Robot-Assisted Renal Surgery with the New Hugo Ras System: Trocar Placement and Docking Settings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Endpoints, Data and Statistical Analysis
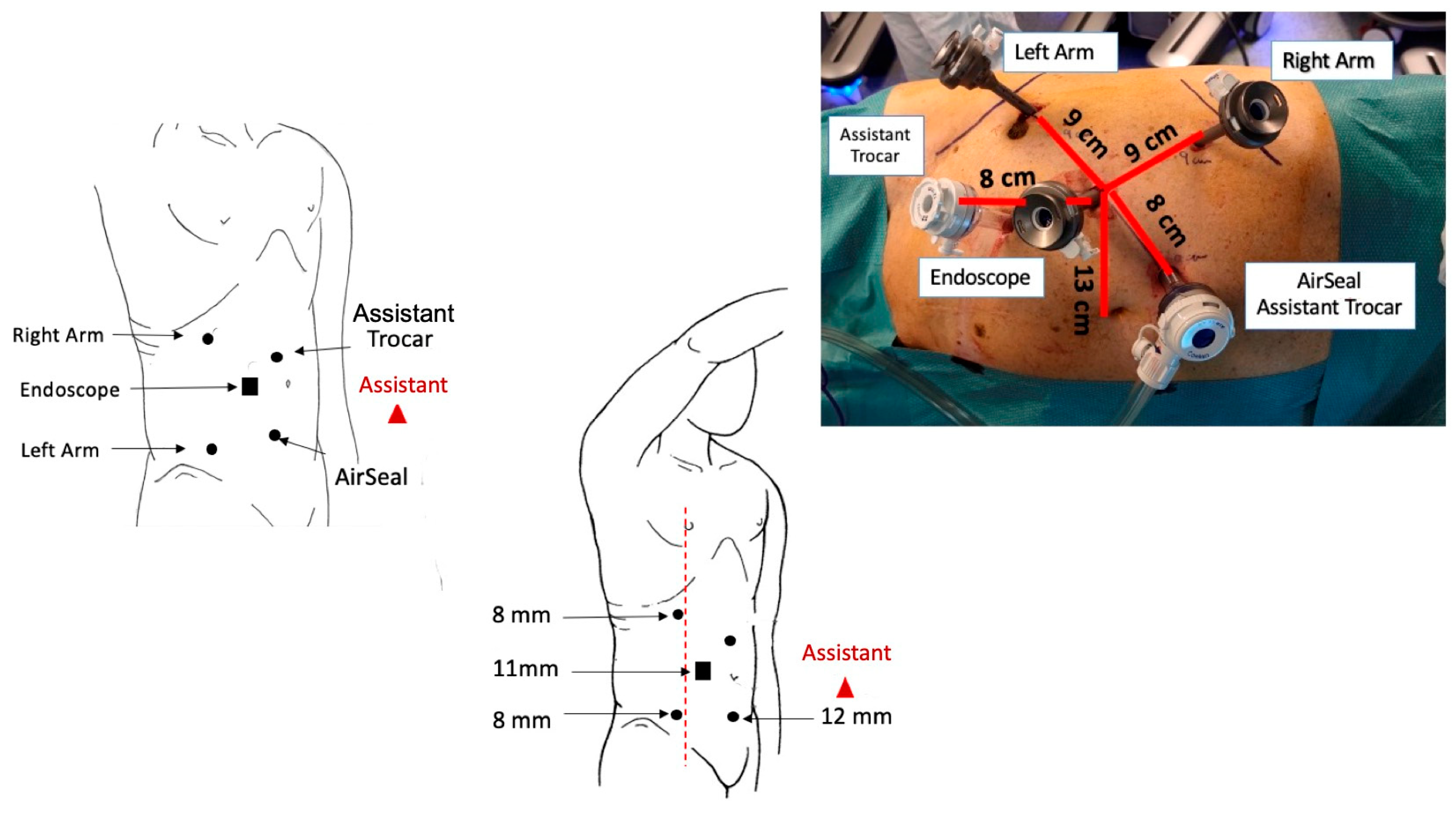
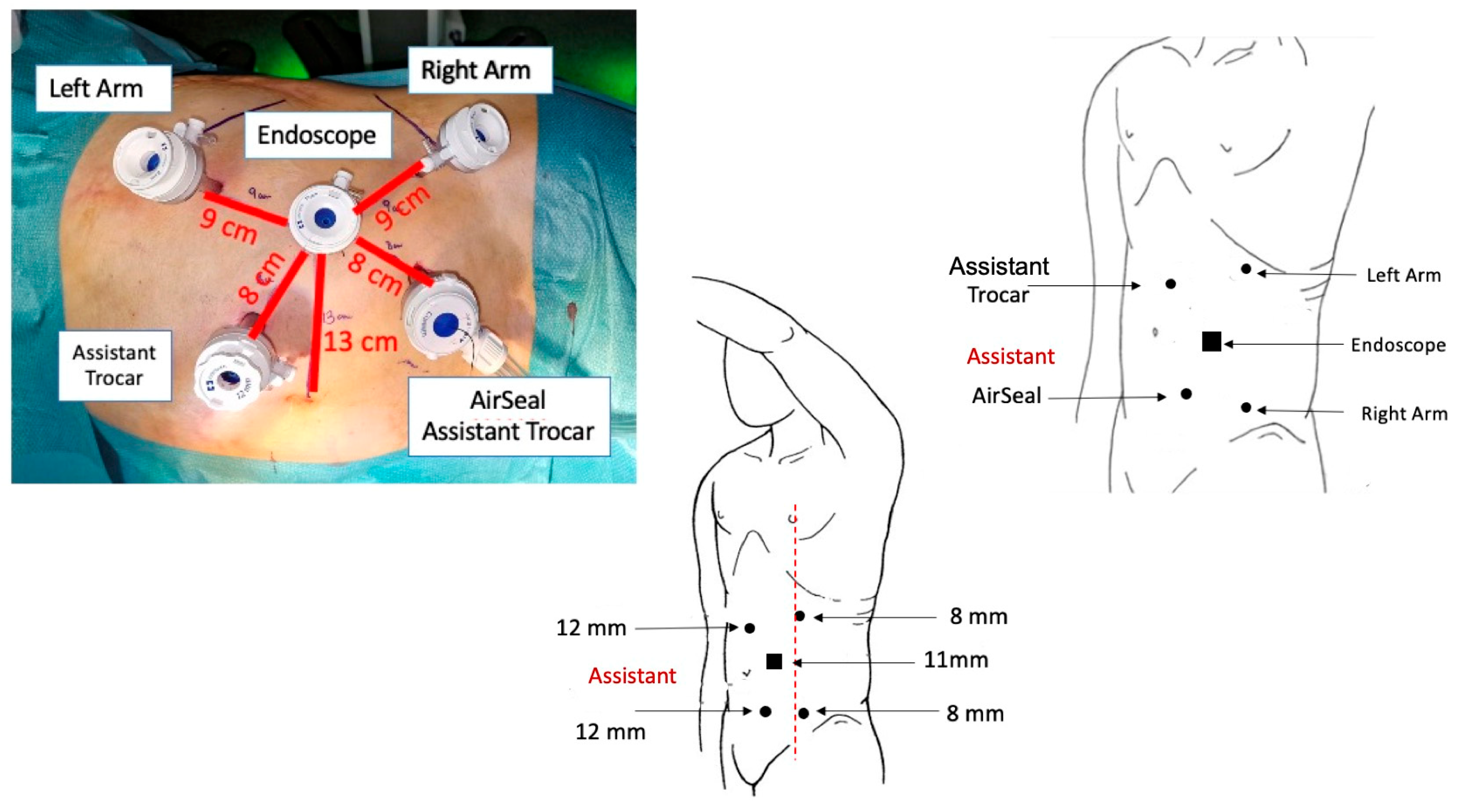
2.3. Trocar Placement and System Docking
| Right RAPN | ||
| Docking angle | Tilt angle | |
| Endoscope | 275° | −45° |
| Right arm | 310° | −15° |
| Left arm | 225° | −15° |
| Left RAPN | ||
| Docking angle | Tilt angle | |
| Endoscope | 90° | −35° |
| Right arm | 135° | −30° |
| Left arm | 45° | −45° |
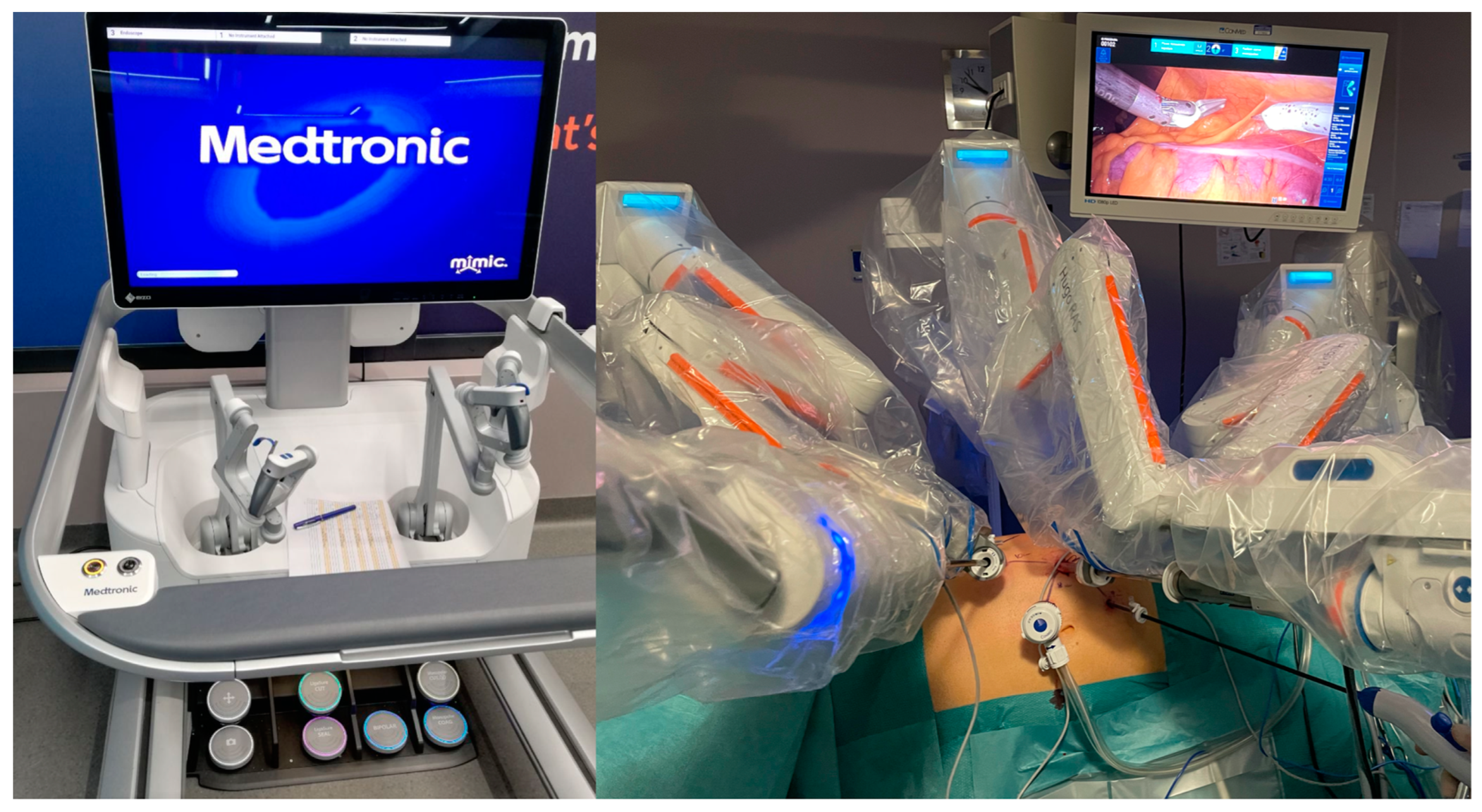
2.4. Surgical Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simone, G.; Tuderti, G.; Anceschi, U.; Papalia, R.; Ferriero, M.; Misuraca, L.; Minisola, F.; Mastroianni, R.; Costantini, M.; Guaglianone, S.; et al. Oncological outcomes of minimally invasive partial versus minimally invasive radical nephrectomy for cT1-2/N0/M0 clear cell renal cell carcinoma: A propensity score-matched analysis. World J. Urol. 2017, 35, 789–794. Available online: https://pubmed.ncbi.nlm.nih.gov/27578234/ (accessed on 30 March 2023). [CrossRef]
- Esperto, F.; Prata, F.; Antonelli, A.; Alloni, R.; Campanozzi, L.; Cataldo, R.; Civitella, A.; Fiori, C.; Ghilardi, G.; Guglielmelli, E.; et al. Bioethical implications of robotic surgery in urology: A systematic review. Minerva Urol. Nephrol. 2022, 73, 700–710. Available online: https://pubmed.ncbi.nlm.nih.gov/34308607/ (accessed on 29 March 2023). [CrossRef]
- Brassetti, A.; Anceschi, U.; Bove, A.M.; Prata, F.; Costantini, M.; Ferriero, M.; Mastroianni, R.; Misuraca, L.; Tuderti, G.; Torregiani, G.; et al. Purely Off-Clamp Laparoscopic Partial Nephrectomy Stands the Test of Time: 15 Years Functional and Oncologic Outcomes from a Single Center Experience. Curr. Oncol. 2023, 30, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Simone, G.; Ferriero, M.; Guaglianone, S.; Costantini, M.; Giannarelli, D.; Maini, C.L.; Forastiere, E.; Gallucci, M. Laparoscopic and robotic partial nephrectomy without renal ischaemia for tumours larger than 4 cm: Perioperative and functional outcomes. World J. Urol. 2012, 30, 671–676. [Google Scholar] [CrossRef]
