Prognostic Impact of Non-Cardiac Comorbidities on Long-Term Prognosis in Patients with Reduced and Preserved Ejection Fraction following Acute Myocardial Infarction
Abstract
1. Introduction
2. Method
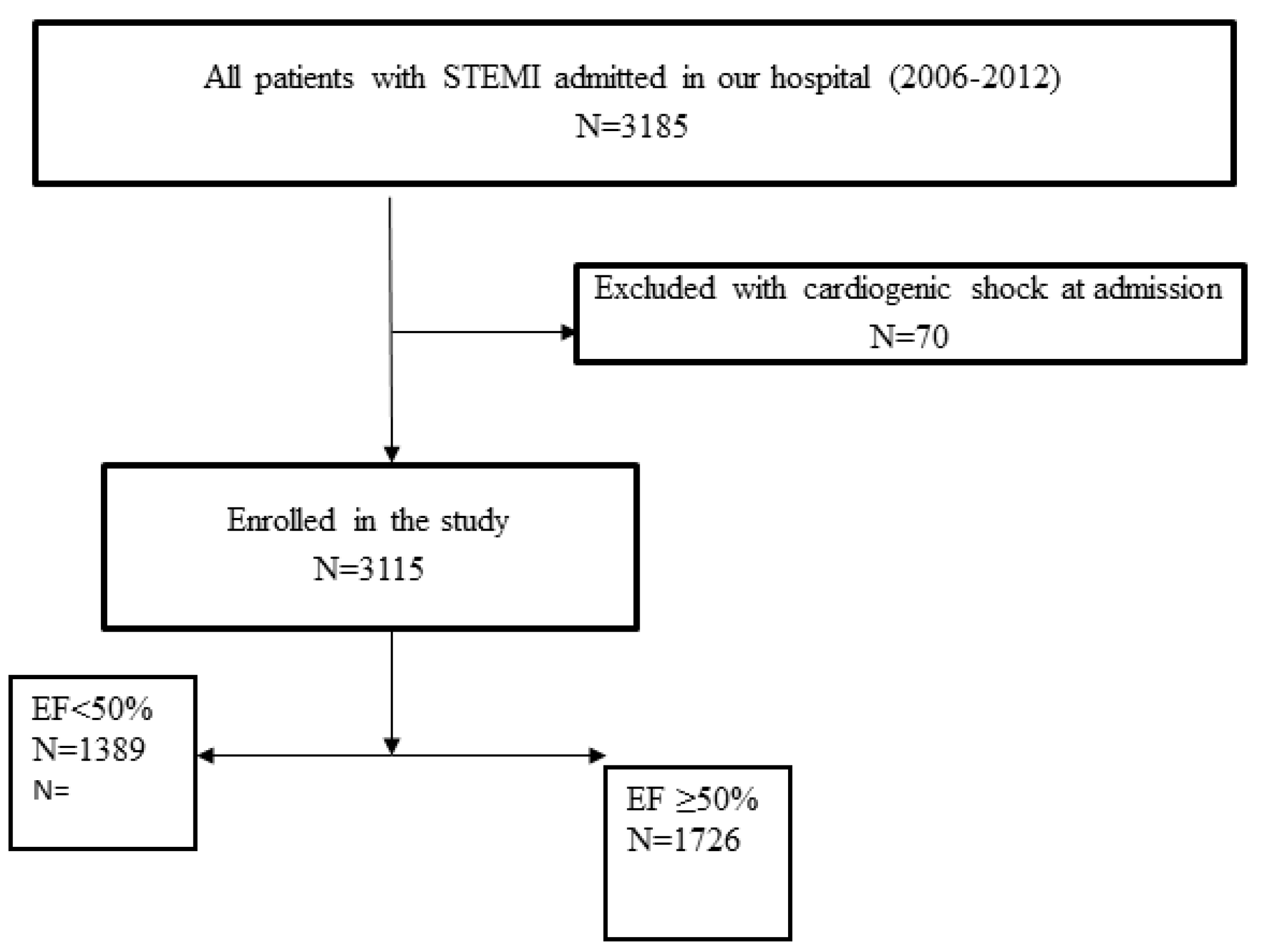
2.1. Study Population, Data Collection and Definitions
2.2. Ethics
2.3. Statistical Analysis
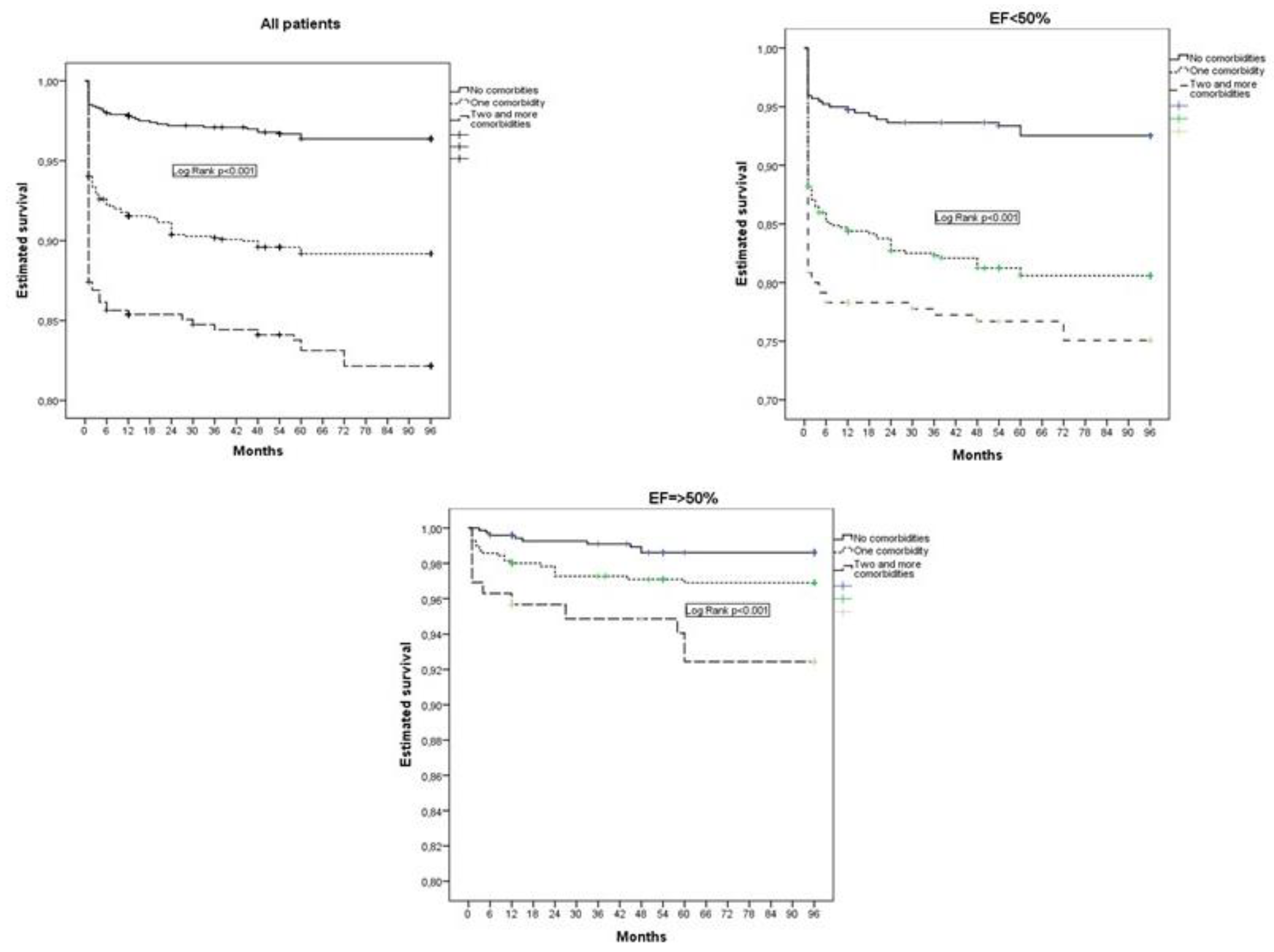
3. Results
4. Discussion
4.1. Non-Cardiac Comorbidities in Patients with Myocardial Infarction
4.2. Non-Cardiac Comorbidities in Relation to EF
4.3. Possible Mechanisms of the Negative Impact of Comorbidities on the Prognosis of Patients with AMI
4.4. Clinical Significance of Our Study
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savic, L.; Mrdovic, I.; Asanin, M.; Stankovic, S.; Krljanac, G.; Lasica, R. Prognostic impact of renal dysfunction on long-term mortality in patients with preserved, moderately impaired, and severely impaired left ventricular systolic function following myocardial infarction. Anatol. J. Cardiol. 2018, 20, 21–28. [Google Scholar] [CrossRef]
- Christensen, D.M.; Schjerning, A.-M.; Smedegaard, L.; Charlot, M.G.; Ravn, P.B.; Ruwald, A.C.; Fosbøl, E.; Køber, L.; Torp-Pedersen, C.; Schou, M.; et al. Long-term mortality, cardiovascular events, and bleeding in stable patients 1 year after myocardial infarction: A Danish nationwide study. Eur. Heart J. 2023, 44, 488–498. [Google Scholar] [CrossRef]
- Gili, M.; Sala, J.; López, J.; Carrión, A.; Béjar, L.; Moreno, J.; Rosales, Á.; Sánchez, G. Impact of comorbidities on in-hospital mortality from Acute myocardial infarction, 2003–2009. Rev. Esp. Cardiol. 2011, 64, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Horváth-Puhó, E.; Ording, A.G.; Bøtker, H.E.; Lash, T.L.; Sørensen, H.T. The interaction effect of cardiac and non-cardiac comorbidity on myocardial infarction mortality: A nationwide cohort study. Int. J. Cardiol. 2020, 308, 1–8. [Google Scholar] [CrossRef]
- Munyombwe, T.; Dondo, T.B.; Aktaa, S.; Wilkinson, C.; Hall, M.; Hurdus, B.; Oliver, G.; West, R.M.; Hall, A.S.; Gale, C.P. Association of multimorbidity and changes in health-related quality of life following myocardial infarction: A UK multicentre longitudinal patient-reported outcomes study. BMC Med. 2021, 19, 227. