Age-Related Changes in Epilepsy Characteristics and Response to Antiepileptic Treatment in Autism Spectrum Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Review Board Approval
2.2. Survey
2.3. Participants
2.4. Statistical Analysis
3. Results
3.1. Seizure Characteristics across the Age Span
3.1.1. Seizure Severity vs. Age of Onset
3.1.2. Epilepsy Resolution
3.1.3. Duration of Epilepsy
3.2. Relation between Patient Characteristics and Seizures
3.2.1. Neurodevelopmental Regression in Epilepsy
3.2.2. Potential Underlying Etiology
3.2.3. Comorbidities and Epilepsy: General Medical Conditions
3.2.4. Comorbidities and Epilepsy: Sleep
3.2.5. Comorbidities and Epilepsy: Gastrointestinal Symptoms
3.2.6. Comorbidities and Epilepsy: Sensory
3.2.7. Comorbidities and Epilepsy: Allergies
3.3. Treatment Effectiveness Ratings
3.3.1. Overall Treatment Effectiveness Ratings
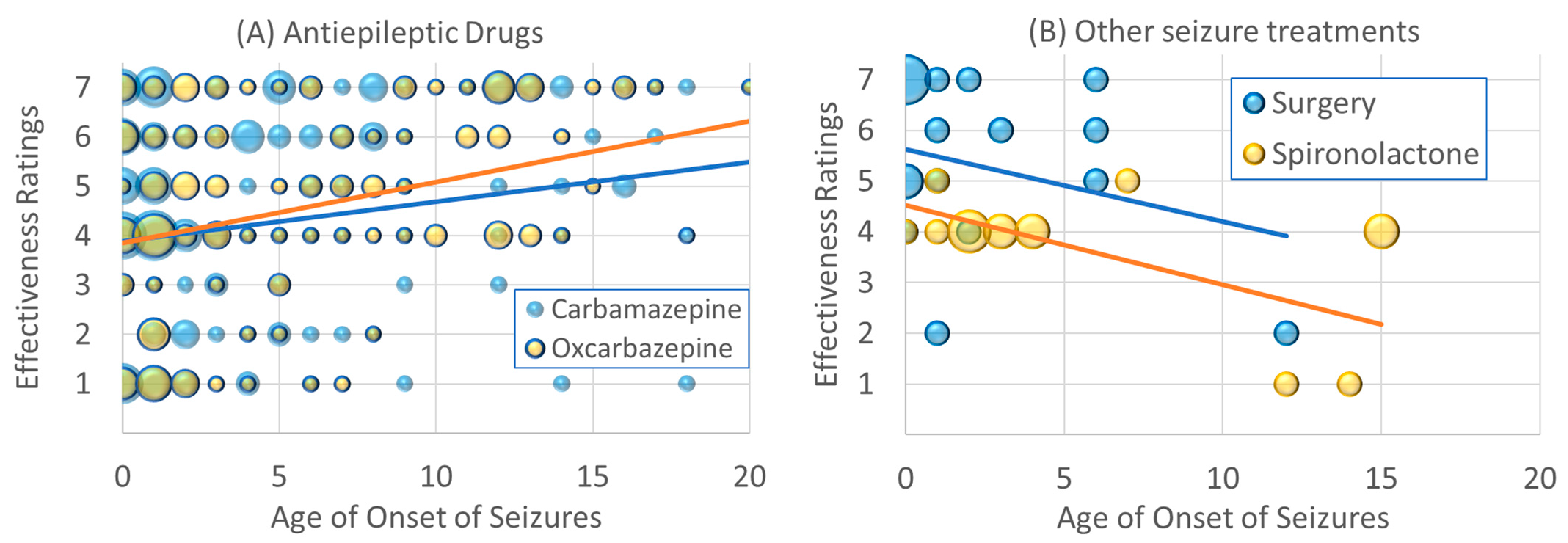
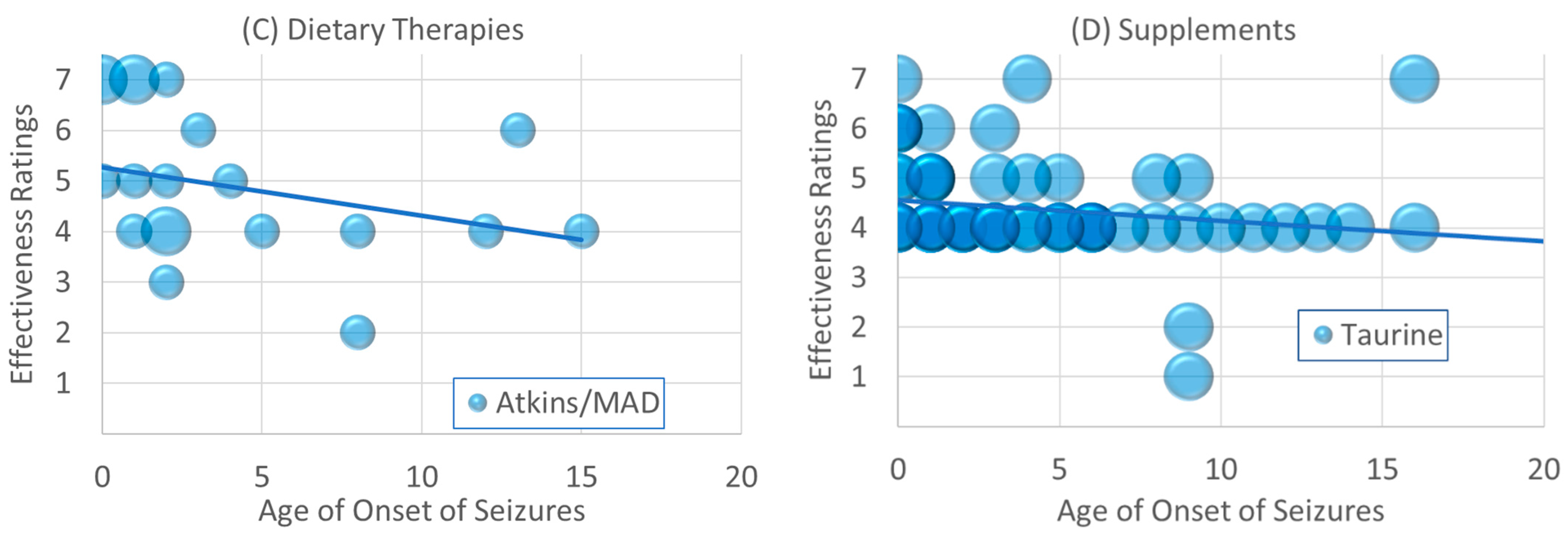
3.3.2. Changes in Effectiveness Rating vs. Age of Onset
3.3.3. Effectiveness Rating across Seizure Types (GTC, Focal, Absence)
3.3.4. Effectiveness Rating in Non-Resolved Seizures
3.3.5. Effectiveness Rating across Seizure Severity
3.3.6. Effectiveness Rating across Autism Diagnosis
3.3.7. Effectiveness Rating with Neurodevelopmental Regression
3.3.8. Effectiveness Rating with Sex
3.4. Specific Epilepsy Syndromes
3.4.1. Seizure Variables
3.4.2. Neurodevelopmental Regression
3.4.3. Potential Underlying Etiology
3.4.4. Comorbidities
3.4.5. Effectiveness of Treatments
4. Discussion
4.1. Seizure Severity Improves with Age
4.2. Epilepsy Duration
4.3. Effectiveness Is Related to Age for Some Treatments
4.4. Potential Mechanisms of Low Carbohydrate Diets
4.5. Effectiveness Is Related to Seizure Severity for Some Treatments
4.6. Epilepsy Syndrome May Require Specific Treatments
4.7. Identifying Patient Comorbidities Could Aid Treatment
4.8. Treatments for Epilepsy in Autism Spectrum Disorder
4.9. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vargason, T.; Frye, R.E.; McGuinness, D.L.; Hahn, J. Clustering of co-occurring conditions in autism spectrum disorder during early childhood: A retrospective analysis of medical claims data. Autism Res. Off. J. Int. Soc. Autism Res. 2019, 12, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Soke, G.N.; Maenner, M.J.; Christensen, D.; Kurzius-Spencer, M.; Schieve, L.A. Prevalence of Co-occurring Medical and Behavioral Conditions/Symptoms Among 4- and 8-Year-Old Children with Autism Spectrum Disorder in Selected Areas of the United States in 2010. J. Autism Dev. Disord. 2018, 48, 2663–2676. [Google Scholar] [CrossRef] [PubMed]