- Muaddi, H.; Hafid MEl Choi, W.J.; Lillie, E.; de Mestral, C.; Nathens, A.; Stukel, T.A.; Karanicolas, P.J. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Ann. Surg. 2021, 273, 467–473. Available online: https://pubmed.ncbi.nlm.nih.gov/32398482/ (accessed on 29 March 2023). [CrossRef] [PubMed]
- Simone, G.; De Nunzio, C.; Ferriero, M.; Cindolo, L.; Brookman-May, S.; Papalia, R.; Sperduti, I.; Collura, D.; Leonardo, C.; Anceschi, U.; et al. Trends in the use of partial nephrectomy for cT1 renal tumors: Analysis of a 10-yr European multicenter dataset. Eur. J. Surg. Oncol. 2016, 42, 1729–1735. Available online: https://pubmed.ncbi.nlm.nih.gov/27106494/ (accessed on 30 March 2023). [CrossRef] [PubMed]
- Larcher, A.; Muttin, F.; Peyronnet, B.; De Naeyer, G.; Khene, Z.E.; Dell’Oglio, P.; Ferreiro, C.; Schatteman, P.; Capitanio, U.; D’Hondt, F.; et al. The Learning Curve for Robot-assisted Partial Nephrectomy: Impact of Surgical Experience on Perioperative Outcomes. Eur. Urol. 2019, 75, 253–256. Available online: https://pubmed.ncbi.nlm.nih.gov/30243798/ (accessed on 29 March 2023). [CrossRef]
- Ferriero, M.; Bove, A.M.; Tuderti, G.; Anceschi, U.; Brassetti, A.; Costantini, M.; Mastroianni, R.; Guaglianone, S.; Gallucci, M.; Simone, G. Impact of learning curve on perioperative outcomes of off-clamp minimally invasive partial nephrectomy: Propensity score matched comparison of outcomes between training versus expert series. Minerva Urol. Nephrol. 2021, 73, 564–571. Available online: https://pubmed.ncbi.nlm.nih.gov/32182230/ (accessed on 30 March 2023). [CrossRef] [PubMed]
- Malthouse, T.; Kasivisvanathan, V.; Raison, N.; Lam, W.; Challacombe, B. The future of partial nephrectomy. Int. J. Surg. 2016, 36, 560–567. Available online: https://pubmed.ncbi.nlm.nih.gov/26975430/ (accessed on 23 May 2023). [CrossRef]
