Comparison of a Peripheral Nerve Block versus Spinal Anesthesia in Foot or Ankle Surgery: A Systematic Review and Meta-Analysis with a Trial Sequential Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Information Source and Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias
2.6. Data Analysis
2.6.1. Conventional Meta-Analysis
2.6.2. Trial Sequential Analysis
2.6.3. Quality of the Evidence
3. Results
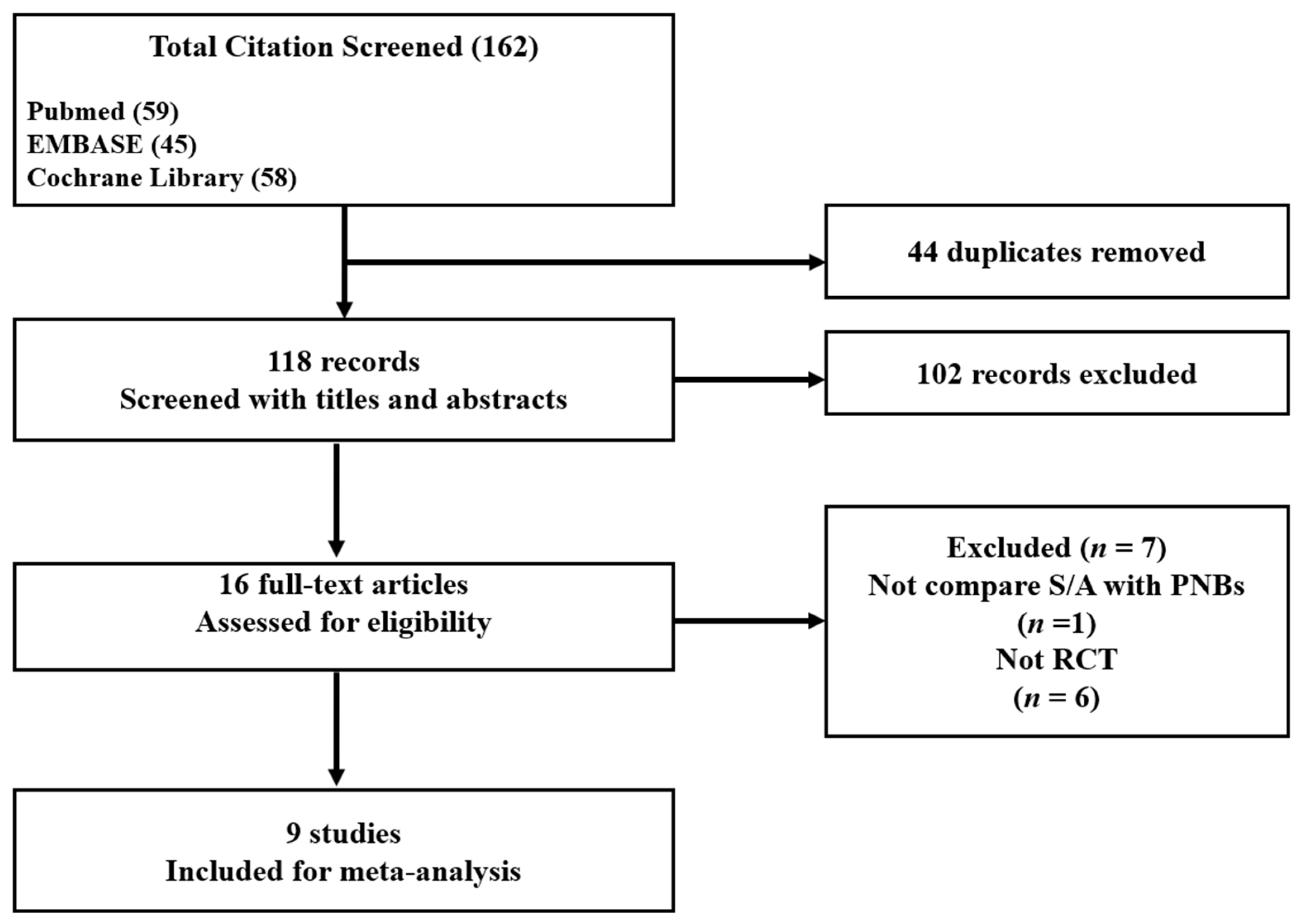
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Conversion to General Anesthesia
3.5. Block Performance Time
3.6. Onset Time of the Sensory Block
3.7. Onset Time of the Motor Block
3.8. Duration of the Sensory Block
3.9. Duration of the Motor Block
3.10. Postoperative Analgesic Requirements
3.11. Incidence of Hypotension
3.12. Use of Vasoactive Drugs
3.13. Systolic Blood Pressure
3.14. Diastolic Blood Pressure
3.15. Heart Rate
3.16. Urinary Retention
3.17. Post-Dural Puncture Headache
3.18. Patient Satisfaction
3.19. Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cozowicz, C.; Poeran, J.; Zubizarreta, N.; Mazumdar, M.; Memtsoudis, S.G. Trends in the use of regional anesthesia: Neuraxial and peripheral nerve blocks. Reg. Anesth. Pain Med. 2016, 41, 43–49. [Google Scholar] [CrossRef]
- Gabriel, R.A.; Ilfeld, B.M. Use of regional anesthesia for outpatient surgery within the United States: A prevalence study using a nationwide database. Anesth. Analg. 2018, 126, 2078–2084. [Google Scholar] [CrossRef]
- Fraser, T.W.; Doty, J.F. Peripheral nerve blocks in foot and ankle surgery. Orthop. Clin. 2017, 48, 507–515. [Google Scholar] [CrossRef]
- Bjørn, S.; Wong, W.Y.; Baas, J.; Nielsen, K.K.; Børglum, J.; Hauritz, R.W.; Bendtsen, T.F. The importance of the saphenous nerve block for analgesia following major ankle surgery: A randomized, controlled, double-blind study. Reg. Anesth. Pain Med. 2018, 43, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.P.; Møller, A.M.; Nielsen, J.K.; Klausen, T.W.; Sort, R. The effects of anesthetic technique on postoperative opioid consumption in ankle fracture surgery. Clin. J. Pain 2016, 32, 870–874. [Google Scholar] [CrossRef]
- Kir, M.C.; Kir, G. Ankle nerve block adjuvant to general anesthesia reduces postsurgical pain and improves functional outcomes in hallux valgus surgery. Med. Princ. Pract. 2018, 27, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Sort, R.; Brorson, S.; Gögenur, I.; Hald, L.L.; Nielsen, J.K.; Salling, N.; Hougaard, S.; Foss, N.B.; Tengberg, P.T.; Klausen, T.W. Peripheral nerve block anaesthesia and postoperative pain in acute ankle fracture surgery: The AnAnkle randomised trial. Br. J. Anaesth. 2021, 126, 881–888. [Google Scholar] [CrossRef]
