Fractionated Stereotactic Radiotherapy with Helical Tomotherapy for Brain Metastases: A Mono-Institutional Experience
Abstract
1. Introduction
2. Methods
Statistical Analysis
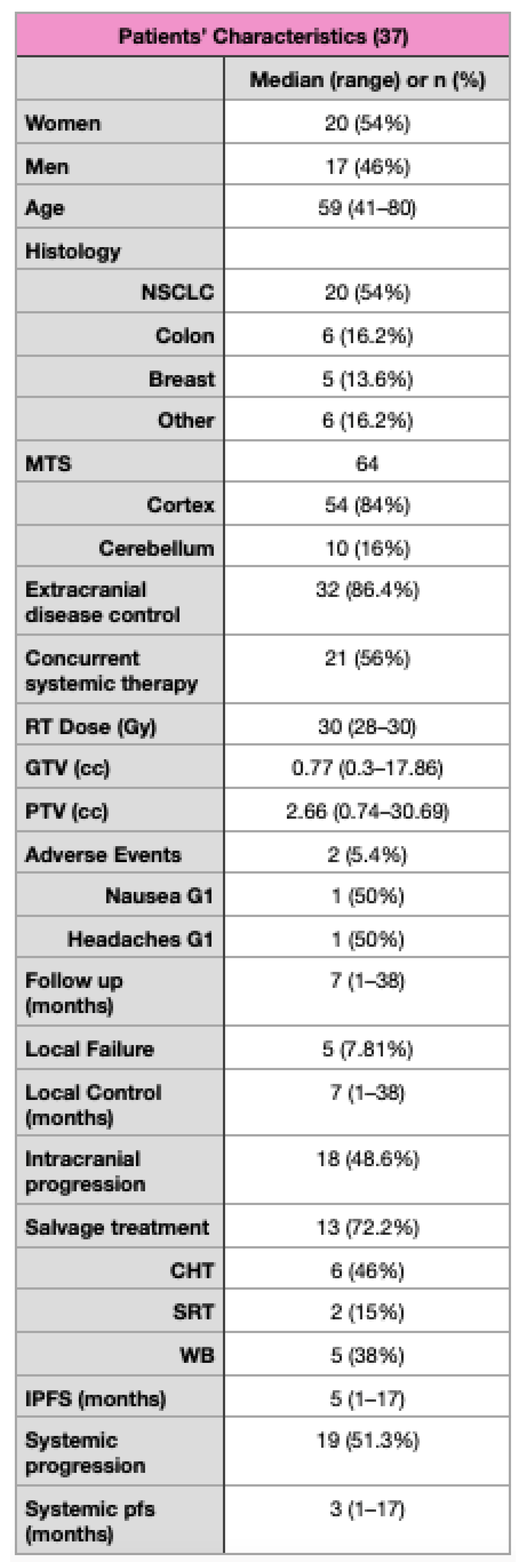
3. Results
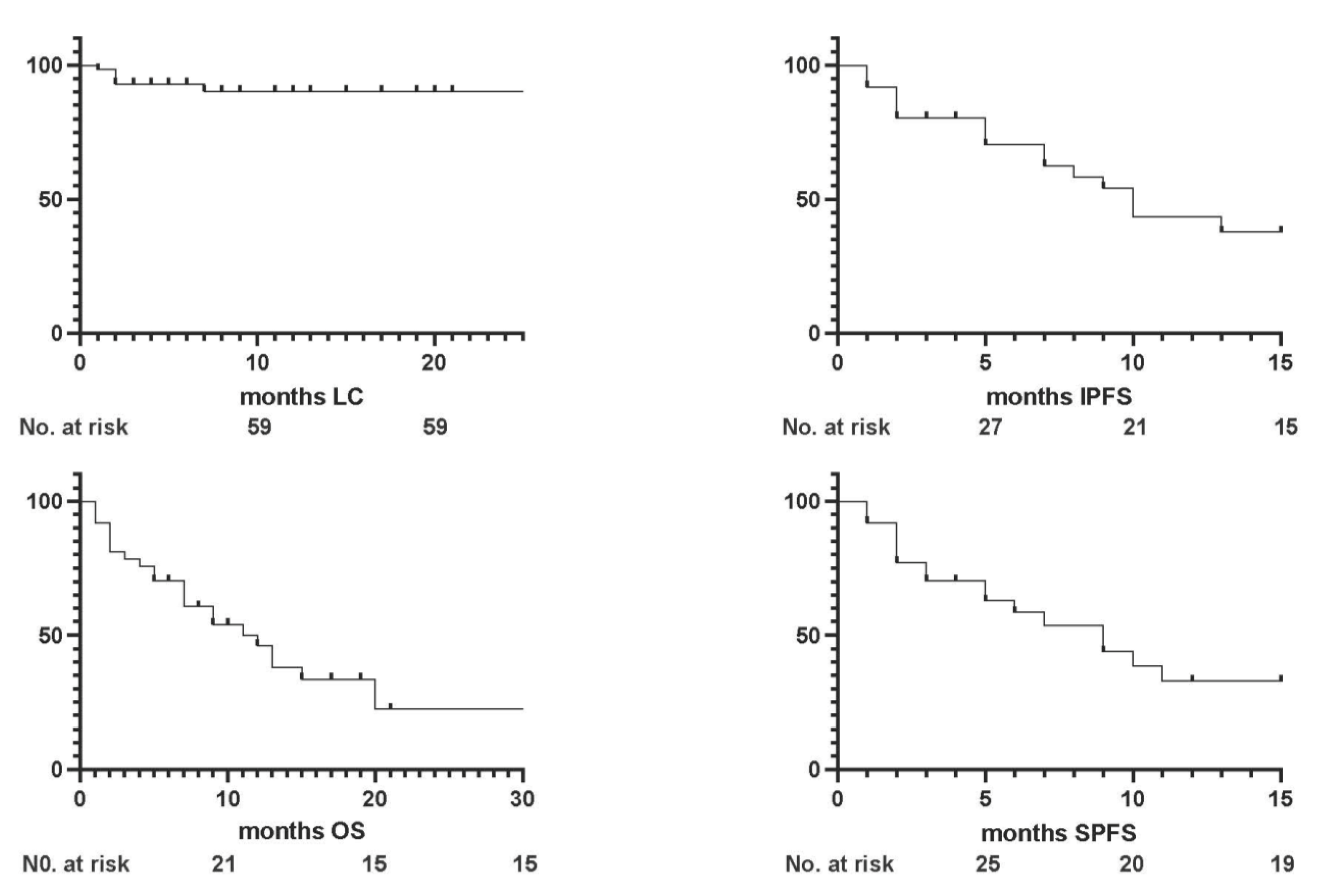
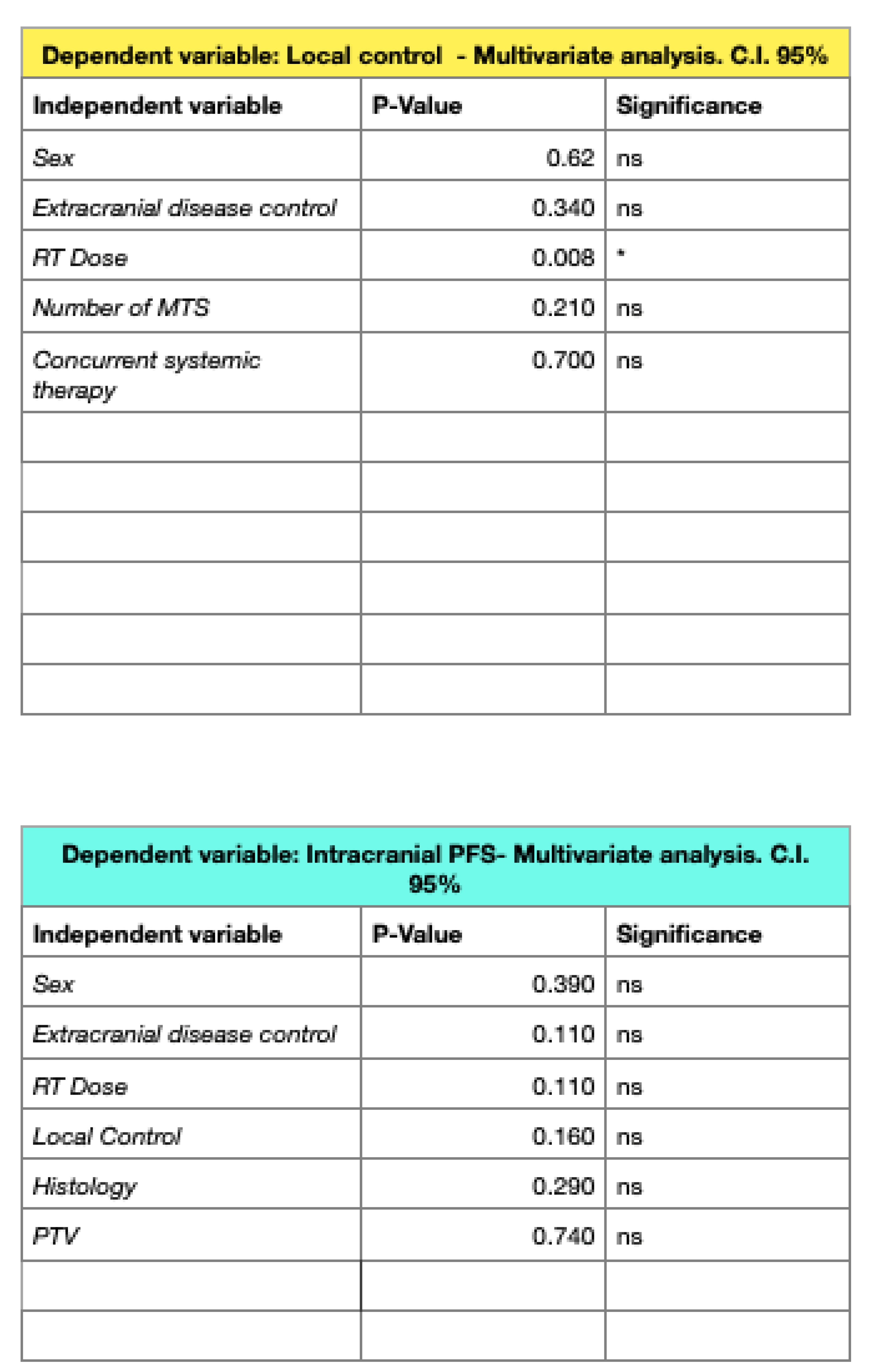
3.1. Survival Outcomes
3.2. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, M.N.; Xu, W.; Wong, R.K.; Lloyd, N.; Laperriere, N.; Sahgal, A.; Rakovitch, E.; Chow, E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2018, 1, CD003869. [Google Scholar] [CrossRef]
- Lassman, A.B.; DeAngelis, L.M. Brain metastases. Neurol. Clin. 2003, 21, 1–23, vii. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.C.; Taksler, G.B.; Kuremsky, J.G.; Lucas, J.T., Jr.; Ayala-Peacock, D.N.; Randolph, D.M., II; Bourland, J.D.; Laxton, A.W.; Tatter, S.B.; Chan, M.D. Clinical and economic outcomes of patients with brain metastases based on symptoms: An argument for routine brain screening of those treated with upfront radiosurgery. Cancer 2014, 120, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Lanier, C.M.; Hughes, R.; Ahmed, T.; LeCompte, M.; Masters, A.H.; Petty, W.J.; Ruiz, J.; Triozzi, P.; Su, J.; O’Neill, S.; et al. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neurooncol. Pract. 2019, 6, 402–409. [Google Scholar] [CrossRef]
- Mu, F.; Lucas, J.T., Jr.; Watts, J.M.; Johnson, A.J.; Daniel Bourland, J.; Laxton, A.W.; Chan, M.D.; Tatter, S.B. Tumor resection with carmustine wafer placement as salvage therapy after local failure of radiosurgery for brain metastasis. J. Clin. Neurosci. 2015, 22, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Das, S.; Larson, D.A.; Sahgal, A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget 2016, 7, 12318–12330. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann. Neurol. 1993, 33, 583–590. [Google Scholar] [CrossRef]
- Noordijk, E.M.; Vecht, C.J.; Haaxma-Reiche, H.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 711–717. [Google Scholar] [CrossRef]