- Brondino, N.; Fusar-Poli, L.; Miceli, E.; Di Stefano, M.; Damiani, S.; Rocchetti, M.; Politi, P. Prevalence of Medical Comorbidities in Adults with Autism Spectrum Disorder. J. Gen. Intern. Med. 2019, 34, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- Holingue, C.; Newill, C.; Lee, L.C.; Pasricha, P.J.; Daniele Fallin, M. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. Off. J. Int. Soc. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Psychotropic Medications for Sleep Disorders in Autism Spectrum Disorders. In Handbook of Autism and Pervasive Developmental Disorder: Assessment, Diagnosis, and Treatment; Matson, J.L., Sturmey, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1191–1217. [Google Scholar] [CrossRef]
- Dizitzer, Y.; Meiri, G.; Flusser, H.; Michaelovski, A.; Dinstein, I.; Menashe, I. Comorbidity and health services’ usage in children with autism spectrum disorder: A nested case-control study. Epidemiol. Psychiatr. Sci 2020, 29, e95. [Google Scholar] [CrossRef]
- Foldes, S.T.; Jensen, A.R.; Jacobson, A.; Vassall, S.; Foldes, E.; Guthery, A.; Brown, D.; Levine, T.; Tyler, W.J.; Frye, R.E. Transdermal Electrical Neuromodulation for Anxiety and Sleep Problems in High-Functioning Autism Spectrum Disorder: Feasibility and Preliminary Findings. J. Pers. Med. 2021, 11, 1307. [Google Scholar] [CrossRef]
- Anukirthiga, B.; Mishra, D.; Pandey, S.; Juneja, M.; Sharma, N. Prevalence of Epilepsy and Inter-Ictal Epileptiform Discharges in Children with Autism and Attention-Deficit Hyperactivity Disorder. Indian J. Pediatr. 2019, 86, 897–902. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Nelson, D.S. Seizure disorders in autism. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 127–129. [Google Scholar] [CrossRef]
- Hara, H. Autism and epilepsy: A retrospective follow-up study. Brain. Dev. 2007, 29, 486–490. [Google Scholar] [CrossRef]
- Hughes, J.R.; Melyn, M. EEG and seizures in autistic children and adolescents: Further findings with therapeutic implications. Clin. EEG Neurosci. 2005, 36, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.J.; Sharieff, G.Q. Seizures in Children. Pediatr. Clin. N. Am. 2006, 53, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Meshram, R.J.; Kumar Singh, R. Febrile Seizures in Children: A Review. Cureus 2022, 14, e31509. [Google Scholar] [CrossRef]
- Strasser, L.; Downes, M.; Kung, J.; Cross, J.H.; De Haan, M. Prevalence and risk factors for autism spectrum disorder in epilepsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lukmanji, S.; Manji, S.A.; Kadhim, S.; Sauro, K.M.; Wirrell, E.C.; Kwon, C.S.; Jetté, N. The co-occurrence of epilepsy and autism: A systematic review. Epilepsy Behav. 2019, 98, 238–248. [Google Scholar] [CrossRef]
- Berg, A.T.; Jallon, P.; Preux, P.M. The epidemiology of seizure disorders in infancy and childhood: Definitions and classifications. Handb. Clin. Neurol. 2013, 111, 391–398. [Google Scholar] [CrossRef]
