The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afanasieva, O.I.; Pokrovsky, S.N. Hyperlipopproteinemia(a) as a dangerous genetically determined violation of lipid metabolism and a risk factor for atherothrombosis and cardiovascular diseases. Russ. J. Cardiol. 2019, 24, 101–108. [Google Scholar] [CrossRef]
- Tmoyan, N.A.; Afanasieva, O.I.; Ezhov, M.V.; Klesareva, E.A.; Balakhonova, T.V.; Pokrovsky, S.N. Lipoprotein(a), immunity, and inflammation in polyvascular atherosclerotic disease. J. Cardiovasc. Dev. Dis. 2021, 8, 11. [Google Scholar] [CrossRef]
- Guedon, A.F.; De Freminville, J.-B.; Mirault, T.; Mohamedi, N.; Rance, B.; Fournier, N.; Paul, J.-L.; Messas, E.; Goudot, G. Association of lipoprotein(a) levels with incidence of major adverse limb events. JAMA Netw. Open. 2022, 5, e2245720. [Google Scholar] [CrossRef] [PubMed]
- Naglic, D.T.; Manojlovic, M.; Pejakovic, S.; Stepanovic, K.; Simeunovic, J.P. Lipoprotein(a): Role in atherosclerosis and new treatment options. Biomol. Biomed. 2023; online ahead of print. [Google Scholar]
- Suwa, S.; Ogita, M.; Miyauchi, K.; Sonoda, T.; Konishi, H.; Tsuboi, S.; Wada, H.; Naito, R.; Dohi, T.; Kasai, T.; et al. Impact of lipoprotein(a) on long-term outcomes in patients with coronary artery disease treated with statin after a first percutaneous coronary intervention. J. Atheroscler. Thromb. 2017, 24, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Dohi, T.; Funamizu, T.; Endo, H.; Wada, H.; Doi, S.; Kato, Y.; Ogitam, M.; Okai, I.; Iwata, H.; et al. Prognostic impact of lipoprotein(a) on long-term clinical outcomes in diabetic patients on statin treatment after percutaneous coronary intervention. J. Cardiol. 2020, 76, 25–29. [Google Scholar] [CrossRef]
- Willeit, P.; Ridker, P.M.; Nestel, P.J.; Simes, J.; Tonkin, A.M.; Pedersen, T.R.; Schwartz, G.G.; Olsson, A.G.; Colhoun, H.M.; Kronenberg, F.; et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: Individual patient-data meta-analysis of statin outcome trials. Lancet 2018, 392, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.E.; Kraaijenhof, J.M.; Stroes, E.S.; Nurmohamed, N.S.; Kroon, J. Lipoprotein(a): An underestimated inflammatory mastermind. Atherosclerosis 2022, 349, 101–109. [Google Scholar] [CrossRef]
- Erqou, S.; Thompson, A.; Angelantonio, E.D.; Saleheen, D.; Kaptoge, S.; Marcovina, S.; Danesh, J. Apolipoprotein(a) isoforms and the risk of vascular disease: Systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 2010, 55, 2160–2167. [Google Scholar] [CrossRef]