- Finkelstein, S.E.; Timmerman, R.; McBride, W.H.; Schaue, D.; Hoffe, S.E.; Mantz, C.A.; Wilson, G.D. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin. Dev. Immunol. 2011, 2011, 439752. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., II; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409, Erratum in JAMA 2018, 320, 510. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Palmer, J.D.; Klamer, B.G.; Ballman, K.V.; Brown, P.D.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; et al. Association of Long-term Outcomes with Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial. JAMA Oncol. 2022, 20, e225049. [Google Scholar] [CrossRef]
- Soike, M.H.; Hughes, R.T.; Farris, M.; McTyre, E.R.; Cramer, C.K.; Bourland, J.D.; Chan, M.D. Does Stereotactic Radiosurgery Have a Role in the Management of Patients Presenting With 4 or More Brain Metastases? Neurosurgery 2019, 84, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.T.; Masters, A.H.; McTyre, E.R.; Farris, M.K.; Chung, C.; Page, B.R.; Kleinberg, L.R.; Hepel, J.; Contessa, J.N.; Chiang, V.; et al. Initial SRS for Patients with 5 to 15 Brain Metastases: Results of a Multi-Institutional Experience. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Sarmey, N.; Kaisman-Elbaz, T.; Mohammadi, A.M. Management Strategies for Large Brain Metastases. Front. Oncol. 2022, 12, 827304. [Google Scholar] [CrossRef]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Ayala-Peacock, D.N.; Peiffer, A.M.; Lucas, J.T.; Isom, S.; Kuremsky, J.G.; Urbanic, J.J.; Bourland, J.D.; Laxton, A.W.; Tatter, S.B.; Shaw, E.G.; et al. A nomogram for predicting distant brain failure in patients treated with gamma knife stereotactic radiosurgery without whole brain radiotherapy. Neuro-Oncology 2014, 16, 1283–1288. [Google Scholar] [CrossRef]
- Gorovets, D.; Ayala-Peacock, D.; Tybor, D.J.; Rava, P.; Ebner, D.; Cielo, D.; Norén, G.; Wazer, D.E.; Chan, M.; Hepel, J.T. Multi-institutional Nomogram Predicting Survival Free from Salvage Whole Brain Radiation After Radiosurgery in Patients with Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 246–253. [Google Scholar] [CrossRef] [PubMed]
