A Prospective Study on Obstructive Sleep Apnea, Clinical Profile and Polysomnographic Variables
Abstract
1. Introduction
2. Methodology
2.1. Study Design and Duration
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Population
2.5. Patients and Predictive Risk Factors
2.6. Polysomnography (PSG) Sleep Study
2.7. Statistical Analysis
3. Results
Polysomnography Sleep Study
4. Discussion
4.1. Prevalence and Age
4.2. Gender
4.3. Obesity
5. Conclusions
6. Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Sunwoo, J.S.; Hwangbo, Y.; Kim, W.J.; Chu, M.K.; Yun, C.H.; Yang, K.I. Prevalence, sleep characteristics, and comorbidities in a population at high risk for obstructive sleep apnea: A nationwide questionnaire study in South Korea. PLoS ONE 2018, 13, e0193549. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Sforza, E.; Roche, F.; Thomas-Anterion, C.; Kerleroux, J.; Beauchet, O.; Celle, S.; Maudoux, D.; Pichot, V.; Laurent, B.; Barthélémy, J.C. Cognitive function and sleep related breathing disorders in a healthy elderly population: The synapse study. Sleep 2010, 33, 515–521. [Google Scholar] [CrossRef]
- Karkoulias, K.; Lykouras, D.; Sampsonas, F.; Drakatos, K.; Sargianou, M.; Drakatos, P.; Spiropoulos, K.; Assimakopoulos, K. The impact of obstructive sleep apnea syndrome severity on physical performance and mental health. The use of SF-36 questionnaire in sleep apnea. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 531–536. [Google Scholar]
- Sampaio, R.; Pereira, M.G.; Winck, J.C. Psychology morbidity, illness representations, and quality of life in female and male patients with obstructive sleep apnea syndrome. Psychol. Health Med. 2012, 17, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Basner, R.C. Continuous positive airway pressure for obstructive sleep apnea. N. Engl. J. Med. 2007, 356, 1751–1758. [Google Scholar] [CrossRef]
- Kato, M.; Adachi, T.; Koshino, Y.; Somers, V.K. Obstructive sleep apnea and cardiovascular disease. Circ. J. 2009, 73, 1363–1370. [Google Scholar] [CrossRef]
- Goyal, M.; Johnson, J. Obstructive Sleep Apnea Diagnosis and Management. Mo. Med. 2017, 114, 120–124. [Google Scholar]
- Karna, B.; Sankari, A.; Tatikonda, G. Sleep disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Badran, M.; Golbidi, S.; Ayas, N.; Laher, I. Nitric Oxide Bioavailability in Obstructive Sleep Apnea: Interplay of Asymmetric Dimethylarginine and Free Radicals. Sleep Disord. 2015, 2015, 387801. [Google Scholar] [CrossRef]
- Victor, L.D. Obstructive sleep apnea. Am. Fam. Physician 1999, 60, 2279–2286. [Google Scholar] [PubMed]
- Devaraj, U.; Maheswari, U.; Balla, S.; Pinto, A.M.; Venkatnarayan, K.; Ramachandran, P.; Veluthat, C.; Souza, G.D. Prevalence and Risk Factors for OSA among Urban and Rural Subjects in Bengaluru District, South India: A Cross-sectional Study. Indian J. Sleep Med. 2021, 16, 5–9. [Google Scholar] [CrossRef]
- Villaneuva, A.T.C.; Buchanan, P.R.; Yee, B.J.; Grunstein, R.R. Ethnicity and obstructive sleep apnoea. Sleep Med. Rev. 2005, 9, 419–436. [Google Scholar] [CrossRef]
- Ghosh, P.; Sapna Varma, N.K.; Ajith, V.V.; Prabha, R.D.; Raj, M. Epidemiological study on prevalent risk factors and craniofacial skeletal patterns in obstructive sleep apnea among South Indian population. Indian J. Dent. Res. 2020, 31, 784–790. [Google Scholar]
- Forcelini, C.M.; Buligon, C.M.; Costa, G.J.K.; Petter, G.D.C.; Scapin, H.P.; Augustin, I.A.; Dal-Piva, L.D.M.; Durgante, R.E.; Lorenzoni, V.P. Age-dependent influence of gender on symptoms of obstructive sleep apnea in adults. Sleep Sci. 2019, 12, 132–137. [Google Scholar]