- Singh, T.P.; Zaman, J.; Cutler, J. Robotic Surgery: At the Crossroads of a Data Explosion. World J. Surg. 2021, 45, 3484–3492. Available online: https://pubmed.ncbi.nlm.nih.gov/34635951/ (accessed on 23 May 2023). [CrossRef] [PubMed]
- Ragavan, N.; Bharathkumar, S.; Chirravur, P.; Sankaran, S.; Mottrie, A. Evaluation of Hugo RAS System in Major Urologic Surgery: Our Initial Experience. J. Endourol. 2022, 36, 1029–1035. Available online: https://pubmed.ncbi.nlm.nih.gov/35156838/ (accessed on 29 March 2023). [CrossRef]
- Totaro, A.; Campetella, M.; Bientinesi, R.; Gandi, C.; Palermo, G.; Russo, A.; Aceto, P.; Bassi, P.; Sacco, E. The new surgical robotic platform HUGOTM RAS: System description and docking settings for robot-assisted radical prostatectomy. Urologia 2022, 89, 603–609. Available online: https://pubmed.ncbi.nlm.nih.gov/35765756/ (accessed on 23 May 2023). [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernandez-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. Available online: https://pubmed.ncbi.nlm.nih.gov/35346519/ (accessed on 30 March 2023). [CrossRef]
- Fiorello, N.; Di Benedetto, A.; Summonti, D.; Mogorovich, A.; Sepich, C.A. Learning curve in robot-assisted partial nephrectomy: Comparison between an expert surgeon and a team in training in single-center experiences. Cent. Eur. J. Urol. 2021, 74, 523–527. Available online: https://pubmed.ncbi.nlm.nih.gov/35083071/ (accessed on 23 May 2023).
- Simone, G.; Papalia, R.; Guaglianone, S.; Gallucci, M. “Zero ischaemia”, sutureless laparoscopic partial nephrectomy for renal tumours with a low nephrometry score. BJU Int. 2012, 110, 124–130. Available online: https://pubmed.ncbi.nlm.nih.gov/22177008/ (accessed on 30 March 2023). [CrossRef] [PubMed]