- McTyre, E.; Ayala-Peacock, D.; Contessa, J.; Corso, C.; Chiang, V.; Chung, C.; Fiveash, J.; Ahluwalia, M.; Kotecha, R.; Chao, S.; et al. Multi-institutional competing risks analysis of distant brain failure and salvage patterns after upfront radiosurgery without whole brain radiotherapy for brain metastasis. Ann. Oncol. 2018, 29, 497–503. [Google Scholar] [CrossRef]
- Tomita, N.; Kodaira, T.; Tachibana, H.; Nakamura, T.; Nakahara, R.; Inokuchi, H.; Shibamoto, Y. Helical tomotherapy for brain metastases: Dosimetric evaluation of treatment plans and early clinical results. Technol. Cancer Res. Treat. 2008, 7, 417–424. [Google Scholar] [CrossRef]
- Rodrigues, G.; Yartsev, S.; Yaremko, B.; Perera, F.; Dar, A.R.; Hammond, A.; Lock, M.; Yu, E.; Ash, R.; Caudrelier, J.M.; et al. Phase I trial of simultaneous in-field boost with helical tomotherapy for patients with one to three brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1128–1133. [Google Scholar] [CrossRef]
- Kirova, Y.M.; Chargari, C.; Zefkili, S.; Campana, F. Could helical tomotherapy do whole brain radiotherapy and radiosurgery? World J. Radiol. 2010, 2, 148–150. [Google Scholar] [CrossRef]
- Levegrün, S.; Pöttgen, C.; Wittig, A.; Lübcke, W.; Abu Jawad, J.; Stuschke, M. Helical tomotherapy for whole-brain irradiation with integrated boost to multiple brain metastases: Evaluation of dose distribution characteristics and comparison with alternative techniques. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 734–742. [Google Scholar] [CrossRef]
- Vanderspek, L.; Bauman, G.; Wang, J.Z.; Yartsev, S.; Ménard, C.; Cho, Y.B.; Mundt, A.J.; Lawson, J.D.; Murphy, K.T. Dosimetric comparison of intensity-modulated radiosurgery and helical tomotherapy for the treatment of multiple intracranial metastases. Technol. Cancer Res. Treat. 2009, 8, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Peñagarícano, J.A.; Yan, Y.; Shi, C.; Linskey, M.E.; Ratanatharathorn, V. Dosimetric comparison of helical tomotherapy and Gamma Knife stereotactic radiosurgery for single brain metastasis. Radiat. Oncol. 2006, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Gérard, M.; Jumeau, R.; Pichon, B.; Biau, J.; Blais, E.; Horion, J.; Noël, G. Contraintes de dose en radiothérapie conformationnelle fractionnée et en radiothérapie stéréotaxique dans les hippocampes, le tronc cérébral et l’encéphale: Limites et perspectives [Hippocampus, brainstem and brain dose-volume constraints for fractionated 3-D radiotherapy and for stereotactic radiation therapy: Limits and perspectives]. Cancer Radiother. 