- Dabir, S.; Mosaffa, F.; Hosseini, B.; Alimoradi, V. Comparison of the combined femoral and lateral femoral cutaneous nerve block plus popliteal block with spinal anesthesia for thigh tourniquet pain during foot or ankle surgery: A randomized clinical trial. Anesthesiol. Pain Med. 2020, 10, e103674. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, S.; Tekgül, Z.T.; Şimşek, E.; Turan, M.; Karaman, Y.; Kaya, A.; Gönüllü, M. Comparison between ultrasonography-guided popliteal sciatic nerve block and spinal anesthesia for hallux valgus repair. Foot Ankle Int. 2016, 37, 85–89. [Google Scholar] [CrossRef]
- Lai, H.Y.; Foo, L.L.; Lim, S.M.; Yong, C.F.; Loh, P.S.; Chaw, S.H.; Hasan, M.S.; Wang, C.Y. The hemodynamic and pain impact of peripheral nerve block versus spinal anesthesia in diabetic patients undergoing diabetic foot surgery. Clin. Auton. Res. 2020, 30, 53–60. [Google Scholar] [CrossRef]
- Protić, A.; Horvat, M.; Komen-Usljebrka, H.; Frkovic, V.; Zuvic-Butorac, M.; Bukal, K.; Sustic, A. Benefit of the minimal invasive ultrasound-guided single shot femoro-popliteal block for ankle surgery in comparison with spinal anesthesia. Wien. Klin. Wochenschr. 2010, 122, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Casati, A.; Grispigni, C.; Aldegheri, G.; Vinciguerra, F.; Sciascia, A.; Fraschini, G.; Fanelli, G. Peripheral or central nerve blocks for foot surgery: A prospective, randomized clinical comparison. Foot Ankle Surg. 2002, 8, 95–100. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Zhang, Z.-J.; Jia, R.; Duan, L.-P. Clinical observation of ultrasound guided popliteal sciatic nerve combined saphenous nerve block for hallux valgus patients with metatarsophalangeal joint dislocation. Zhongguo Gu Shang China J. Orthop. Traumatol. 2018, 31, 907–911. [Google Scholar]
- Yang, L.; Ji, J.; Zhao, Z.; Wang, W.; Luo, S.; Mo, Y.; Wang, J. Effect comparison of ultrasound-guided lower extremity nerve block and spinal anesthesia in ankle surgery. Zhonghua Yi Xue Za Zhi 2016, 96, 3337–3341. [Google Scholar]
- Chauhan, D.; Bhamri, S.; Shah, N.; Syed, A.N. A peripheral nerve stimulator guided popliteal sciatic nerve block combined with adductor canal block in lower leg surgery—A sole anesthetic technique. Indian J. Clin. Anaesth. 2023, 10, 53–57. [Google Scholar] [CrossRef]
