Surgical Site Infections in Glioblastoma Patients—A Retrospective Analysis
Abstract
1. Introduction
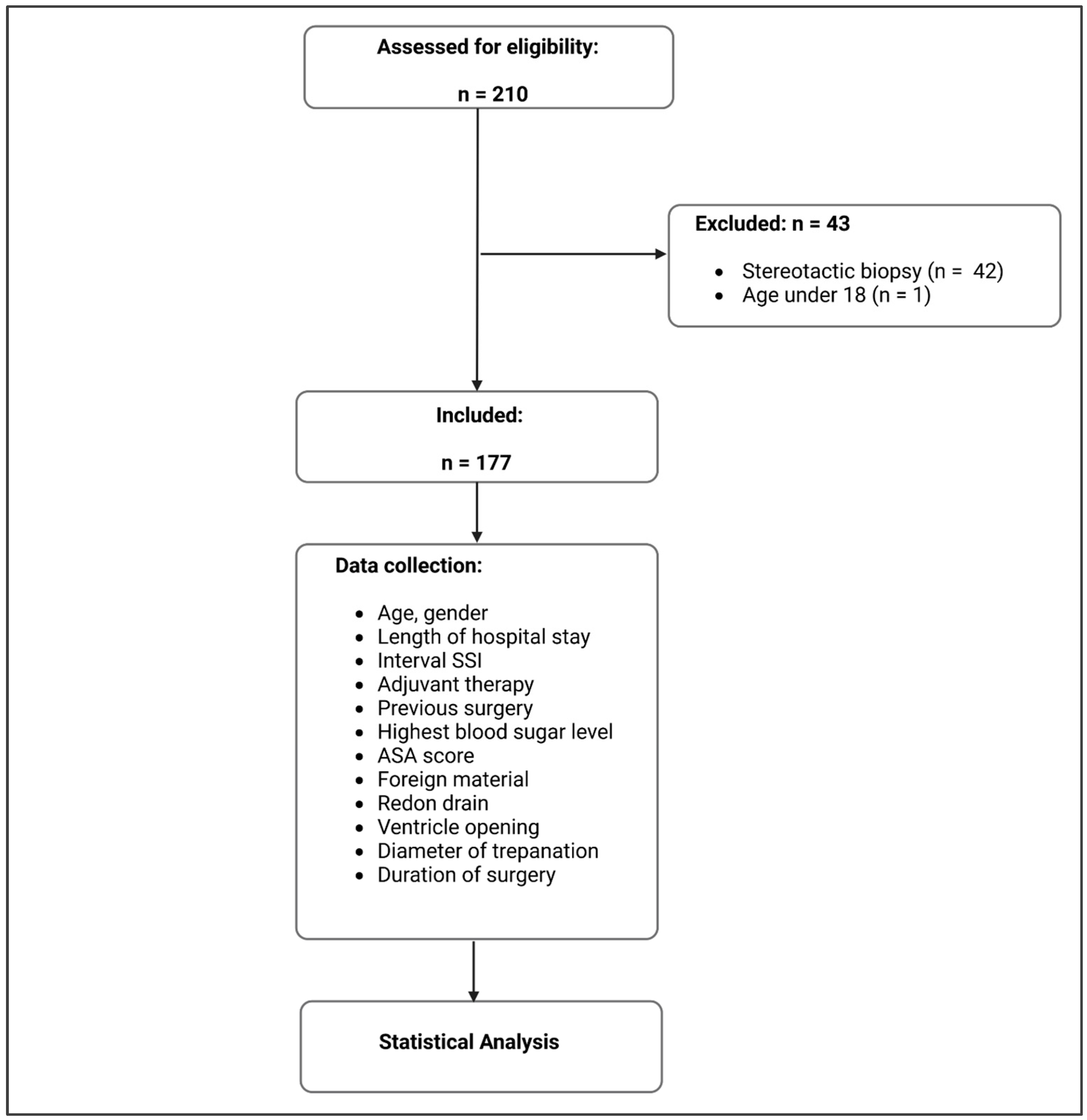
2. Methods
2.1. Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, C.; Zhu, T.; Zhang, P.; Xia, L.; Sun, C. Risk factors of neurosurgical site infection after craniotomy: A systematic review and meta-analysis. Am. J. Infect. Control 2017, 45, e123–e134. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. Available online: https://pubmed.ncbi.nlm.nih.gov/26948367/ (accessed on 1 July 2023).