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Zomer, E.; Chin, K.L.; Markey, P.; Si, S.; Ademi, Z.; Curtis, A.J.; Zoungas, S.; Liew, D. Prevalence and impact of non-cardiovascular comorbidities among older adults hospitalized for non-ST segment elevation Acute coronary syndrome. Cardiovasc. Diagn. Ther. 2019, 9, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Rothnie, K.; Bloom, C.; Zakeri, R.; Sahadevan, J.; Singh, A.; Nagai, T.; Potts, J.; Wedzicha, J.; Smeeth, L.; et al. Impact of comorbidities on peak troponin levels and mortality in Acute myocardial infarction. Heart 2020, 106, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Rapsomaniki, E.; Thuresson, M.; Yang, E.; Blin, P.; Hunt, P.; Chung, S.C.; Stogiannis, D.; Pujades-Rodriguez, M.; Timmis, A.; Denaxas, S.C.; et al. Using big data from health records from four countries to evaluate chronic disease outcomes: A study in 114 364 survivors of myocardial infarction. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 2, 172–183. [Google Scholar] [CrossRef]
- Schmidt, M.; Jacobsen, J.B.; Lash, T.L.; Bøtker, H.E.; Sørensen, H.T. 25 year trends in first time hospitalisation for Acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: A Danish nationwide cohort study. BMJ 2012, 344, e356. [Google Scholar] [CrossRef]
- Yadegarfar, M.E.; Gale, C.P.; Dondo, T.B.; Wilkinson, C.G.; Cowie, M.R.; Hall, M. Association of treatments for Acute myocardial infarction and survival for seven common comorbidity states: A nationwide cohort study. BMC Med. 2020, 18, 231. [Google Scholar] [CrossRef]
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and morbidity trends after the first year in survivors of Acute myocardial infarction: A systematic review. BMC Cardiovasc. Disord. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.G.; Lansky, A.J.; Meller, S.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.; Shah, R.; Mehran, R.; Stone, G.W. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction:the HORIZONS-AMI trial. Eur. Heart J. Acute Cardiovasc. Care. 2014, 3, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mrdovic, I.; Savic, L.; Lasica, R.; Krljanac, G.; Asanin, M.; Brdar, N.; Djuricic, N.; Marinkovic, J.; Perunicic, J. Efficacy and safety of tirofiban-supported primary percutaneous coronary intervention in patients pretreated with 600 mg clopidogrel: Results of propensity analysis using the clinical center of serbia STEMI register. Eur. Heart J. Acute Cardiovasc. Care. 2014, 3, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lawler, P.R.; Filion, K.B.; Dourian, T.; Atallah, R.; Garfinkle, M.; Eisenberg, M.J. Anemia and mortality in Acute coronary syndromes: A systematic review and meta-analysis. Am. Heart J. 2013, 165, 143–153.e5. [Google Scholar] [CrossRef] [PubMed]