- Bixler, E.O.; Vgontzas, A.N.; Ten Have, T.; Tyson, K.; Kales, A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am. J. Respir. Crit. Care Med. 1998, 157, 144–148. [Google Scholar] [CrossRef]
- Ernst, G.; Mariani, J.; Blanco, M.; Finn, B.; Salvado, A.; Borsini, E. Increase in the frequency of obstructive sleep apnea in elderly people. Sleep Sci. 2019, 12, 222–226. [Google Scholar]
- Deng, X.; Gu, W.; Li, Y.; Liu, M.; Li, Y.; Gao, X. Age-group-specific associations between the severity of obstructive sleep apnea and relevant risk factors in male and female patients. PLoS ONE 2014, 9, e107380. [Google Scholar] [CrossRef]
- Mohsenin, V. Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep Med. 2003, 4, 523. [Google Scholar] [CrossRef] [PubMed]
- Erdemir Işık, M.; Gulbay, B.; Çiftci, F.; Acıcan, T. Polysomnographic, demographic and clinic differences between male and female obstructive sleep apnea patients. Tuberk. Toraks 2020, 68, 361–370. [Google Scholar] [CrossRef]
- Perger, E.; Mattaliano, P.; Lombardi, C. Menopause and sleep apnea. Maturitas 2019, 124, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Sigurðardóttir, E.S.; Gislason, T.; Benediktsdottir, B.; Hustad, S.; Dadvand, P.; Demoly, P.; Franklin, K.A.; Heinrich, J.; Holm, M.; van der Plaat, D.A.; et al. Female sex hormones and symptoms of obstructive sleep apnea in European women of a population-based cohort. PLoS ONE 2022, 17, e0269569. [Google Scholar] [CrossRef]
- Wimms, A.; Woehrle, H.; Ketheeswaran, S.; Ramanan, D.; Armitstead, J. Obstructive Sleep Apnea in Women: Specific Issues and Interventions. BioMed Res. Int. 2016, 2016, 1764837. [Google Scholar] [CrossRef]
- Glicksman, A.; Hadjiyannakis, S.; Barrowman, N.; Walker, S.; Hoey, L.; Katz, S.L. Body Fat Distribution Ratios and Obstructive Sleep Apnea Severity in Youth with Obesity. J. Clin. Sleep Med. 2017, 13, 545–550. [Google Scholar] [CrossRef]
- Lim, D.C.; Pack, A.I. Obstructive Sleep Apnea: Update and Future. Annu. Rev. Med. 2016, 68, 99–112. [Google Scholar]
- Chetan, I.M.; Maierean, A.D.; Domokos Gergely, B.; Cabau, G.; Tomoaia, R.; Chis, A.F.; Albu, A.; Stoia, M.A.; Vesa, S.C.; Blendea, D.; et al. A Prospective Study of CPAP Therapy in Relation to Cardiovascular Outcome in a Cohort of Romanian Obstructive Sleep Apnea Patients. J. Pers. Med. 2021, 11, 1001. [Google Scholar] [CrossRef]
- Harsch, I.; Konturek, P.; Koebnick, C.; Kuehnlein, P.; Fuchs, F.; Pour Schahin, S.; Wiest, G.; Hahn, E.; Lohmann, T.; Ficker, J. Leptin and ghrelin levels in patients with obstructive sleep apnoea: Effect of CPAP treatment. Eur. Respir. J. 2003, 22, 251–257. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Patil, S.P.; Laffan, A.M.; Polotsky, V.; Schneider, H.; Smith, P.L. Obesity and obstructive sleep apnea: Pathogenic mechanisms and therapeutic approaches. Proc. Am. Thorac. Soc. 2008, 5, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Wolk, R.; Shamsuzzaman, A.S.M.; Somers, V.K. Obesity, Sleep Apnea, and Hypertension. Hypertension 2003, 42, 1067–1074. [Google Scholar] [CrossRef]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017, 1, 00019. [Google Scholar]
- Ciavarella, D.; Tepedino, M.; Chimenti, C.; Troiano, G.; Mazzotta, M.; Foschino Barbaro, M.P.; Lo Muzio, L.; Cassano, M. Correlation between body mass index and obstructive sleep apnea severity indexes—A retrospective study. Am. J. Otolaryngol. 2018, 39, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Huang, J.; Zheng, M.; Zhao, W.; Lao, L.; Li, H.; Wang, Z.; Lu, J.; Chen, W.; Deng, H.; et al. Association of healthy lifestyle with risk of obstructive sleep apnea: A cross-sectional study. BMC Pulm. Med. 2022, 22, 33. [Google Scholar] [CrossRef]