- Gianakos, A.L.; Romanelli, F.; Rao, N.; Badri, M.; Lubberts, B.; Guss, D.; DiGiovanni, C.W. Combination lower extremity nerve blocks and their effect on postoperative pain and opioid consumption: A systematic review. J. Foot Ankle Surg. 2021, 60, 121–131. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: New York, NY, USA, 2019. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Kang, H. Trial sequential analysis: Novel approach for meta-analysis. Anesth. Pain Med. 2021, 16, 138–150. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar]
- Kullenberg, B.; Topalis, C.; Resch, S. Ankle nerve block-perioperative pain relief in surgery of the forefoot. Foot 2006, 16, 135–137. [Google Scholar] [CrossRef]
- Arnold, H.; Weber, J.; Von Hoesslin, H. The concept of pre-emptive balanced analgesia in ambulatory foot surgery: Results of a prospective study. Foot Ankle Surg. 2000, 6, 39–43. [Google Scholar] [CrossRef]
- Filimonov, R.V.; Filimonova, I.V.; Shapoval, S.D.; Kobeliatskyi, Y.Y. Comparative analysis of the methods of anesthetic maintenance in patients with diabetes with the syndrome of diabetic foot requiring operative intervention. Wiad. Lek. 2019, 72, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Eshelman, M.R.; Cracchiolo, A., III. Popliteal fossa neural blockade as the sole anesthetic technique for outpatient foot and ankle surgery. Foot Ankle Int. 2000, 21, 38–44. [Google Scholar] [CrossRef]
- Sarrafian, S.; Ibrahim, I.; Breihan, J. Ankle-foot peripheral nerve block for mid and forefoot surgery. Foot Ankle. 1983, 4, 86–90. [Google Scholar] [CrossRef]
- Shah, S.; Tsai, T.; Iwata, T.; Hadzic, A. Outpatient regional anesthesia for foot and ankle surgery. Int. Anesthesiol. Clin. 2005, 43, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Marhofer, P.; Harrop-Griffiths, W.; Kettner, S.; Kirchmair, L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 1. Br. J. Anaesth. 2010, 104, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.G.; Bronsert, M.R.; Henderson, W.G.; Mangione, M.P.; Sum-Ping, S.J.; Bentt, D.R.; Nguyen, J.D.; Richman, J.S.; Meguid, R.A.; Hammermeister, K.E. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology 2015, 123, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Asao, Y.; Nasu, K.; Shimizu, T.; Higuchi, T. Five Cases of Anesthetic Management for Femoral Neck Fracture Repair Complicated with Severe Aortic Stenosis. Masui Jpn. J. Anesthesiol. 2016, 65, 965–968. [Google Scholar]
- Asao, Y.; Higuchi, T.; Tsubaki, N.; Shimoda, Y. Combined paravertebral lumbar plexus and parasacral sciatic nerve block for reduction of hip fracture in four patients with severe heart failure. Masui Jpn. J. Anesthesiol. 2005, 54, 648–652. [Google Scholar]




| Author, Year, Country | Participants | Sample Size/Intervention | Primary Outcome | Major Findings |
|---|---|---|---|---|