2017, 21, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Wiggenraad, R.; Kanter, A.V.; De Kal, H.B.; Taphoorn, M.; Vissers, T.; Struikmans, H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother. Oncol. 2011, 98, 292–297. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Figlia, V.; Mazzola, R.; Napoli, G.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Lunardi, G.; Tomasini, D.; Bonù, M.L.; et al. Repeated stereotactic radiosurgery (SRS) using a non-coplanar mono-isocenter (HyperArc™) technique versus upfront whole-brain radiotherapy (WBRT): A matched-pair analysis. Clin. Exp. Metastasis 2020, 37, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Capone, L.; Nardiello, B.; El Gawhary, R.; Raza, G.; Scaringi, C.; Bianciardi, F.; Gentile, P.; Paolini, S. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J. Neurooncol. 2020, 148, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Nicosia, L.; Figlia, V.; Giaj-Levra, N.; Cuccia, F.; Mazzola, R.; Ricchetti, F.; Rigo, M.; Vitale, C.; De Simone, A.; et al. Long-term disease outcome and volume-based decision strategy in a large cohort of multiple brain metastases treated with a mono-isocentric linac-based Stereotactic Radiosurgery technique. Clin. Transl. Oncol. 2021, 23, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Mortellaro, G.; Ognibene, L.; Craparo, G.; Lo Casto, A.; Ferrera, G. Salvage Re-irradiation Options in Adult Medulloblastoma: A Case Report and Review of the Literature. In Vivo 2020, 34, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Mortellaro, G.; Cespuglio, D.; Valenti, V.; DEGregorio, G.; Quartuccio, E.; Blasi, L.; Francaviglia, N.; Gallo, C.; Lo Casto, A.; et al. A Case Report of Adult Pineoblastoma Occurring in a Pregnant Woman. Anticancer. Res. 2019, 39, 2627–2631. [Google Scholar] [CrossRef]