- Koschinsky, M.L.; Boffa, M.B. Oxidized phospholipid modification of lipoprotein(a): Epidemiology, biochemistry and pathophysiology. Atherosclerosis 2022, 349, 92–100. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Hoogeveen, R.M.; Lubna, A.; Prange, K.; Waissi, F.; van Weeghel, M.; Bachmann, J.C.; Versloot, M.; Borrelli, M.J.; Yeang, C.; et al. Atherogenic lipoprotein(a) increases vascular glycolysis, thereby facilitating inflammation and leukocyte extravasation. Circ. Res. 2020, 126, 1346–1359. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Poels, K.; Stiekema, L.; Yeang, C.; Tsimikas, S.; Kroom, J.; Stroes, E.; Lutgens, E.; Seijkens, T. Short-term regulation of hematopoiesis by lipoprotein(a) results in the production of pro-inflammatory monocytes. Int. J. Cardiol. 2020, 315, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Knowlden, S.; Georas, S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 2014, 192, 851–857. [Google Scholar] [CrossRef]
- Bourgeois, R.; Bourgault, J.; Despres, A.A.; Perrot, N.; Guertin, J.; Girard, A.; Mitchell, P.L.; Gotti, C.; Bourassa, S.; Scipione, C.A.; et al. Lipoprotein proteomics and aortic valve transcriptomics identify biological pathways linking lipoprotein(a) levels to aortic stenosis. Metabolites 2021, 11, 459. [Google Scholar] [CrossRef]
- Bobryshev, Y.V. Monocyte recruitment and foam cell formation in atherosclerosis. Micron 2006, 37, 208–222. [Google Scholar] [CrossRef]
- Rajamaki, K.; Lappalainen, J.; Oorni, K.; Valimaki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. Blood monocytes and their subsets: Established features and open questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemund, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Arnold, K.A.; Blair, J.E.; Paul, J.D.; Shah, A.P.; Nathan, S.; Alenghat, F.J. Monocyte and macrophage subtypes as paired cell biomarkers for coronary artery disease. Exp. Physiol. 2019, 104, 134–1352. [Google Scholar] [CrossRef] [PubMed]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Groβe-Dunker, G.; Hornof, F.; Jeken, J.; Rebling, N.M.; et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Filatova AYu Potekhina, A.V.; Radyukhina, N.V.; Ruleva NYu Provatorov, S.I.; Arefieva, T.I. Circulating monocyte populations in patients with coronary atherosclerosis. Future Cardiol. 2022, 18, 455–460. [Google Scholar] [CrossRef]