- Breen, K.; Finnegan, L.; Vuckovic, K.; Fink, A.; Rosamond, W.; DeVon, H.A. Multimorbidity in Patients With Acute Coronary Syndrome Is Associated With Greater Mortality, Higher Readmission Rates, and Increased Length of Stay: A Systematic Review. J. Cardiovasc. Nurs. 2020, 35, E99–E110. [Google Scholar] [CrossRef]
- Rashid, M.; Kwok, C.S.; Gale, C.P.; Doherty, P.; Olier, I.; Sperrin, M.; Kontopantelis, E.; Peat, G.; Mamas, M.A. Impact of co-morbid burden on mortality in patients with coronary Heart disease, Heart failure, and cerebrovascular accident: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2017, 3, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.; Jaguszewski, M.; Nallamothu, B.N.; Lüscher, T.F.; Urban, P.; Pedrazzini, G.B.; Erne, P.; Radovanovic, D.; AMIS Plus Investigators. Acute multivessel revascularization improves 1-year outcome in ST-elevation myocardial infarction: A nationwide study cohort from the AMIS Plus registry. Int. J. Cardiol. 2014, 172, 76–81. [Google Scholar] [CrossRef]
- Sanchis, J.; Núñez, J.; Bodí, V.; Núñez, E.; García-Alvarez, A.; Bonanad, C.; Regueiro, A.; Bosch, X.; Heras, M.; Sala, J.; et al. Influence of comorbid conditions on one-year outcomes in non-ST-segment elevation Acute coronary syndrome. Mayo Clin. Proc. 2011, 86, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Senni, M.; Barbati, G.; Greene, S.J.; Poli, S.; Zambon, E.; Di Nora, C.; Cioffi, G.; Tarantini, L.; Gavazzi, A.; et al. Prevalence and prognostic impact of non-cardiac co-morbidities in Heart failure outpatients with preserved and reduced ejection fraction: A community-based study. Eur. J. Heart Fail. 2018, 20, 1257–1266. [Google Scholar] [CrossRef]
- van Deursen, V.M.; Urso, R.; Laroche, C.; Damman, K.; Dahlström, U.; Tavazzi, L.; Maggioni, A.P.; Voors, A.A. Co-morbidities in patients with Heart failure: An analysis of the European Heart Failure Pilot Survey. Eur. J. Heart Fail. 2014, 16, 103–111. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Wu, B.; Lu, J.; Xiu, J.; Tu, J.; Chen, S.; Pan, Y.; Bao, K.; Wang, J.; et al. Predictors of mortality in Heart failure with reduced ejection fraction: Interaction between diabetes mellitus and impaired renal function. Int. Urol. Nephrol. 2023; ahead of print. [Google Scholar] [CrossRef]