- Papalia, R.; Simone, G.; Ferriero, M.; Costantini, M.; Guaglianone, S.; Forastiere, E.; Gallucci, M. Laparoscopic and Robotic Partial Nephrectomy with Controlled Hypotensive Anesthesia to Avoid Hilar Clamping: Feasibility, Safety and Perioperative Functional Outcomes. J. Urol. 2012, 187, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Petros, F.G.; Patel, M.N.; Kheterpal, E.; Siddiqui, S.; Ross, J.; Bhandari, A.; Diaz, M.; Menon, M.; Rogers, C.G. Robotic partial nephrectomy in the setting of prior abdominal surgery. BJU Int. 2010, 108, 413–419. Available online: https://bjui-journals.onlinelibrary.wiley.com/doi/10.1111/j.1464-410X.2010.09803.x (accessed on 23 May 2023). [CrossRef]
- Garisto, J.D.; Bertolo, R.; Kaouk, J. Technique for Docking and Port Placement Using a Purpose-built Robotic System (SP1098) in Human Cadaver. Urology 2018, 119, 91–96. [Google Scholar] [CrossRef]
- Sarchi, L.; Mottaran, A.; Bravi, C.A.; Paciotti, M.; Farinha, R.; Piazza, P.; Puliatti, S.; De Groote, R.; De Naeyer, G.; Gallagher, A.; et al. Robot-assisted radical prostatectomy feasibility and setting with the HugoTM robot-assisted surgery system. BJU Int. 2022, 130, 671–675. Available online: https://pubmed.ncbi.nlm.nih.gov/35689414/ (accessed on 23 May 2023). [CrossRef] [PubMed]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. Available online: https://pubmed.ncbi.nlm.nih.gov/19616235/ (accessed on 29 March 2023). [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. Available online: https://pubmed.ncbi.nlm.nih.gov/19638912/ (accessed on 29 March 2023). [CrossRef] [PubMed]
- Brassetti, A.; Cacciamani, G.E.; Mari, A.; Garisto, J.D.; Bertolo, R.; Sundaram, C.P.; Derweesh, I.; Bindayi, A.; Dasgupta, P.; Porter, J.; et al. On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for cT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis. Cancers 2022, 14, 4431. [Google Scholar] [CrossRef]
- Gallioli, A.; Uleri, A.; Gaya, J.M.; Territo, A.; Aumatell, J.; Verri, P.; Basile, G.; Fontanet, S.; Tedde, A.; Diana, P.; et al. Initial experience of robot-assisted partial nephrectomy with HugoTM RAS system: Implications for surgical setting. World J. Urol. 2023, 41, 1085–7091. Available online: https://pubmed.ncbi.nlm.nih.gov/36847815/ (accessed on 29 March 2023). [CrossRef] [PubMed]