| Lai et al., 2020, Malaysia [10] | ASA II–III diabetic patients aged >18 years for wound debridement, amputation | PNB (n = 45), ultrasound-guided sciatic and femoral or saphenous NB with or without a nerve stimulator SAB (n = 50) | Significant hypotension (reduction of ≥30% of SBP) | The SAB group had a large number of patients with significant hypotension. |
| Chauhan et al., 2023, India [15] | ASA I–III 18–70 years old patients for foot or ankle surgery | PNB (n = 27),popliteal sciatic and adductor canal block with nerve stimulator SAB (n = 30) | Duration of sensory and motor block | PNB can be alternative technique with advantage of prolonged post-operative analgesia and hemodynamic stability. |
| Karaarslan et al., 2016, Turkey [9] | ASA I–II 18–60 years old patients for hallux valgus repair | PNB (n = 30), ultrasound-guided popliteal sciatic NB with a nerve stimulator and saphenous block as infiltration anesthesia SAB (n = 30), unilateral spinal block | Pain VAS at 2 h | VAS at 2, 4, 6, and 12 h were significantly lower, and no adverse effects (hypotension, bradycardia, PDPH, urinary retention) in the PNB group. |
| Sort et al., 2021, Denmark [7] | ASA I–III patients >18 years old for ankle fracture surgery | PNB (n = 77), ultrasound-guided popliteal and saphenous blocks SAB (n = 73) | Postoperative pain and opioid consumption | 0–27 h of morphine consumption and pain scores were significantly lower in the PNB group. |
| Dabir et al., 2020, Iran [8] | ASA I–II patients ≥18 for foot or ankle surgery using a pneumatic thigh tourniquet. | PNB (n = 30), ultrasound-guided popliteal, femoral, lateral femoral cutaneous blocks SAB (n = 30) | Tourniquet pain score | Mean tourniquet pain scores and the total amount of fentanyl and ketamine administered during surgery were significantly higher in the PNB group. |
| Casati et al., 2002, Italy [12] | ASA I–II patients for foot surgery | PNB (2% mepivacaine, n = 50 0.75% ropivacain, n = 50), sciatic and femoral NB with nerve stimulator SAB (bilateral, n = 50, unilateral, n = 50) | Not defined | PNB is an effective and safe as spinal anesthesia with less urinary retention. |
| Protic et al., 2010, Croatia [11] | Adult trauma patients with bimalleolar fracture | PNB (n= 20), ultrasound-guided femoropopliteal block SAB (n = 20) | Not defined | PNB provides sufficient anesthesia for ankle fracture. |
| Xu et al., 2018, China [13] | ASA I–II 20–76 years old patients for hallux valgus surgery | PNB (n = 30), ultrasound-guided popliteal and saphenous block SAB (n = 30) | Not defined | PNB provides sufficient anesthesia for hallux valgus surgery with maintaining hemodynamic stability. |