- ter Maaten, J.M.; Damman, K.; Verhaar, M.C.; Paulus, W.J.; Duncker, D.J.; Cheng, C.; Heerebeek, L.; Hillege, H.L.; Lam, C.S.; Navis, G.; et al. Connecting Heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur. J. Heart Fail. 2016, 18, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Prud’Homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.-M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction Through the Galectin-3 Pathway. JACC Basic. Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.Y.; Chin, L.H.; Tsai, C.S.; Lin, F.Z.; Chen, Y.H.; Chang, Y.L.; Huang, S.M.; Chen, Y.C.; Lin, C.Y. Cardiac calcium dysregulation in mice with chronic kidney disease. J. Cell Mol. Med. 2020, 24, 3669–3677. [Google Scholar] [CrossRef]
- Jernberg, T.; Hasvold, P.; Henriksson, M.; Hjelm, H.; Thuresson, M.; Janzon, M. Cardiovascular risk in post-myocardial infarction patients: Nationwide real world data demonstrate the importance of a long-term perspective. Eur. Heart J. 2015, 36, 1163–1170. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef]
- Moukarbel, G.V.; Yu, Z.-F.; Dickstein, K.; Hou, Y.; Wittes, J.T.; McMurray, J.J.; Pitt, B.; Zannad, F.; Pfeffer, M.A.; Solomon, S.D. The impact of kidney function on outcomes following high risk myocardial infarction:findings from 27,610 patients. Eur. J. Heart Fail. 2014, 16, 289–299. [Google Scholar] [CrossRef]
- Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with Heart failure with presserved or reduced left ventricular ejection fraction; an individual patient data meta-analysis. Eur. Heart J. 2012, 33, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Søholm, H.; Lønborg, J.; Andersen, M.J.; Vejlstrup, N.; Engstrøm, T.; Møller, J.E.; Hassager, C. Repeated echocardiography after first ever ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention–is it necessary? Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 528–536. [Google Scholar] [CrossRef]
| Characteristics | All Patients N = 3115 | Reduced EF N = 1389 | Preserved EF N = 1726 | p Value (Reduced vs. Preserved EF) |
|---|---|---|---|---|
| Age, years med(IQR) | 60 (52, 59) | 61 (54, 71) | 57 (50, 67.5) | <0.001 |
| Female, n (%) | 867 (27.8) | 407 (29.3) | 460 (26.7) | 0.160 |