- Anceschi, U.; Brassetti, A.; Bertolo, R.; Tuderti, G.; Ferriero, M.C.; Mastroianni, R.; Flammia, R.S.; Costantini, M.; Kaouk, J.; Leonardo, C.; et al. On-clamp versus purely off-clamp robot-assisted partial nephrectomy in solitary kidneys: Comparison of perioperative outcomes and chronic kidney disease progression at two high-volume centers. Minerva Urol. Nephrol. 2022, 73, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Capitanio, U.; Tuderti, G.; Presicce, F.; Leonardo, C.; Ferriero, M.; Misurace, L.; Costantini, M.; Larcher, A.; Minisola, F.; et al. On-clamp versus off-clamp partial nephrectomy: Propensity score-matched comparison of long-term functional outcomes. Int. J. Urol. 2019, 26, 985–991. Available online: https://pubmed.ncbi.nlm.nih.gov/31342589/ (accessed on 30 March 2023). [CrossRef]
- Anceschi, U.; Flammia, R.S.; Mattevi, D.; Tufano, A.; Brassetti, A.; Ferriero, M.C.; Tuderti, G.; Misuraca, L.; Bove, A.M.; Mastroianni, R.; et al. External Validation of a Novel Comprehensive Trifecta System in Predicting Oncologic and Functional Outcomes of Partial Nephrectomy: Results of a Multicentric Series. J. Clin. Med. 2022, 11, 796. Available online: https://pubmed.ncbi.nlm.nih.gov/35160248/ (accessed on 30 March 2023). [CrossRef]
- Anceschi, U.; Brassetti, A.; Tuderti, G.; Consiglia Ferriero, M.; Minervini, A.; Mari, A.; Grosso, A.A.; Carni, M.; Capitanio, U.; Larcher, A.; et al. Risk factors for progression of chronic kidney disease after robotic partial nephrectomy in elderly patients: Results from a multi-institutional collaborative series. Minerva Urol. Nephrol. 2022, 74, 452–460. [Google Scholar] [CrossRef]