- Frye, R.E.; Rossignol, D.; Casanova, M.F.; Brown, G.L.; Martin, V.; Edelson, S.; Coben, R.; Lewine, J.; Slattery, J.C.; Lau, C.; et al. A review of traditional and novel treatments for seizures in autism spectrum disorder: Findings from a systematic review and expert panel. Front. Public Health 2013, 1, 31. [Google Scholar] [CrossRef]
- Frye, R.E.; Sreenivasula, S.; Adams, J.B. Traditional and non-traditional treatments for autism spectrum disorder with seizures: An on-line survey. BMC Pediatr. 2011, 11, 37. [Google Scholar] [CrossRef]
- Precenzano, F.; Parisi, L.; Lanzara, V.; Vetri, L.; Operto, F.F.; Pastorino, G.M.G.; Ruberto, M.; Messina, G.; Risoleo, M.C.; Santoro, C.; et al. Electroencephalographic Abnormalities in Autism Spectrum Disorder: Characteristics and Therapeutic Implications. Medicina 2020, 56, 419. [Google Scholar] [CrossRef]
- Lewine, J.D.; Andrews, R.; Chez, M.; Patil, A.A.; Devinsky, O.; Smith, M.; Kanner, A.; Davis, J.T.; Funke, M.; Jones, G.; et al. Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics 1999, 104, 405–418. [Google Scholar] [CrossRef]
- Yara, M.-D.; Karina, A.G.O.; Elizabeth, M.B.; Stephan, U.S. Indications and yield of ambulatory EEG recordings. Epileptic Disord. 2021, 23, 94–103. [Google Scholar] [CrossRef]
- Barger, B.D.; Campbell, J.M.; McDonough, J.D. Prevalence and onset of regression within autism spectrum disorders: A meta-analytic review. J. Autism Dev. Disord. 2013, 43, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, R.F.; Rapin, I. Regression in pervasive developmental disorders: Seizures and epileptiform electroencephalogram correlates. Pediatrics 1997, 99, 560–566. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Cerebral Folate Deficiency, Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1141. [Google Scholar] [CrossRef]
- Specchio, N.; Di Micco, V.; Trivisano, M.; Ferretti, A.; Curatolo, P. The epilepsy-autism spectrum disorder phenotype in the era of molecular genetics and precision therapy. Epilepsia 2022, 63, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, X.; Sun, C.; Zou, M.; Chen, Y.; Huang, J.; Wu, L.; Chen, W.X. Prevalence of epilepsy in autism spectrum disorders: A systematic review and meta-analysis. Autism 2022, 26, 33–50. [Google Scholar] [CrossRef]
- Juric-Sekhar, G.; Hevner, R.F. Malformations of Cerebral Cortex Development: Molecules and Mechanisms. Annu. Rev. Pathol. 2019, 14, 293–318. [Google Scholar] [CrossRef]
- Simmons, R.; Martinez, A.B.; Barkovich, J.; Numis, A.L.; Cilio, M.R.; Glenn, O.A.; Gano, D.; Rogers, E.E.; Glass, H.C. Disorders of Neuronal Migration/Organization Convey the Highest Risk of Neonatal Onset Epilepsy Compared with Other Congenital Brain Malformations. Pediatr. Neurol. 2022, 127, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wiwattanadittakul, N.; Suwannachote, S.; You, X.; Cohen, N.T.; Tran, T.; Phuackchantuck, R.; Tsuchida, T.N.; Depositario-Cabacar, D.F.; Zelleke, T.; Schreiber, J.M.; et al. Spatiotemporal distribution and age of seizure onset in a pediatric epilepsy surgery cohort with cortical dysplasia. Epilepsy Res. 2021, 172, 106598. [Google Scholar] [CrossRef]