| Previous MI, n (%) | 327 (10.5) | 193 (13.9) | 134 (7.8) | <0.001 |
| Previous PCI, n (%) | 84 (2.7) | 57 (4.1) | 27 (1.6) | <0.001 |
| Previous CABG, n (%) | 52 (1.7) | 28 (2) | 24 (1.4) | 0.176 |
| Hypertension, n (%) | 2089 (67.1) | 967 (69.1) | 1122 (62.5) | 0.012 |
| HLP, n (%) | 1890 (60.7) | 814 (58.7) | 1076 (62.3) | 0.034 |
| Smoking, n (%) | 1656 (53.2) | 648 (46.7) | 1008 (58.4) | <0.001 |
| Pain duration, hours med(IQR) | 2.5 (1.5, 4) | 3 (1.5, 4) | 2.5 (1.5, 4) | 0.091 |
| Atrial fibrillation on initial ECG, n (%) | 215 (6.9) | 160 (11.5) | 55 (3.2) | <0.001 |
| Complete AV block, n (%) | 144 (4.6) | 75 (5.4) | 68 (3.9) | 0.081 |
| Killip class > 1, n (%) | 390 (12.5) | 349 (25.1) | 41 (2.4) | <0.001 |
| Systolic BP at admission, med(IQR) | 135 (120, 150) | 130 (120, 150) | 140 (120, 150) | <0.001 |
| Heart rate at admission med(IQR) | 78 (70, 90) | 80 (70, 96) | 75 (66, 82) | 0.005 |
| BBB on initial ECG, n (%) | 119 (3.8) | 85 (6.2) | 134 (7.8) | <0.001 |
| Multivessel disease, n (%) | 1763 (56.6) | 868 (62.5) | 895 (51.9) | <0.001 |
| Three-vessel disease, n (%) | 837 (27.9) | 436 (31.4) | 401 (23.2) | <0.001 |
| LM stenosis, n (%) | 191 (6.1) | 108 (7.8) | 83 (4.8) | <0.001 |
| Pre-procedural flow TIMI 0, n (%) | 2148 (69) | 1031 (74.2) | 1113 (64.8) | <0.001 |
| Post-procedural flow TIMI < 3, n (%) | 146 (4.7) | 109 (7.8) | 37 (2.1) | <0.001 |
| CK MB, med (IQR) | 1869 (986, 3475) | 2671 (1333, 4671) | 1529 (877, 2779) | <0.001 |
| eGFR, med (IQR) | 90.3 (69.6, 110.6) | 83.2 (64.1, 104.5) | 93.6 (75.1, 114) | <0.001 |
| EF, med(IQR) | 50 (40, 55) | 40 (35, 45) | 55 (50, 58) | <0.001 |
| Non-cardiac comorbidities, n (%) | 1014 (32.5) | 541 (38.9) | 473 (27.4) | <0.001 |
| One comorbidity, n (%) | 565 (18.1) | 279 (20) | 286 (16.6) | <0.001 |
| Two or more comorbidities, n (%) | 449 (14.4) | 262 (18.9) | 187 (10.9) | <0.001 |
| Diabetes, n (%) | 610 (19.6) | 330 (23.7) | 280 (16.2) | <0.001 |
| CKD, n (%) | 489 (15.7) | 292 (21) | 197 (11.4) | <0.001 |
| Obesity, n (%) | 490 (15.7) | 206 (14.9) | 284 (16.3) | 0.047 |
| Anaemia, n (%) | 250 (8) | 138 (9.1) | 112 (6.5) | <0.001 |
| Previous stroke, n (%) | 126 (4) | 71 (5.1) | 55 (3.2) | 0.007 |
| COPD, n (%) | 40 (1.3) | 12 (0.8) | 28 (1.6) | <0.001 |
| PAD, n (%) | 29 (0.9) | 18 (1.3) | 11 (0.6) | 0.469 |
| Peptic ulcer disease, n (%) | 14 (0.4) | 8 (0.5) | 6 (0.3) | <0.001 |