- Barra, S.; Agostinelli, S.; Vagge, S.; Giannelli, F.; Siccardi, D.; Garelli, S.; Aloi, D.; Belgioia, L.; Bosetti, D.; Zeverino, M.; et al. Radiosurgery with Helical Tomotherapy: Outcomes for Patients with One or Multifocal Brain Metastasis. Technol. Cancer Res. Treat. 2015, 14, 693–699. [Google Scholar] [CrossRef]
- Putz, F.; Weissmann, T.; Oft, D.; Schmidt, M.A.; Roesch, J.; Siavooshhaghighi, H.; Filimonova, I.; Schmitter, C.; Mengling, V.; Bert, C.; et al. FSRT vs. SRS in Brain Metastases-Differences in Local Control and Radiation Necrosis-A Volumetric Study. Front. Oncol. 2020, 10, 559193. [Google Scholar] [CrossRef]
- Nagai, A.; Shibamoto, Y.; Yoshida, M.; Wakamatsu, K.; Kikuchi, Y. Treatment of Single or Multiple Brain Metastases by Hypofractionated Stereotactic Radiotherapy Using Helical Tomotherapy. Int. J. Mol. Sci. 2014, 15, 6910–6924. [Google Scholar] [CrossRef]
- Kornhuber, C.; Ensminger, S.; Hübsch, P.; Janich, M.; Leucht, C.A.; Vordermark, D.; Dietzel, C.T. Feasibility of a simultaneously integrated boost concept for hypofractionated stereotactic radiotherapy of unresected brain metastases. Radiat. Oncol. 2023, 18, 88. [Google Scholar] [CrossRef]
- Di Perri, D.; Tanguy, R.; Malet, C.; Robert, A.; Sunyach, M.P. Risk of radiation necrosis after hypofractionated stereotactic radiotherapy (HFSRT) for brain metastases: A single center retrospective study. J. Neurooncol. 2020, 149, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Layer, J.P.; Layer, K.; Sarria, G.R.; Röhner, F.; Dejonckheere, C.S.; Friker, L.L.; Zeyen, T.; Koch, D.; Scafa, D.; Leitzen, C.; et al. Five-Fraction Stereotactic Radiotherapy for Brain Metastases—A Retrospective Analysis. Curr. Oncol. 2023, 30, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Soni, Y.S.; Rich, B.J.; Kwon, D.; Zhao, W.; John, D.L.; Seldon, C.; Meshman, J.; Benveniste, R.; Komotar, R.J.; de la Fuente, M.; et al. Factors associated with the use of salvage whole brain radiation therapy versus salvage stereotactic radiosurgery after initial stereotactic radiosurgery for brain metastases. J. Radiosurgery SBRT 2022, 8, 85–94. [Google Scholar]
- Gruber, I.; Stark, P.; Weidner, K.; Treutwein, M.; Koelbl, O. Fractionated stereotactic radiotherapy of brain metastases: Results of a retrospective study. Radiat. Oncol. 2023, 18, 85. [Google Scholar] [CrossRef]