- Yankowitz, L.D.; Herrington, J.D.; Yerys, B.E.; Pereira, J.A.; Pandey, J.; Schultz, R.T. Evidence against the “normalization” prediction of the early brain overgrowth hypothesis of autism. Mol. Autism. 2020, 11, 51. [Google Scholar] [CrossRef]
- Guang, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Tachibana, M.; Rahman, S.; Kagitani-Shimono, K. Atypical structural connectivity of language networks in autism spectrum disorder: A meta-analysis of diffusion tensor imaging studies. Autism Res. Off. J. Int. Soc. Autism Res. 2022, 15, 1585–1602. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, K.; Lynn, A. Age differences and brain maturation provide insight into heterogeneous results in autism spectrum disorder. Front. Hum. Neurosci. 2022, 16, 957375. [Google Scholar] [CrossRef]
- Zhang, P.; Omanska, A.; Ander, B.P.; Gandal, M.J.; Stamova, B.; Schumann, C.M. Neuron-specific transcriptomic signatures indicate neuroinflammation and altered neuronal activity in ASD temporal cortex. Proc. Natl. Acad. Sci. USA 2023, 120, e2206758120. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.M.; Adams, J.B.; Anderson, A.L.; Frye, R.E. Rating of the Effectiveness of 26 Psychiatric and Seizure Medications for Autism Spectrum Disorder: Results of a National Survey. J. Child Adolesc. Psychopharmacol. 2019, 29, 107–123. [Google Scholar] [CrossRef]
- Licchetta, L.; Vignatelli, L.; Toni, F.; Teglia, A.; Belotti, L.M.B.; Ferri, L.; Menghi, V.; Mostacci, B.; Di Vito, L.; Bisulli, F.; et al. Long-term Outcome of Epilepsy and Cortical Malformations Due to Abnormal Migration and Postmigrational Development: A Cohort Study. Neurology 2022, 99, e23–e32. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Dobyns, W.B.; Barkovich, A.J. Abnormal development of the human cerebral cortex: Genetics, functional consequences and treatment options. Trends Neurosci. 2008, 31, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef]
- Shoffner, J.; Hyams, L.; Langley, G.N.; Cossette, S.; Mylacraine, L.; Dale, J.; Ollis, L.; Kuoch, S.; Bennett, K.; Aliberti, A.; et al. Fever plus mitochondrial disease could be risk factors for autistic regression. J. Child Neurol. 2010, 25, 429–434. [Google Scholar] [CrossRef]
- Singh, K.; Singh, I.N.; Diggins, E.; Connors, S.L.; Karim, M.A.; Lee, D.; Zimmerman, A.W.; Frye, R.E. Developmental regression and mitochondrial function in children with autism. Ann. Clin. Transl. Neurol. 2020, 7, 683–694. [Google Scholar] [CrossRef]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.; Adams, J.; Hanagan, K.; Kang, D.W.; Krajmalnik-Brown, R.; Hahn, J. Multivariate Analysis of Fecal Metabolites from Children with Autism Spectrum Disorder and Gastrointestinal Symptoms before and after Microbiota Transfer Therapy. J. Pers. Med. 2020, 10, 152. [Google Scholar] [CrossRef]