- Kraaijenhof, J.M.; Hovingh, G.K.; Stroes, E.S.; Kroon, J. The iterative lipid impact on inflammation in atherosclerosis. Curr. Oin Lipidol. 2021, 32, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Krychtiuk, K.A.; Kastl, S.P.; Hofbauer, S.L.; Wonnerth, A.; Goliasch, G.; Ozsvar-Kozma, M.; Katsaros, K.M.; Maurer, G.; Huber, K.; Dostal, E.; et al. Monocyte subset distribution in patients with stable atherosclerosis and elevated levels of lipoprotein(a). J. Clin. Lipidol. 2015, 9, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Afanasieva, O.I.; Filatova AYu Arefieva, T.I.; Klesareva, E.A.; Tyurina, A.V.; Radyukhina, N.V.; Ezhov, M.V.; Pokrovsky, S.N. The association of lipoprotein(a)and circulating monocyte subsets with severe coronary atherosclerosis. J. Cardiovasc. Dev. Dis. 2021, 8, 63. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 biology and its role in atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef]
- Leander, K.; Mälarstig, A.; Van’t Hooft, F.M.; Hyde, C.; Hellénius, M.L.; Troutt, J.S.; Konrad, R.J.; Öhrvik, J.; Hamsten, A.; de Faire, U. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Hoffmann, M.M.; Winkler, K.; Böhm, M.; Laufs, U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vasc. Pharmacol. 2014, 62, 94–102. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Ferri, N.; Tibolla, G.; Pirillo, A.; Cipollone, F.; Mezzetti, A.; Pacia, S.; Corsini, A.; Catapano, A.L. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Secreted by Cultured Smooth Muscle Cells Reduces Macrophages LDLR Levels. Atherosclerosis 2012, 220, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Shahanawaz, J.; Shmookler Reis, R.J.; Varughese, K.I.; Sawamura, T.; Mehta, J.L. Cross-Talk between LOX-1 and PCSK9 in Vascular Tissues. Cardiovasc. Res. 2015, 107, 556–567. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Mathur, P.; Dai, Y.; Theus, S.; Deng, X.; Fan, Y.; Mehta, J.L. Cross-Talk Between PCSK9 and Damaged MtDNA in Vascular Smooth Muscle Cells: Role in Apoptosis. Antioxid. Redox Signal. 2016, 25, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.U.; Kee, P.; Danila, D.; Teng, B.B. A critical role of PCSK9 in mediating IL-17-producing T cell responses in hyperlipidemia. Immune Netw. 2019, 19, e41. [Google Scholar] [CrossRef]
- Liu, A.; Frostegard, J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J. Intern. Med. 2018, 284, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Theus, S.; Deng, X.; Fan, Y.; Zhou, S.; Mehta, J.L. PCSK9 regulates expression of scavenger receptors and ox-LDL uptake in macrophages. Cardiovasc. Res. 2018, 114, 1145–1153. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Goes “DAMP”. Circulation 2021, 143, 62–64. [Google Scholar] [CrossRef]