| Yang et al., 2016, China [14] | ASA I–II 18–70 years old patients for ankle surgery | PNB (n = 40), ultrasound-guided femoral, obturator, lateral femoral cutaneous and sacral plexus block SAB (n = 40) | Not defined | PNB may be safe and effective in patients undergoing ankle surgery. |
| Author, Year | Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Intervention | Bias Due to Missing Outcome Data | Bias in Measurement of the Outcome | Bias in Selection of the Reported Results | Overall Bias |
|---|---|---|---|---|---|---|
| Lai et al., 2020, Malaysia [10] | Low risk | Some concern | Some concern | Low risk | Low risk | High risk |
| Chauhan et al., 2023, India [15] | Low risk | Some concern | Some concern | Some concern | Low risk | High risk |
| Karaarslan et al., 2016, Turkey [9] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Sort et al., 2021, Denmark [7] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Dabir et al., 2020, Iran [8] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Casati et al., 2002, Italy [12] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Protic et al., 2010, Croatia [11] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Xu et al., 2018, China [13] | Low risk | Some concern | Low risk | Low risk | Low risk | Some concern |
| Yang et al., 2016, China [14] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| No of Studies | No of Patients | Conventional Meta-Analysis | Trial Sequential Analysis | NNT | ||||
|---|---|---|---|---|---|---|---|---|
| RR or WMD, or SMD with 95% CI | Heterogeneity (I2) | Conventional Test Boundary | Monitoring Boundary | RIS | ||||
| Conversion to GA | 8 | 602 | Not significant (RR: 2.261; 95% CI 0.514 to 9.953) | 0.0 | Not cross | Not cross | 2.9% (602 of 20,771 patients) | Significant (NNTH: 43; 95% CI NNTH 22 to NNTH 478) |
| Block performance time | 4 | 260 | Significant (WMD: 7.470; 95% CI 6.072 to 8.868) | 88.66 | Cross | Cross | 99.2% (260 of 262) patients) | NR |
| Onset time of sensory block | 4 | 234 | Significant (WMD: 7.483; 95% CI 2.837 to 12.130) | 99.67 | Cross | Cross | 18.9% (234 of 1236 patients) | NR |
| Onset time of motor block | 7 | 554 | Significant (WMD: 9.071; 95% CI 4.049 to 14.094) | 99.40 | Cross | Cross | 93.7% (554 of 591 patients) | NR |
| Duration of sensory block | 7 | 679 | Significant (WMD: 458.53; 95% CI 328.296 to 588.765) | 97.02 | Cross | Cross | exceeds RIS (679 of 246 patients) | NR |
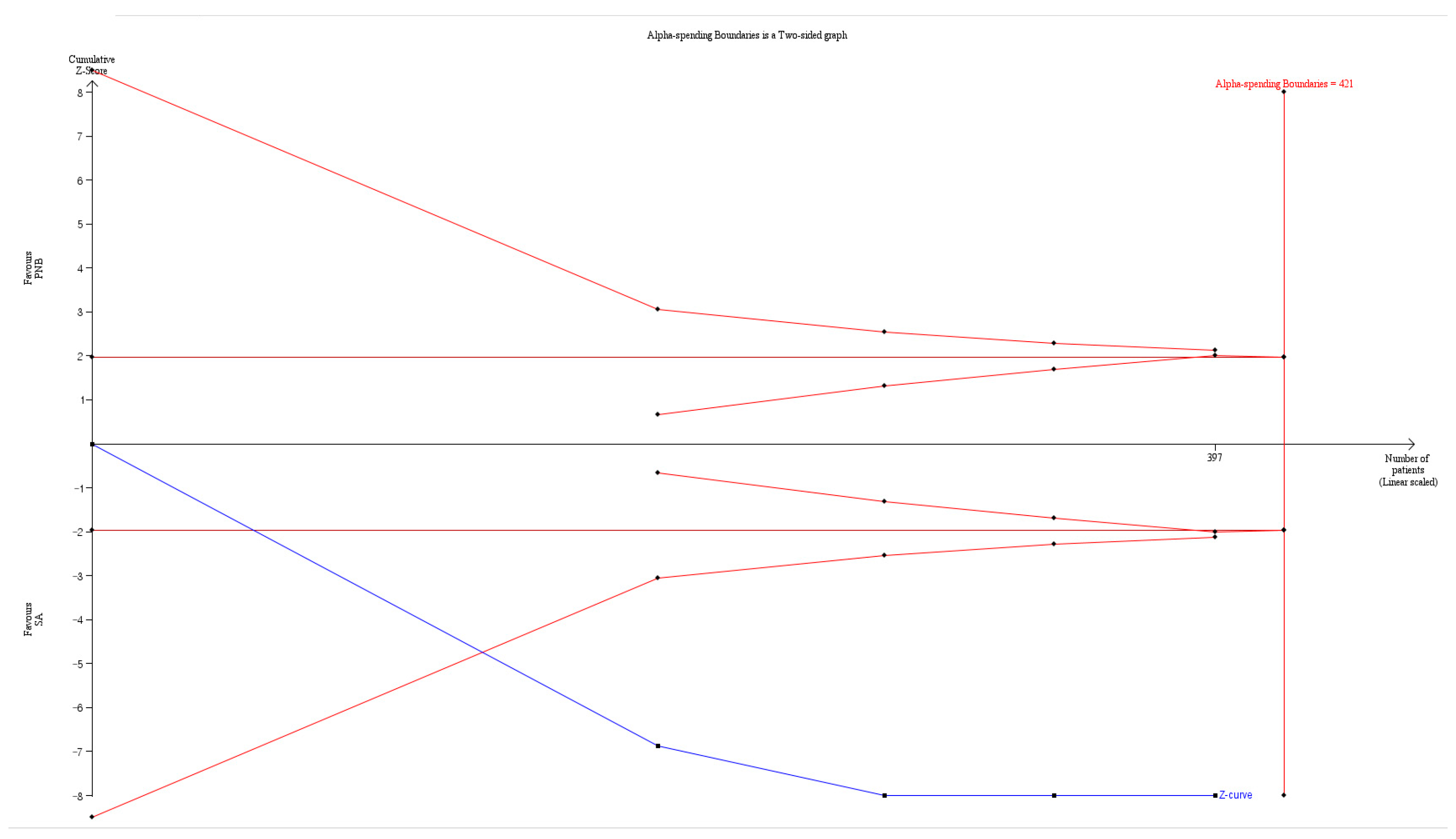
| Duration of motor block | 4 | 397 | Significant (WMD: 247.416; 95% CI 95.625 to 399.208) | 97.325 | Cross | Cross | 94.3% (397 of 421 patients) | NR |