- Lim, J.M.; Letchumanan, V.; Tan, L.T.; Hong, K.W.; Wong, S.H.; Ab Mutalib, N.S.; Lee, L.H.; Law, J.W. Ketogenic Diet: A Dietary Intervention via Gut Microbiome Modulation for the Treatment of Neurological and Nutritional Disorders (a Narrative Review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef] [PubMed]
- Tilford, J.M.; Payakachat, N.; Kuhlthau, K.A.; Pyne, J.M.; Kovacs, E.; Bellando, J.; Williams, D.K.; Brouwer, W.B.; Frye, R.E. Treatment for Sleep Problems in Children with Autism and Caregiver Spillover Effects. J. Autism Dev. Disord. 2015, 45, 3613–3623. [Google Scholar] [CrossRef] [PubMed]
- Nobili, L.; Beniczky, S.; Eriksson, S.H.; Romigi, A.; Ryvlin, P.; Toledo, M.; Rosenzweig, I. Expert Opinion: Managing sleep disturbances in people with epilepsy. Epilepsy Behav. 2021, 124, 108341. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.M.; Soke, G.N.; Sabourin, K.R.; Hepburn, S.; Katz, T.; Wiggins, L.D.; Schieve, L.A.; Levy, S.E. Sleep Problems in 2-to 5-Year-Olds with Autism Spectrum Disorder and Other Developmental Delays. Pediatrics 2019, 143, e20180492. [Google Scholar] [CrossRef]
- Tammimies, K. Genetic mechanisms of regression in autism spectrum disorder. Neurosci. Biobehav. Rev. 2019, 102, 208–220. [Google Scholar] [CrossRef]
- Spagnoli, C.; Fusco, C.; Pisani, F. Rett Syndrome Spectrum in Monogenic Developmental-Epileptic Encephalopathies and Epilepsies: A Review. Genes 2021, 12, 1157. [Google Scholar] [CrossRef]
- Scheffer, I.E. Diagnosis and long-term course of Dravet syndrome. Eur. J. Paediatr. Neurol. 2012, 16 (Suppl. S1), S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Kahler, S.G. Approach to the Diagnosis of the Inborn Errors of Metabolism Associated with Epilepsy and their Clinical Genetics. In The Causes of Epilepsy: Common and Uncommon Causes in Adults and Children, 2nd ed.; Shorvon, S., Guerrini, R., Schachter, S., Trinka, E., Eds.; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Stöckler-Ipsiroglu, S.; Plecko, B. Metabolic epilepsies: Approaches to a diagnostic challenge. Can. J. Neurol. Sci. 2009, 36 (Suppl. S2), S67–S72. [Google Scholar] [PubMed]
- Muzio, M.R.; Cascella, M.; Al Khalili, Y. Landau-Kleffner Syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tassinari, C.A.; Rubboli, G.; Volpi, L.; Meletti, S.; d’Orsi, G.; Franca, M.; Sabetta, A.R.; Riguzzi, P.; Gardella, E.; Zaniboni, A.; et al. Encephalopathy with electrical status epilepticus during slow sleep or ESES syndrome including the acquired aphasia. Clin. Neurophysiol. 2000, 111 (Suppl. S2), S94–S102. [Google Scholar] [CrossRef] [PubMed]