- Anceschi, U.; Ferriero, M.C.; Tuderti, G.; Brassetti, A.; Bertolo, R.; Capitanio, U.; Larcher, A.; Garisto, J.; Antonelli, A.; Mottrie, A.; et al. Head to Head Impact of Margin, Ischemia, Complications, Score Versus a Novel Trifecta Score on Oncologic and Functional Outcomes After Robotic-assisted Partial Nephrectomy: Results of a Multicenter Series. Eur. Urol. Focus. 2021, 7, 1391–1399. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Guaglianone, S.; Forestiere, E.; Gallucci, M. Preoperative Superselective Transarterial Embolization in Laparoscopic Partial Nephrectomy: Technique, Oncologic, and Functional Outcomes. J. Endourol. 2009, 23, 1473–1478. [Google Scholar] [CrossRef]
- Gallucci, M.; Guaglianone, S.; Carpanese, L.; Papalia, R.; Simone, G.; Forestiere, E.; Leonardo, C. Superselective Embolization as First Step of Laparoscopic Partial Nephrectomy. Urology 2007, 69, 642–645. [Google Scholar] [CrossRef]
| Variable | Cohort (n = 25) |
|---|---|
| Age (n, median, IQR) | 69 (60–73) |
| Gender (n, %) | |
| Male | 19 (76%) |
| Female | 6 (24%) |
| BMI (kg/m2, median, IQR) | 27.3 (25.7–28.1) |
| ASA score (n, %) | |
| I | 1 (4%) |
| II | 18 (72%) |
| III | 5 (20%) |
| IV | 1 (4%) |
| Charlson Comorbidity Index (median, IQR) | 4.5 (3.25–5) |
| Diabetes (n, %) | 3 (12%) |
| Hypertension (n, %) | 14 (56%) |
| Preoperative Hemoglobin (g/dl, median, IQR) | 15 (13.8–15.5) |
| Preoperative Creatinine (mg/dL, median, IQR) | 0.92 (0.81–1.07) |
| Preoperative eGFR (ml/min/1.73 m2, median, IQR) | 84.6 (64.5–90.9) |
| Clinical Tumor Size (mm, median, IQR) | 32.5 (26–43.7) |
| Number of Lesion (n, %) | |
| 1 | 24 (100%) |
| 2 | 1 (0%) |
| cT (n, %) | |
| T1a | 19 (76%) |
| T1b | 4 (16%) |
| T2 | 2 (8%) |
| Side (n, %) | |
| Right | 14 (56%) |
| Left | 11 (44%) |
| R.E.N.A.L. score (median, IQR) | 6 (5–7) |
| Variable | Cohort (n = 7) |
|---|---|
| Docking Time (min, median, IQR) | 5 (5–6) |
| Console Time (min, median, IQR) | 90 (68–135.75) |
| Estimated blood loss (ml, median, IQR) | 175 (100–400) |
| Perioperative complications (n, %) | 4 (16%) |
| Length of Stay (days, median, IQR) | 3 (3–4) |
| Hemoglobin at discharge (g/dl, median, IQR) | 11.5 (10.2–12.8) |
| Creatinine at discharge (mg/dL, median, IQR) | 0.9 (0.82–1.12) |