| Liver disease, n (%) | 3 (0.09) | 0 | 3 (0.2) | <0.001 |
| Psychiatric disorder, n (%) | 2 (0.09) | 1 (0.07) | 1 (0.05) | 0.123 |
| Carcinoma, n (%) | 9 (0.2) | 5 (0.3) | 4 (0.2) | 0.154 |
| Therapy at discharge * | ||||
| Beta blockers, n (%) | 2987 (85.5) | 1211 (95.9) | 1647 (95.8) | 0.895 |
| ACE inhibitors, n (%) | 2784 (89.4) | 1034 (74.4) | 1370 (79.4) | 0.001 |
| Statin, n (%) | 3033 (97.4) | 1300 (93.6) | 1725 (99.9) | 0.004 |
| Diuretic, n (%) | 478 (15.3) | 385 (27.2) | 93 (5.4) | <0.001 |
| Amiodarone, n (%) | 82 (2.6) | 42 (3.1) | 40 (2.4) | 0.211 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| All Patients | ||||
| Age (years) | 1.04 (1.02–1.05) | <0.001 | 1.04 (1.02–1.05) | <0.001 |
| Previous infarction | 1.07 (0.83–1.55) | 0.730 | ||
| Systolic blood pressure at admission mmHg | 0.99 (0.99–1.00) | 0.190 | ||
| Heart rate at admission | 1.0 (0.99–1.0) | 0.691 | ||
| CK max | 1.00 (1.00–1.01) | 0.802 | ||
| EF < 50% | 3.66 (2.49–5.41) | <0.001 | 2.28 (2.28–4.07) | <0.001 |
| Killip > 1 at admission | 2.59 (1.81–3.29) | <0.001 | 2.14 (1.51–3.05) | <0.001 |
| Pre-procedural TIMI 0 | 1.25 (0.79–1.95) | 0.336 | ||
| Post-procedural TIMI < 3 | 2.30 (1.58–3.32) | <0.001 | 2.0 (1.10–3.66) | 0.023 |
| Acute bundle branch block | 1.62 (1.15–2.20) | 0.020 | ||
| Atrial fibrillation on initial ECG | 1.55 (1.12–1.99) | 0.032 | ||
| Complete AV block at admission | 2.07 (1.06–4.24) | 0.033 | 1.97 (1.06–2.08) | 0.042 |
| Three-vessel disease | 1.51 (1.14–1.99) | 0.040 | ||
| Diabetes mellitus | 2.08 (1.30–2.78) | 0.010 | 1.79 (1.18–2.48) | 0.015 |
| CKD | 2.68 (1.37–3.68) | <0.001 | 1.69 (1.17–2.68) | 0.010 |
| Anemia | 1.42 (1.12–1.98) | 0.054 | ||
| Preserved EF ≥ 50% | ||||
| Age (years) | 1.03 (1.01–1.07) | 0.050 | 1.04 (1.01–1.08) | 0.040 |
| Systolic blood pressure at admission mmHg | 1.0 (0.98–1.01) | 0.721 | ||
| Heart rate at admission | 1.01 (0.98–1.04) | 0.091 | ||
| CK max | 1.0 (1.0–1.01) | 0.779 | ||
| Killip > 1 at admission | 1.32 (1.08–3.65) | 0.053 | ||
| Three-vessel disease | 1.25 (0.69–2.64) | 0.071 | ||
| Pre-procedural TIMI 0 | 0.89 (0.85–1.28) | 0.861 | ||
| Post-procedural TIMI < 3 | 2.91 (0.99–12.3) | 0.055 | ||
| Diabetes mellitus | 2.24 (1.11–4.64) | 0.027 | 1.93 (1.21–3.75) | 0.032 |
| CKD | 1.56 (1.28–2.24) | 0.017 | 1.55 (1.07–2.23) | 0.047 |
| Anemia | 1.39 (1.02–2.89) | 0.051 | ||