| Antiepileptic Drugs | Other Seizure Treatments | Supplements | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | N | Effectiveness | Treatment | N | Effectiveness | Treatment | N | Effectiveness |
| Primidone | 7 | 5.29 (0.64) | Surgery | 17 | 5.41 (0.40) | GABA | 47 | 4.51 (0.158) |
| Valproic Acid | 247 | 5.21 (0.12) | Steroids | 33 | 5.09 (0.25) | Vitamin B6 | 82 | 4.51 (0.12) |
| Levetiracetam | 151 | 5.07 (0.14) | VNS | 25 | 4.80 (0.34) | Magnesium | 96 | 4.49 (0.08) |
| Lamotrigine | 171 | 4.91 (0.14) | IVIG | 15 | 4.27 (0.40) | L-carnosine | 29 | 4.41 (0.15) |
| Phenobarbital | 78 | 4.90 (0.21) | Neurofeedback | 14 | 4.21 (0.40) | Vit B12 | 111 | 4.37 (0.09) |
| Topiramate | 141 | 4.81 (0.13) | TMS/DCS | 2 | 4.00 (0.00) | Taurine | 60 | 4.35 (0.13) |
| Felbamate | 28 | 4.68 (0.33) | Spironolactone | 14 | 3.71 (0.32) | DMG | 50 | 4.30 (0.125) |
| Zonisamide | 58 | 4.67 (0.23) | CoQ10 | 69 | 4.29 (0.08) | |||
| Carbamazepine | 149 | 4.65 (0.16) | Diet therapies | Bacopa | 4 | 4.25 (0.25) | ||
| Oxcarbazepine | 125 | 4.59 (0.18) | Treatment | N | Effectiveness | Glutathione | 66 | 4.24 (0.10) |
| Tiagabine | 7 | 4.57 (0.30) | Ketogenic | 48 | 5.38 (0.21) | L-carnitine | 101 | 4.24 (0.09) |
| Phenytoin | 66 | 4.52 (0.19) | Atkins/MAD | 20 | 5.00 (0.33) | 5-HTP | 33 | 4.15 (0.13) |
| Ethosuximide | 27 | 4.44 (0.32) | SCD | 34 | 4.74 (0.21) | Alternative therapies | ||
| Clonazepam | 86 | 4.41 (0.19) | GFCF | 154 | 4.59 (0.08) | Treatment | N | Effectiveness |
| Vigabatrin | 10 | 4.40 (0.50) | HBOT | 44 | 4.39 (0.19) | |||
| Gabapentin | 43 | 3.79 (0.27) | Pioglitazone | 6 | 4.33 (0.21) | |||
| Chelation | 62 | 4.08 (0.16) | ||||||
| Minocycline | 1 | 4.00 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gundogdu, B.S.; Gaitanis, J.; Adams, J.B.; Rossignol, D.A.; Frye, R.E. Age-Related Changes in Epilepsy Characteristics and Response to Antiepileptic Treatment in Autism Spectrum Disorders. J. Pers. Med. 2023, 13, 1167. https://doi.org/10.3390/jpm13071167
Gundogdu BS, Gaitanis J, Adams JB, Rossignol DA, Frye RE. Age-Related Changes in Epilepsy Characteristics and Response to Antiepileptic Treatment in Autism Spectrum Disorders. Journal of Personalized Medicine. 2023; 13(7):1167. https://doi.org/10.3390/jpm13071167
Chicago/Turabian StyleGundogdu, Beliz Su, John Gaitanis, James B. Adams, Daniel A. Rossignol, and Richard E. Frye. 2023. "Age-Related Changes in Epilepsy Characteristics and Response to Antiepileptic Treatment in Autism Spectrum Disorders" Journal of Personalized Medicine 13, no. 7: 1167. https://doi.org/10.3390/jpm13071167
APA StyleGundogdu, B. S., Gaitanis, J., Adams, J. B., Rossignol, D. A., & Frye, R. E. (2023). Age-Related Changes in Epilepsy Characteristics and Response to Antiepileptic Treatment in Autism Spectrum Disorders. Journal of Personalized Medicine, 13(7), 1167. https://doi.org/10.3390/jpm13071167