| eGFR at discharge (ml/min/1.73 m2, median, IQR) | 81.9 (60.6–89.5) |
| Readmission (n, %) | 0 (0%) |
| Pathological Size (mm, median, IQR) | 30 (18.5–40) |
| Pathology (n, %) | |
| Benign | 8 (32%) |
| Malignant | 17 (68%) |
| Histology subtype (n, %) | |
| Oncocytoma | 6 (24%) |
| Clear Cell | 10 (40%) |
| Papillary | 6 (24%) |
| Angiomyolipoma | 2 (8%) |
| Chromophobe | 1 (4%) |
| Positive Margins (n, %) | 0 (0%) |
| pT Stage (n, %) | |
| 1a | 21 (84%) |
| 1b | 2 (8%) |
| 2a | 2 (8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prata, F.; Raso, G.; Ragusa, A.; Iannuzzi, A.; Tedesco, F.; Cacciatore, L.; Civitella, A.; Tuzzolo, P.; D’Addurno, G.; Callè, P.; et al. Robot-Assisted Renal Surgery with the New Hugo Ras System: Trocar Placement and Docking Settings. J. Pers. Med. 2023, 13, 1372. https://doi.org/10.3390/jpm13091372
Prata F, Raso G, Ragusa A, Iannuzzi A, Tedesco F, Cacciatore L, Civitella A, Tuzzolo P, D’Addurno G, Callè P, et al. Robot-Assisted Renal Surgery with the New Hugo Ras System: Trocar Placement and Docking Settings. Journal of Personalized Medicine. 2023; 13(9):1372. https://doi.org/10.3390/jpm13091372
Chicago/Turabian StylePrata, Francesco, Gianluigi Raso, Alberto Ragusa, Andrea Iannuzzi, Francesco Tedesco, Loris Cacciatore, Angelo Civitella, Piergiorgio Tuzzolo, Giuseppe D’Addurno, Pasquale Callè, and et al. 2023. "Robot-Assisted Renal Surgery with the New Hugo Ras System: Trocar Placement and Docking Settings" Journal of Personalized Medicine 13, no. 9: 1372. https://doi.org/10.3390/jpm13091372
APA StylePrata, F., Raso, G., Ragusa, A., Iannuzzi, A., Tedesco, F., Cacciatore, L., Civitella, A., Tuzzolo, P., D’Addurno, G., Callè, P., Basile, S., Fantozzi, M., Pira, M., Prata, S. M., Anceschi, U., Simone, G., Scarpa, R. M., & Papalia, R. (2023). Robot-Assisted Renal Surgery with the New Hugo Ras System: Trocar Placement and Docking Settings. Journal of Personalized Medicine, 13(9), 1372. https://doi.org/10.3390/jpm13091372