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. Available online: https://pubmed.ncbi.nlm.nih.gov/15758009/ (accessed on 1 July 2023). [CrossRef] [PubMed]
- Ening, G.; Huynh, M.T.; Schmieder, K.; Brenke, C. Repeat-surgery at Glioblastoma recurrence, when and why to operate? Clin. Neurol. Neurosurg. 2015, 136, 89–94. [Google Scholar] [CrossRef]
- Gold, C.; Kournoutas, I.; Seaman, S.C.; Greenlee, J. Bone flap management strategies for postcraniotomy surgical site infection. Surg. Neurol. Int. 2021, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Ening, G.; Osterheld, F.; Capper, D.; Schmieder, K.; Brenke, C. Risk factors for glioblastoma therapy associated complications. Clin. Neurol. Neurosurg. 2015, 134, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; Botros, D.; Chakravarti, S.; Mao, Y.; Wu, E.; Lu, B.; Liu, S.; Elshareif, M.; Jackson, C.M.; Gallia, G.L.; et al. Predictors of surgical site infection in glioblastoma patients undergoing craniotomy for tumor resection. J. Neurosurg. 2022, 138, 1227–1234. [Google Scholar] [CrossRef]
- Jiménez-Martínez, E.; Cuervo, G.; Hornero, A.; Ciercoles, P.; Gabarrós, A.; Cabellos, C.; Pelegrin, I.; García-Somoza, D.; Adamuz, J.; Carratalà, J.; et al. Risk factors for surgical site infection after craniotomy: A prospective cohort study. Antimicrob. Resist. Infect. Control 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Akalin, E.; Doods, J.; Ewelt, C.; Stummer, W.; Suero Molina, E. When the Infection Hits the Wound: Matched Case-Control Study in a Neurosurgical Patient Collective Including Systematic Literature Review and Risk Factors Analysis. World Neurosurg. 2016, 95, 178–189. [Google Scholar] [CrossRef]
- Corsini Campioli, C.; Challener, D.; Comba, I.Y.; Shah, A.; Wilson, W.R.; Sohail, M.R.; Van Gompel, J.J.; O’Horo, J.C. Overview and risk factors for postcraniotomy surgical site infection: A four-year experience. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e14. [Google Scholar] [CrossRef]
- Korinek, A.M.; Golmard, J.L.; Elcheick, A.; Bismuth, R.; Van Effenterre, R.; Coriat, P.; Puybasset, L. Risk factors for neurosurgical site infections after craniotomy: A critical reappraisal of antibiotic prophylaxis on 4578 patients. Br. J. Neurosurg. 2005, 19, 155–162. [Google Scholar] [CrossRef]
- Dormand, E.-L.; Banwell, P.E.; Goodacre, T.E.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- ASA Physical Status Classification System. 2014. Available online: http://napanc.ca/assets/forms/appendices%202018.pdf (accessed on 3 April 2023).