| Postoperative analgesics requirement | 3 | 407 | Significant (SMD: −1.091; 95% CI −1.634 to −0.549) | 83.10 | Cross | Cross | 23.4% (407 of 1741 patients) | NR |
| Incidence of hypotension | 4 | 272 | Significant (RR: 0.152; 95% CI 0.042 to 0.548) | 0.0 | Cross | Not cross | 6.7% (272 of 4043 patients) | Significant (NNTB: 7; 95% CI NNTB 5 to NNTH 14) |
| Vasoactive drug | 3 | 305 | Significant (RR: 0.253; 95% CI 0.101 to 0.638) | 0.0 | Cross | Not cross | 8.2% (305 of 3698 patients) | Significant (NNTB: 8; 95% CI NNTB 5 to NNTH 15) |
| SBP at baseline (T0) | 3 | 212 | Not significant (WMD: −0.112; 95% CI −5.329 to 5.168) | 31.501 | Cross | Not cross | 1.6% (212 of 9127 patients) | NR |
| SBP at 30 min after the beginning of surgery (T30) | 3 | 212 | Significant (WMD: 13.950; 95% CI 4.603 to 23.298) | 74.829 | Cross | Cross | exceeds RIS (212 of 75 patients) | NR |
| DBP at baseline (T0) | 3 | 212 | Not significant (WMD: −1.297; 95% CI −3.931 to 1.337) | 0.0 | Not cross | Not cross | 11.8% (212 of 1802 patients) | NR |
| DBP at 30 min after the beginning of surgery (T30) | 3 | 212 | Not significant (WMD: 4.535; 95% CI −0.969 to 10.039) | 75.674 | Not cross | Not cross | 32.8 (212 of 646 patients) | NR |
| Heart rate (T0) | 3 | 212 | Not significant (WMD: −0.204; 95% CI −3.696 to 3.288) | 0.0621 | Not cross | Not cross | 11.8% (212 of 1802 patients) | NR |
| Heart rate (T30) | 3 | 212 | Not significant (WMD: 2.617; 95% CI −3.265 to 8.599) | 66.401 | Cross | Not cross | 9.5% (212 of 2237 patients) | NR |
| Urinary retention | 4 | 354 | Not significant (RR: 0.089; 95% CI 0.002 to 3.297) | 0.0 | Not cross | Not cross | 18.5% (354 of 1912 patients | Significant NNTB: 18; 95% CI NNTB 11 to NNTH 45 |
| PDPH | 4 | 234 | Not significant (RR: 0.159; 95% CI 0.004 to 6.071) | 0.0 | Not cross | Not cross | 1.2% (234 of 14,721 patients) | Significant (NNTB: 30; 95% CI NNTB 15 to NNTH 813) |
| Patients’ satisfaction | 2 | 350 | Not significant (RR: 1.047; 95% CI 0.989 to 1.108) | 25.04 | Not cross | Not cross | 83.7% (350 of 418 patients) | Not significant (NNTH: 13; 95% CI NNTH −131 to ∞ to NNTB 226) |
| Outcomes | Number of Studies | Quality Assessment | Quality | ||||
|---|---|---|---|---|---|---|---|
| ROB | Inconsistency | Indirectness | Imprecision | Publication Bias | |||
| Conversion to GA | 8 | not serious | not serious | not serious | not serious | NA | ⨁⨁⨁⨁ High |
| Block performance time | 4 | serious | serious | not serious | not serious | NA | ⨁⨁◯◯ Low |
| The onset of sensory block | 4 | not serious | serious | not serious | serious | NA | ⨁⨁◯◯ Low |