| Reduced EF < 50% | ||||
| Age (years) | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 |
| Previous infarction | 1.59 (1.11–1.97) | 0.072 | ||
| Systolic blood pressure at admission, mmHg | 0.99 (0.98–0.99) | 0.056 | ||
| Heart rate at admission | 1.0 (0.99–1.0) | 0.131 | ||
| CK max | 1.0 (0.99–1.01) | 0.269 | ||
| Pre-procedural TIMI = 0 | 1.23 (0.78–2.08) | 0.423 | ||
| Post-procedural TIMI < 3 | 4.42 (3.12–6.09) | <0.001 | 2.49 (1.80–3.28) | <0.001 |
| Killip > 1 at admission | 3.68 (2.81–4.23) | <0.001 | 2.40 (1.90–3.74) | <0.001 |
| Atrial fibrillation on initial ECG | 1.49 (1.11–2.09) | 0.030 | ||
| Acute bundle branch block | 1.89 (1.28–2.38) | 0.020 | ||
| Three-vessel disease | 1.51 (1.11–2.04) | 0.008 | 1.71 (1.30–2.18) | 0.035 |
| Diabetes mellitus | 1.69 (1.22–2.19) | 0.001 | ||
| CKD | 3.20 (2.14–4.35) | <0.001 | 2.09 (1.18–2.52) | 0.030 |
| Anemia | 2.23 (1.56–3.20) | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savic, L.; Mrdovic, I.; Asanin, M.; Stankovic, S.; Lasica, R.; Matic, D.; Simic, D.; Krljanac, G. Prognostic Impact of Non-Cardiac Comorbidities on Long-Term Prognosis in Patients with Reduced and Preserved Ejection Fraction following Acute Myocardial Infarction. J. Pers. Med. 2023, 13, 1110. https://doi.org/10.3390/jpm13071110
Savic L, Mrdovic I, Asanin M, Stankovic S, Lasica R, Matic D, Simic D, Krljanac G. Prognostic Impact of Non-Cardiac Comorbidities on Long-Term Prognosis in Patients with Reduced and Preserved Ejection Fraction following Acute Myocardial Infarction. Journal of Personalized Medicine. 2023; 13(7):1110. https://doi.org/10.3390/jpm13071110
Chicago/Turabian StyleSavic, Lidija, Igor Mrdovic, Milika Asanin, Sanja Stankovic, Ratko Lasica, Dragan Matic, Damjan Simic, and Gordana Krljanac. 2023. "Prognostic Impact of Non-Cardiac Comorbidities on Long-Term Prognosis in Patients with Reduced and Preserved Ejection Fraction following Acute Myocardial Infarction" Journal of Personalized Medicine 13, no. 7: 1110. https://doi.org/10.3390/jpm13071110
APA StyleSavic, L., Mrdovic, I., Asanin, M., Stankovic, S., Lasica, R., Matic, D., Simic, D., & Krljanac, G. (2023). Prognostic Impact of Non-Cardiac Comorbidities on Long-Term Prognosis in Patients with Reduced and Preserved Ejection Fraction following Acute Myocardial Infarction. Journal of Personalized Medicine, 13(7), 1110. https://doi.org/10.3390/jpm13071110