- Tavori, H.; Christian, D.; Minnier, J.; Plubell, D.; Shapiro, M.D.; Yeang, C.; Giunzioni, I.; Croyal, M.; Duell, P.B.; Lambert, G.; et al. PCSK9 Association with lipoprotein(a). Circ. Res. 2016, 119, 29–35. [Google Scholar] [CrossRef]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus; Abbreviated Report of a WHO Consultation (WHO/NMH/CHP/CPM/111); World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Dahlen, G.H. Incidence of Lp(a) among populations. In Lipoprotein(a); Scanu, A.M., Ed.; Academic Press: New York, NY, USA, 1990; pp. 151–173. ISBN 0-12-620990-1. [Google Scholar]
- Afanasieva, O.I.; Ezhov, M.V.; Razova, O.A.; Afanasieva, M.I.; Utkina, E.A.; Pokrovsky, S.N. Apolipoprotein(a) phenotype determines the correlations of liporprotein(a) and proprotein convertase subtilisin/kexin type 9 levels in patients with potential familial hypercholesterolemia. Atherosclerosis 2018, 277, 477–482. [Google Scholar] [CrossRef]
- Razova, O.A.; Afanasieva, O.I.; Egiazaryan, M.; Sherstyuk, E.; Klesareva, E.A.; Pokrovsky, S.N. Circulating complex of lipoprotein(a) and proprotein convertase subtilisin kexin type 9 in the serum measured by ELISA. Bull. Exp. Biol. Med. 2020, 169, 639–643. [Google Scholar] [CrossRef]
- Tsimikas, S. A test in context: Lipoprotein(a): Diagnosis, prognosis, controversies, and emerging therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef]
- Shaya, G.E.; Leucker, T.M.; Jones, S.R.; Martin, S.S.; Toth, P.P. Coronary heart disease risk: Low-density lipoprotein and beyond. Trends Cardiovasc. Med. 2022, 32, 181–194. [Google Scholar] [CrossRef]
- Cho, K.I.; Yu, J.; Hayashi, T.; Han, S.H.; Koh, K.K. Strategies to overcome residual risk during statins era. Circ. J. 2019, 83, 1973–1979. [Google Scholar] [CrossRef]
- Weber, C.; Shantsila, E.; Hristov, M.; Caligiuri, G.; Guzik, T.; Heine, G.H.; Hoefer, I.E.; Monaco, C.; Peter, K.; Rainger, E.; et al. Role and analysis of monocyte subsets in cardiovascular disease, Joint consensus document of the European Society of Cardiology (ESC) working grouos “Atherosclerosis & Vascular Biology” and “Thrombosis”. Thromb. Haemost. 2016, 116, 626–637. [Google Scholar] [PubMed]
- Franca, C.N.; Izar, M.C.; Hortencio, M.N.; do Amaral, J.B.; Ferreira, C.E.; Tuleta, I.D.; Fonseca, F.A. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin. Sci. 2017, 131, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Afanasieva, O.I.; Tyurina, A.V.; Klesareva, E.A.; Arefieva, T.I.; Ezhov, M.V.; Pokrovsky, S.N. Lipoprotein(a), immune cells and cardiovascular outcomes in patients with premature coronary heart disease. J. Pers. Med. 2022, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, F.M.; Bekkering, S.; Kroon, J.; Yeang, C.; Van den Bossche, J.; Van Buul, J.D.; Ravandi, A.; Nederveen, A.J.; Verberne, H.J.; Scipione, C.; et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016, 134, 611–624. [Google Scholar] [CrossRef]