- Johannwerner, L.; Werner, E.M.; Blanck, O.; Janssen, S.; Cremers, F.; Yu, N.Y.; Rades, D. Radiation Necrosis Following Stereotactic Radiosurgery or Fractionated Stereotactic Radiotherapy with High Biologically Effective Doses for Large Brain Metastases. Biology 2023, 12, 655. [Google Scholar] [CrossRef]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Putz, F.; Pirschel, W.; Fietkau, R. FSRT-Trial: Erste Phase-III-Studie zum Vergleich fraktionierte stereotaktische Radiotherapie (FSRT) versus Einzeitradiochirurgie (SRS) bei Hirnmetastasen. Forum 2022, 37, 241–245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuccia, F.; D’Alessandro, S.; Carruba, G.; Figlia, V.; Spera, A.; Cespuglio, D.; Mortellaro, G.; Iacoviello, G.; Lo Casto, A.; Tringali, G.; et al. Fractionated Stereotactic Radiotherapy with Helical Tomotherapy for Brain Metastases: A Mono-Institutional Experience. J. Pers. Med. 2023, 13, 1099. https://doi.org/10.3390/jpm13071099
Cuccia F, D’Alessandro S, Carruba G, Figlia V, Spera A, Cespuglio D, Mortellaro G, Iacoviello G, Lo Casto A, Tringali G, et al. Fractionated Stereotactic Radiotherapy with Helical Tomotherapy for Brain Metastases: A Mono-Institutional Experience. Journal of Personalized Medicine. 2023; 13(7):1099. https://doi.org/10.3390/jpm13071099
Chicago/Turabian StyleCuccia, Francesco, Salvatore D’Alessandro, Giuseppe Carruba, Vanessa Figlia, Antonio Spera, Daniela Cespuglio, Gianluca Mortellaro, Giuseppina Iacoviello, Antonio Lo Casto, Giovanni Tringali, and et al. 2023. "Fractionated Stereotactic Radiotherapy with Helical Tomotherapy for Brain Metastases: A Mono-Institutional Experience" Journal of Personalized Medicine 13, no. 7: 1099. https://doi.org/10.3390/jpm13071099
APA StyleCuccia, F., D’Alessandro, S., Carruba, G., Figlia, V., Spera, A., Cespuglio, D., Mortellaro, G., Iacoviello, G., Lo Casto, A., Tringali, G., Craparo, G., Blasi, L., & Ferrera, G. (2023). Fractionated Stereotactic Radiotherapy with Helical Tomotherapy for Brain Metastases: A Mono-Institutional Experience. Journal of Personalized Medicine, 13(7), 1099. https://doi.org/10.3390/jpm13071099