| The onset of motor block | 7 | not serious | serious | not serious | not serious | NA | ⨁⨁⨁◯ Moderate |
| Duration of sensory block | 7 | not serious | serious | not serious | not serious | NA | ⨁⨁⨁◯ Moderate |
| Duration of motor block | 4 | not serious | serious | not serious | serious | NA | ⨁⨁◯◯ Low |
| Postoperative analgesics requirement | 3 | not serious | serious | not serious | serious | NA | ⨁⨁◯◯ Low |
| Incidence of hypotension | 4 | serious | not serious | not serious | not serious | NA | ⨁⨁⨁◯ Moderate |
| Vasoactive drug | 3 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| SBP at baseline (T0) | 3 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| SBP at 30 min after the beginning of surgery (T30) | 3 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| DBP at baseline (T0) | 3 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| DBP at 30 min after the beginning of surgery (T30) | 3 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| Heart rate (T0) | 3 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| Heart rate (T30) | 3 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| Urinary retention | 4 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| PDPH | 4 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
| Patient satisfaction | 2 | not serious | not serious | not serious | serious | NA | ⨁⨁⨁◯ Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Lee, C.; Lim, J.; Kim, H.; Choi, Y.-S.; Kang, H. Comparison of a Peripheral Nerve Block versus Spinal Anesthesia in Foot or Ankle Surgery: A Systematic Review and Meta-Analysis with a Trial Sequential Analysis. J. Pers. Med. 2023, 13, 1096. https://doi.org/10.3390/jpm13071096
Lee M, Lee C, Lim J, Kim H, Choi Y-S, Kang H. Comparison of a Peripheral Nerve Block versus Spinal Anesthesia in Foot or Ankle Surgery: A Systematic Review and Meta-Analysis with a Trial Sequential Analysis. Journal of Personalized Medicine. 2023; 13(7):1096. https://doi.org/10.3390/jpm13071096
Chicago/Turabian StyleLee, Myeongjong, Cheol Lee, Junsung Lim, Hyungtae Kim, Yoo-Shin Choi, and Hyun Kang. 2023. "Comparison of a Peripheral Nerve Block versus Spinal Anesthesia in Foot or Ankle Surgery: A Systematic Review and Meta-Analysis with a Trial Sequential Analysis" Journal of Personalized Medicine 13, no. 7: 1096. https://doi.org/10.3390/jpm13071096
APA StyleLee, M., Lee, C., Lim, J., Kim, H., Choi, Y.-S., & Kang, H. (2023). Comparison of a Peripheral Nerve Block versus Spinal Anesthesia in Foot or Ankle Surgery: A Systematic Review and Meta-Analysis with a Trial Sequential Analysis. Journal of Personalized Medicine, 13(7), 1096. https://doi.org/10.3390/jpm13071096