- Stiekema, L.C.; Prange, K.H.; Hoogeveen, R.M.; Verweij, S.L.; Kroon, J.; Schnitzler, J.G.; Dzobo, K.E.; Cupido, A.J.; Tsimikas, S.; Stroes, E.S.; et al. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur. Heart J. 2020, 41, 2262–2271. [Google Scholar] [CrossRef]
- Adorni, M.P.; Cipollari, E.; Favari, E.; Zanotti, I.; Zimetti, F.; Corsini, A.; Ricci, C.; Bernini, F.; Ferri, N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2017, 256, 1–6. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, S.; Shahanawaz, J.; Theus, S.; Fan, Y.; Deng, X.; Zhou, S.; Mehta, J.L. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 2018, 114, 1738–1751. [Google Scholar] [CrossRef]
- Cheng, J.M.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; Boersma, E.; van Geuns, R.J.; Serruys, P.W.; Kardys, I.; Akkerhuis, K.M. PCSK9 in relation to coronary plaque inflammation: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2016, 248, 117–122. [Google Scholar] [CrossRef]
- Moens Bernelot, S.J.; Neele, A.E.; Kroon, J.; van der Valk, F.M.; van den Bossche, J.; Hoeksema, M.A.; Hoogeveen, R.M.; Schnitzler, J.G.; Baccara-Dinet, M.T.; Manvelian, G.; et al. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur. Heart J. 2017, 38, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Meyborg, H.; Bezhaeva, T.; Kappert, K.; Hillmeister, P.; Kintscher, U.; Pieske, B.; Stawowy, P. PCSK9 regulates the chemokine receptor CCR2 on monocytes. Biochem. Biophys. Res. Commun. 2017, 485, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Krychtiuk, K.A.; Lenz, M.; Hohensinner, P.; Distelmaier, K.; Schrutka, L.; Kastl, S.P.; Huber, K.; Dostal, E.; Oravec, S.; Hengstenberg, C.; et al. Circulating levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) are associated with monocyte subsets in patients with stable coronary artery disease. J. Clin. Lipidol. 2021, 15, 512–521. [Google Scholar] [CrossRef] [PubMed]
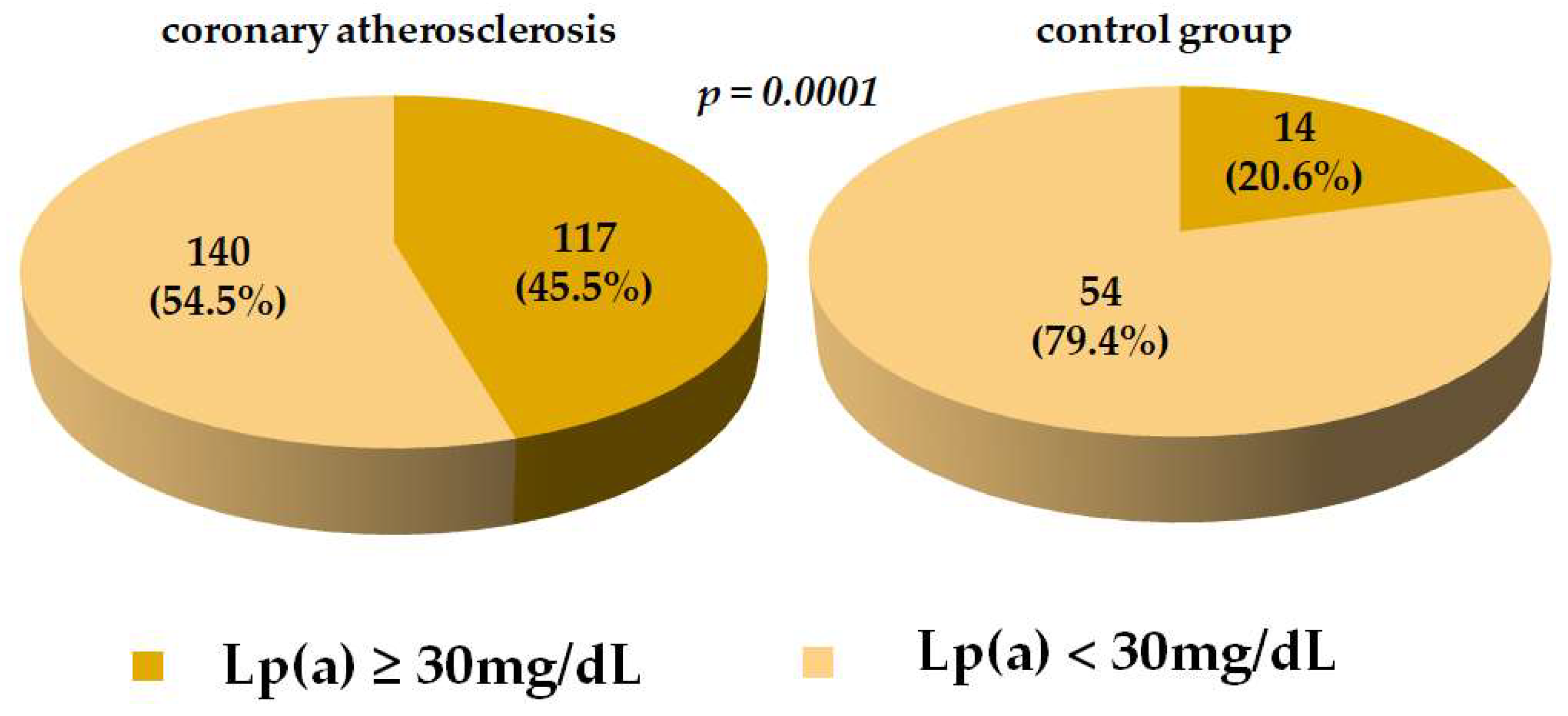
| Parameter | Coronary Atherosclerosis Group (n = 257) | Control Group (n = 68) |
|---|---|---|
| Age (years) | 60 (53; 66) | 60 (53; 65) |
| Smoking | 166 (64) | 38 (56) |
| Arterial hypertension | 213 (83) | 50 (74) |
| Myocardial infraction | 193 (75) | no |
| Coronary atherosclerosis severity | ||
| 1 vessel | 52 (20) | no |
| 2 vessels | 77 (30) | no |
| 3 and multivessel | 128 (50) | no |
| CABG | 53 (21) | no |
| Coronary stenting | 210 (82) | no |
| Type 2 diabetes | 61 (24) | 11 (16) |
| Aspirin | 230 (89) | 55 (81) |
| ACEI or ARB | 200 (78) | 48 (70) |
| β blockers | 228 (88) | 55 (81) |
| Diuretics | 64 (25) | 14 (21) |
| Calcium channel blockers | 79 (31) | 15 (22) |
