value | |
| Intraoperative variables | ||||||
| Anesthesia time, min | 155 (135, 190) | 190 (140, 225) | 0.005 * | 155 (135, 190) | 190 (140, 230) | 0.012 * |
| Operation time, min | 105 (84.5, 138) | 133 (87, 165) | 0.018 * | 104 (84, 137) | 124 (81, 165) | 0.085 |
| Blood loss, ml | 0 (0, 50) | 0 (0, 20) | 0.113 | 0 (0, 50) | 0 (0, 20) | 0.400 |
| Intraoperative RBC transfusion | 0 | 0 | 1.000 | 0 | 0 | 1.000 |
| Administered rocuronium, mg | 50 (27.5, 50) | 50 (50, 50) | 0.002 * | 50 (25, 50) | 50 (50, 50) | 0.006 * |
| Administered neostigmine, mg | 1 (1, 1) | - | - | 1 (1, 1) | - | - |
| Administered sugammadex, mg | - | 200 (200, 200) | - | - | 200 (200, 200) | - |
| Thymic pathology | 0.372 | 0.231 | ||||
| Normal thymus | 5 (6) | 2 (2) | 5 (6) | 1 (1) | ||
| Thymoma | 40 (45) | 48 (58) | 39 (46) | 49 (59) | ||
| Thymic/Follicular hyperplasia | 36 (41) | 28 (34) | 34 (40) | 26 (31) | ||
| Others | 7 (8) | 5 (6) | 7 (8) | 7 (8) | ||
| Thymoma size, cm | 3.6 (2.5, 5) | 4 (2.95, 5.45) | 0.254 | 3.7 (2.6, 5) | 4 (2.9, 5.5) | 0.380 |
| Variables | Original Dataset before IPTW | Pseudo Dataset after IPTW | ||||
|---|---|---|---|---|---|---|
| Neostigmine (n = 88) | Sugammadex (n = 83) | p-Value | Neostigmine (n = 84) | Sugammadex (n = 83) | p-Value | |
| Length of postoperative hospital stay, days | 5 (3, 7) | 4 (2, 5) | 0.001 * | 5 (3, 6) | 4 (2, 4) | 0.003 * |
| Mortality | 0 | 0 | 1.000 | 0 | 0 | 1.000 |
| Postoperative complication | ||||||
| Postoperative myasthenic crisis | 3 (3) | 3 (4) | 1.000 | 2 (2) | 2 (3) | 0.908 |
| Nerve palsy | 1 (1) | 0 (0) | 1.000 | 1 (1) | 0 (0) | 1.000 |
| Atelectasis | 0 (0) | 1 (1) | 0.485 | 0 (0) | 1 (1) | 1.000 |
| Pleural effusion | 1 (1) | 0 (0) | 1.000 | 1 (1) | 0 (0) | 1.000 |
| Extubated patients in the OR | 84 (95) | 81 (98) | 0.683 | 80 (96) | 82 (99) | 0.176 |
| Reintubated patients in the OR | 4 (5) | 1 (1) | 0.369 | 3 (3) | 1 (2) | 0.533 |
| Finally extubated patients in the OR | 80 (91) | 80 (96) | 0.145 | 78 (93) | 81 (97) | 0.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
No, H.-J.; Yoo, Y.C.; Oh, Y.J.; Lee, H.S.; Jeon, S.; Kweon, K.H.; Kim, N.Y. Comparison between Sugammadex and Neostigmine after Video-Assisted Thoracoscopic Surgery–Thymectomy in Patients with Myasthenia Gravis: A Single-Center Retrospective Exploratory Analysis. J. Pers. Med. 2023, 13, 1380. https://doi.org/10.3390/jpm13091380
No H-J, Yoo YC, Oh YJ, Lee HS, Jeon S, Kweon KH, Kim NY. Comparison between Sugammadex and Neostigmine after Video-Assisted Thoracoscopic Surgery–Thymectomy in Patients with Myasthenia Gravis: A Single-Center Retrospective Exploratory Analysis. Journal of Personalized Medicine. 2023; 13(9):1380. https://doi.org/10.3390/jpm13091380
Chicago/Turabian StyleNo, Hyun-Joung, Young Chul Yoo, Young Jun Oh, Hye Sun Lee, Soyoung Jeon, Ki Hong Kweon, and Na Young Kim. 2023. "Comparison between Sugammadex and Neostigmine after Video-Assisted Thoracoscopic Surgery–Thymectomy in Patients with Myasthenia Gravis: A Single-Center Retrospective Exploratory Analysis" Journal of Personalized Medicine 13, no. 9: 1380. https://doi.org/10.3390/jpm13091380
APA StyleNo, H.-J., Yoo, Y. C., Oh, Y. J., Lee, H. S., Jeon, S., Kweon, K. H., & Kim, N. Y. (2023). Comparison between Sugammadex and Neostigmine after Video-Assisted Thoracoscopic Surgery–Thymectomy in Patients with Myasthenia Gravis: A Single-Center Retrospective Exploratory Analysis. Journal of Personalized Medicine, 13(9), 1380. https://doi.org/10.3390/jpm13091380