- Salle, H.; Deluche, E.; Couvé-Deacon, E.; Beaujeux, A.-C.; Pallud, J.; Roux, A.; Dagain, A.; de Barros, A.; Voirin, J.; Seizeur, R.; et al. Surgical Site Infections after glioblastoma surgery: Results of a multicentric retrospective study. Infection 2020, 49, 267–275. [Google Scholar] [CrossRef]
- Seidelman, J.L.; Mantyh, C.R.; Anderson, D.J. Surgical Site Infection Prevention: A Review. JAMA 2023, 329, 244–252. [Google Scholar] [CrossRef]
- Patel, D.M.; Agarwal, N.; Tomei, K.L.; Hansberry, D.R.; Goldstein, I.M. Optimal Timing of Whole-Brain Radiation Therapy Following Craniotomy for Cerebral Malignancies. World Neurosurg. 2015, 84, 412–419. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, B.A.; Ubl, D.S.; Babu, M.; Maloney, P.; Murphy, M.; Kerezoudis, P.; Bydon, M.; Habermann, E.B.; Parney, I. Predictors of Surgical Site Infection Following Craniotomy for Intracranial Neoplasms: An Analysis of Prospectively Collected Data in the American College of Surgeons National Surgical Quality Improvement Program Database. World Neurosurg. 2016, 88, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, I.A.; Donoho, D.A.; Patel, A.; Lin, M.; Wen, T.; Ding, L.; Giannotta, S.L.; Mack, W.J.; Attenello, F. Predictors of Surgical Site Infection After Nonemergent Craniotomy: A Nationwide Readmission Database Analysis. World Neurosurg. 2018, 120, e440–e452. [Google Scholar] [CrossRef]
- Martin, E.T.; Kaye, K.S.; Knott, C.; Nguyen, H.; Santarossa, M.; Evans, R.; Bertran, E.; Jaber, L. Diabetes and Risk of Surgical Site Infection: A systematic review and meta-analysis. Infect. Control. Hosp. Epidemiol. 2015, 37, 88–99. [Google Scholar] [CrossRef]
- Ee, W.W.G.; Lau, W.L.J.; Yeo, W.; Von Bing, Y.; Yue, W.M. Does minimally invasive surgery have a lower risk of surgical site infections compared with open spinal surgery? Clin. Orthop. Relat. Res. 2014, 472, 1718–1724. [Google Scholar] [CrossRef]
- Mueller, K.; Zhao, D.; Johnson, O.; Sandhu, F.A.; Voyadzis, J.-M. The Difference in Surgical Site Infection Rates Between Open and Minimally Invasive Spine Surgery for Degenerative Lumbar Pathology: A Retrospective Single Center Experience of 1442 Cases. Oper. Neurosurg. 2019, 16, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Grundy, T.J.; Davies, B.M.; Patel, H.C. When should we measure surgical site infection in patients undergoing a craniotomy? A consideration of the current practice. Br. J. Neurosurg. 2020, 34, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, M.; de Ghellinck, L.; De Greef, J.; Di Santo, M.; Ribeiro Vaz, J.G.; Zech, F.; Belkhir, L. Outcome of Patients with Surgical Site Infection after Craniotomy. Surg. Infect. 2022, 23, 388–393. Available online: https://pubmed.ncbi.nlm.nih.gov/35333641/ (accessed on 3 April 2023). [CrossRef]
- Drewes, C.; Sagberg, L.M.; Jakola, A.S.; Solheim, O. Quality of life in patients with intracranial tumors: Does tumor laterality matter? J. Neurosurg. 2016, 125, 1400–1407. [Google Scholar] [CrossRef]
- Tunthanathip, T.; Sae-Heng, S.; Oearsakul, T.; Sakarunchai, I.; Kaewborisutsakul, A.; Taweesomboonyat, C. Machine learning applications for the prediction of surgical site infection in neurological operations. Neurosurg. Focus 2019, 47, E7. [Google Scholar] [CrossRef]
- Rucinska, M.; Nawrocki, S. COVID-19 Pandemic: Impact on Cancer Patients. Int. J. Environ. Res. Public Health 2022, 19, 12470. [Google Scholar] [CrossRef] [PubMed]