| Glucose (mmol/L) | 5.5 (5.0; 6.1) | 5.2 (4.6; 6.2) |
| BMI (kg/m2) | 29.0 (26.0; 32.0) | 28.5 (27.0; 32.0) |
| Obesity | 106 (41) | 32 (47) |
| TC (mmol/L) | 3.8 (3.2; 4.5) * | 4.8 (3.9; 5.9) |
| TG (mmol/L) | 1.5 (1.1; 2.1) | 1.5 (0.9; 2.0) |
| HDL-C (mmol/L) | 1.1 (0.9; 1.3) | 1.2 (1.0; 1.5) |
| LDL-C (mmol/L) | 2.0 (1.5; 2.6) * | 2.8 (2.0; 3.7) |
| LDL-C corr (mmol/L) | 1.8 (1.3; 2.4) * | 2.7 (1.8; 3.5) |
| Lp(a) (mg/dL) | 23.0 (9.1; 73.3) * | 10.7 (4.7; 25.0) |
| PCSK9 (ng/mL) | 288.0 (237.4; 361.4) * | 214.7 (161.2; 300.0) |
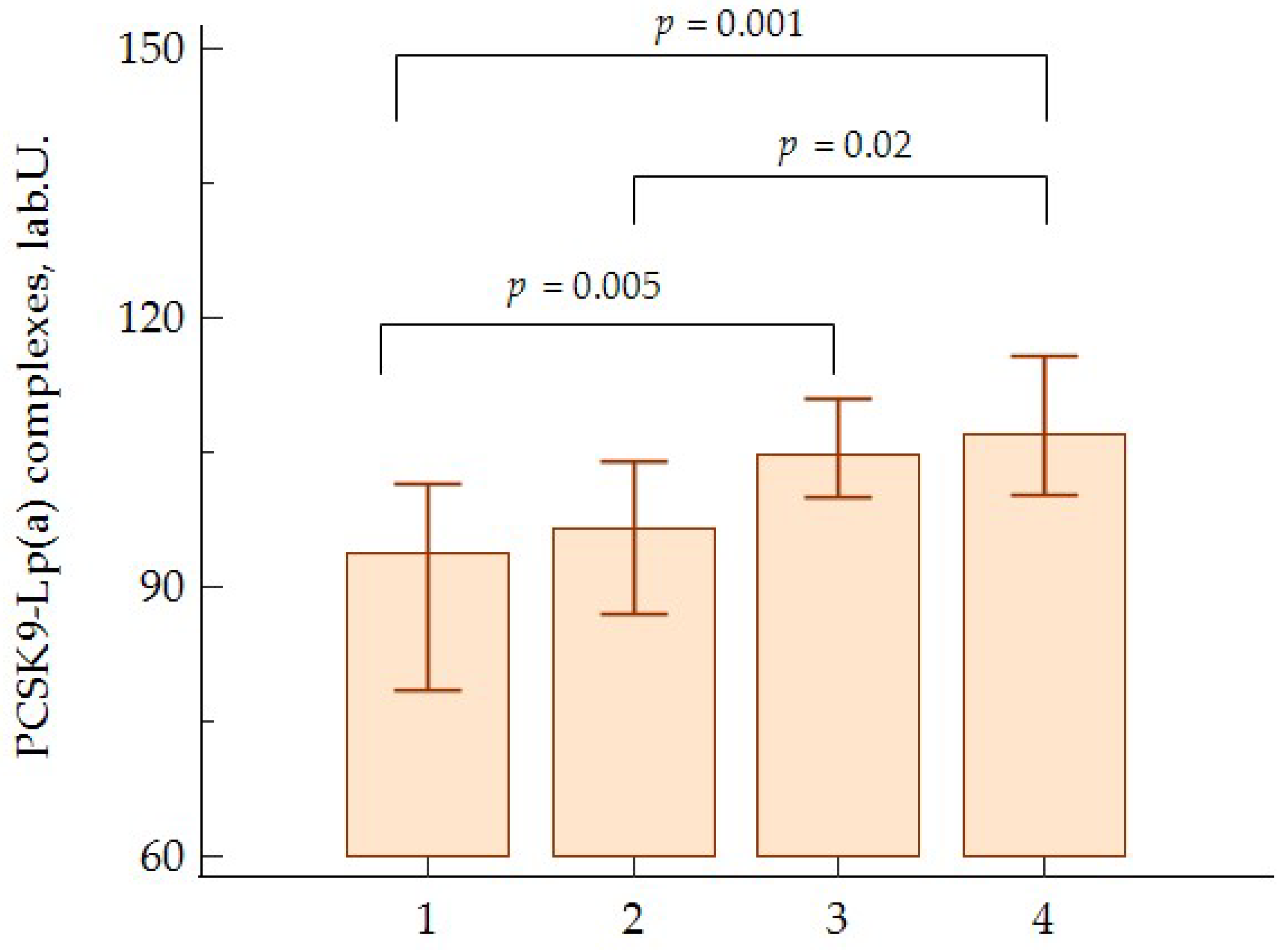
| PCSK9-Lp(a) (lab.U.) | 101 (82; 118) | 92 (81; 110) |
| Leukocytes (109/L) | 7.6 (6.3; 8.7) | 7.1 (6.1; 8.4) |
| Monocytes (106/mL) | 0.59 (0.45; 0.72) | 0.55 (0.44; 0.64) |
| Classical monocytes (103/mL) | 360.1 (293.2; 473.1) | 385.2 (297.5; 480.2) |
| Intermediate monocytes (103/mL) | 41.3 (24.4; 67.0) | 33.6 (23.7; 53.5) |
| Non-classical monocytes (103/mL) | 83.2 (68.1; 126.3) | 92.6 (71.4; 134.7) |
| Parameter | Simple | Multiple | ||
|---|---|---|---|---|
| Coefficient | p-Level | Coefficient | p-Level | |
| PCSK9-Lp(a) complexes | 0.23 | 0.0004 | 0.27 | <0.0001 |
| BMI | 0.19 | 0.003 | 0.25 | 0.0001 |
| Age | −0.02 | <0.0001 | 0.01 | 0.856 |
| Hypertension | −0.014 | - | −0.07 | 0.247 |
| Diabetes | −0.047 | - | 0.02 | 0.761 |
| Lp(a) | −0.008 | - | −0.01 | 0.876 |
| LDL-C | −0.073 | - | 0.11 | 0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filatova, A.Y.; Afanasieva, O.I.; Arefieva, T.I.; Potekhina, A.V.; Tyurina, A.V.; Klesareva, E.A.; Razova, O.A.; Ezhov, M.V.; Pokrovsky, S.N. The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis. J. Pers. Med. 2023, 13, 1077. https://doi.org/10.3390/jpm13071077
Filatova AY, Afanasieva OI, Arefieva TI, Potekhina AV, Tyurina AV, Klesareva EA, Razova OA, Ezhov MV, Pokrovsky SN. The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis. Journal of Personalized Medicine. 2023; 13(7):1077. https://doi.org/10.3390/jpm13071077
Chicago/Turabian StyleFilatova, Anastasiia Yu., Olga I. Afanasieva, Tatiana I. Arefieva, Alexandra V. Potekhina, Alexandra V. Tyurina, Elena A. Klesareva, Oksana A. Razova, Marat V. Ezhov, and Sergey N. Pokrovsky. 2023. "The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis" Journal of Personalized Medicine 13, no. 7: 1077. https://doi.org/10.3390/jpm13071077
APA StyleFilatova, A. Y., Afanasieva, O. I., Arefieva, T. I., Potekhina, A. V., Tyurina, A. V., Klesareva, E. A., Razova, O. A., Ezhov, M. V., & Pokrovsky, S. N. (2023). The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis. Journal of Personalized Medicine, 13(7), 1077. https://doi.org/10.3390/jpm13071077