- Zaben, M.; Richards, A.; Merola, J.; Patel, C.; Leach, P. Surgical site infection in paediatric posterior fossa surgery: Does pathology matter? Childs Nerv. Syst. 2021, 37, 1859–1861. [Google Scholar] [CrossRef] [PubMed]

| SSIs | No SSI | p Value | Test | |
|---|---|---|---|---|
| Number | 14 (7.9%) | 163 (92.1%) | ||
| Female | 6 (42.9%) | 102 (62.6%) | 0.163 | Fisher |
| Age (years, mean, SD) | 59 (±11) | 63 (±11) | 0.180 | t-Test |
| Hospital stay (days, mean, SD) | 18 (±9) | 21 (±10) | 0.215 | t-Test |
| Interval SSI (days, mean, SD) | 45 (±41) | |||
| Previous surgery | 7 (50.0%) | 46 (28.2%) | 0.125 | Fisher |
| Chemotherapy | 10 (71.4%) | 105 (64.4%) | 0.775 | Fisher |
| Irradiation | 8 (57.1%) | 131 (80.4%) | 0.034 | Fisher |
| Interval irradiation (days, mean, SD) | 22 (±14) | 23 (±9) | 0.950 | t-Test |
| Highest blood glucose level (mmol/L, mean, SD) | 13 (±4) | 13 (±5) | 0.938 | t-Test |
| ASA score (mean, SD) | 2 (0–2) | 2 (0–2) | 0.930 | Fisher |
| Foreign material | 10 (71.4%) | 95 (58.3%) | 0.405 | Fisher |
| Subgaleal suction drain | 6 (42.9%) | 59 (36.2%) | 0.773 | Fisher |
| Ventricle opening | 6 (42.9%) | 54 (33.1%) | 0.558 | Fisher |
| Diameter of trepanation (mm, mean, SD) | 65 (±17) | 64 (±18) | 0.731 | t-Test |
| Duration of surgery (min, mean, SD) | 267 (±120) | 258 (±87) | 0.793 | t-Test |
| Number | Sex | Age (Years) | Stay (Days) | Interval SSI (Days) | Previous Surgery | Chemotherapy | Irradiation | Interval Irradiation (Days) | Highest Blood Glucose Level | ASA Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | female | 56 | 32 | 22 | no | no | no | 19.5 | 2 | |
| 2 | female | 52 | 33 | 65 | yes | yes | yes | 26 | 12.1 | 2 |
| 3 | female | 65 | 18 | 25 | no | no | yes | 23 | 12.0 | 3 |
| 4 | female | 76 | 35 | 16 | no | no | no | 18.9 | 3 | |
| 5 | male | 55 | 17 | 44 | no | yes | yes | 13 | 17.0 | 2 |
| 6 | male | 72 | 17 | 21 | no | yes | yes | 13 | 16.1 | 3 |
| 7 | male | 43 | 11 | 172 | no | yes | yes | 20 | 16.0 | 2 |
| 8 | female | 48 | 12 | 68 | yes | yes | no | 13.0 | 2 | |
| 9 | female | 50 | 10 | 63 | yes | yes | yes | 26 | 6.3 | 2 |
| 10 | male | 66 | 24 | 15 | no | yes | yes | 33 | 11.5 | 3 |
| 11 | male | 56 | 11 | 15 | yes | yes | no | 8.2 | 2 | |
| 12 | male | 71 | 10 | 35 | yes | no | no | 10.5 | 3 | |
| 13 | male | 71 | 10 | 24 | yes | yes | no | 14.0 | 2 | |
| 14 | male | 47 | 8 | 48 | yes | yes | yes | 47 | 8.3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheer, M.; Spindler, K.; Strauss, C.; Schob, S.; Dietzel, C.T.; Leisz, S.; Prell, J.; Rampp, S. Surgical Site Infections in Glioblastoma Patients—A Retrospective Analysis. J. Pers. Med. 2023, 13, 1117. https://doi.org/10.3390/jpm13071117
Scheer M, Spindler K, Strauss C, Schob S, Dietzel CT, Leisz S, Prell J, Rampp S. Surgical Site Infections in Glioblastoma Patients—A Retrospective Analysis. Journal of Personalized Medicine. 2023; 13(7):1117. https://doi.org/10.3390/jpm13071117
Chicago/Turabian StyleScheer, Maximilian, Kai Spindler, Christian Strauss, Stefan Schob, Christian T. Dietzel, Sandra Leisz, Julian Prell, and Stefan Rampp. 2023. "Surgical Site Infections in Glioblastoma Patients—A Retrospective Analysis" Journal of Personalized Medicine 13, no. 7: 1117. https://doi.org/10.3390/jpm13071117
APA StyleScheer, M., Spindler, K., Strauss, C., Schob, S., Dietzel, C. T., Leisz, S., Prell, J., & Rampp, S. (2023). Surgical Site Infections in Glioblastoma Patients—A Retrospective Analysis. Journal of Personalized Medicine, 13(7), 1117. https://doi.org/10.3390/jpm13071117