- Ioannidou, D.; Kalamaras, G.; Kotoulas, S.-C.; Pataka, A. Smoking and Obstructive Sleep Apnea: Is There An Association between These Cardiometabolic Risk Factors?—Gender Analysis. Medicina 2021, 57, 1137. [Google Scholar] [CrossRef]
- Yang, S.; Guo, X.; Liu, W.; Li, Y.; Liu, Y. Alcohol as an independent risk factor for obstructive sleep apnea. Ir. J. Med. Sci. 2022, 191, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Sowho, M.; Sgambati, F.; Guzman, M.; Schneider, H.; Schwartz, A. Snoring: A source of noise pollution and sleep apnea predictor. Sleep 2020, 15, zsz305. [Google Scholar] [CrossRef] [PubMed]
- Lucia, S.; Caruso, D.; Di Maria, G. Obstructive sleep apnoea syndrome and its management. Ther. Adv. Chronic Dis. 2015, 6, 273–285. [Google Scholar]
- Rajendran, A.; Danyluk, A.B.; Smith, K.L.; Boeve, A.R.; Bukartyk, J.; Singh, P.R.; Somers, V.K.; Covassin, N.K. Family History of Obstructive Sleep Apnea and Risk of Obesity. Physiology 2019, 33, lb520. [Google Scholar] [CrossRef]
- Budhiraja, R.; Javaheri, S.; Parthasarathy, S.; Berry, R.B.; Quan, S.F. The Association Between Obstructive Sleep Apnea Characterized by a Minimum 3 Percent Oxygen Desaturation or Arousal Hypopnea Definition and Hypertension. J. Clin. Sleep Med. 2019, 15, 1261–1270. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Zucconi, M.; Palazzi, S.; Castronovo, V.; Oldani, A.; della Marca, G.; Smirne, S. Snoring and nocturnal oxygen desaturations in an Italian middle-aged male population. Epidemiologic study with an ambulatory device. Chest 1994, 105, 1759–1764. [Google Scholar] [CrossRef]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef]
- Pokharel, M.; Shrestha, B.L.; Dhakal, A.; Rajbhandari, P.; Shrestha, K.S.; Kc, A.K.; Bhattarai, A.; Karki, D.R. Clinical Profile and Diagnosis of Obstructive Sleep Apnea Syndrome using Overnight Polysomnography in a Tertiary Care Hospital. Kathmandu Univ. Med. J. 2021, 19, 361–365. [Google Scholar] [CrossRef]
- Wali, S.O.; Abaalkhail, B.; AlQassas, I.; Alhejaili, F.; Spence, D.W.; Pandi-Perumal, S.R. The correlation between oxygen saturation indices and the standard obstructive sleep apnea severity. Ann. Thorac. Med. 2020, 15, 70–75. [Google Scholar] [CrossRef]
- Young, T.; Shahar, E.; Nieto, F.J.; Redline, S.; Newman, A.B.; Gottlieb, D.J.; Walsleben, J.A.; Finn, L.; Enright, P.; Samet, J.M.; et al. Predictors of sleep-disordered breathing in community-dwelling adults: The sleep heart health study. Arch. Intern. Med. 2002, 162, 893–900. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and leep-disordered breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Almaghaslah, D.; Sivanandy, P.; Arumugam, S. Effectiveness of nasal continuous airway pressure therapy in patients with obstructive sleep apnea. Int. J. Health Plann. Manag. 2019, 34, e1200–e1207. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Sánchez-de-la-Torre, M.; White, D.P.; Azarbarzin, A. Hypoxic Burden in Obstructive Sleep Apnea: Present and Future. Arch. Broconeumol. 2023, 59, 36–43. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Sex differences in respiratory function. Breathe 2018, 14, 131–140. [Google Scholar] [CrossRef]
- Pham, L.V.; Schwartz, A.R. The pathogenesis of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 1358–1372. [Google Scholar] [PubMed]


| Category | Non-OSA (41) | OSA Patients (175) |
|---|---|---|
| Number (%) | Number (%) | |
| Gender | ||
| Male | 32 (78.0) | 143 (81.7) |
| Female | 9 (22.0) | 32 (18.3) |
| Age | ||
| Male | 55.41 ± 11.24 | |
| Female | 52.11 ± 3.12 |
| Variables | Non-OSA (AHI < 5) n (%) | Mild OSA (AHI >5–15) n (%) | Moderate OSA (AHI16–29) n (%) | Severe OSA (AHI > 30) n (%) |
|---|---|---|---|---|
| AHI (events/hour) | 41 (18.6) | 30 (17.1) | 60 (34.3) | 85 (48.6) |
| BMI Kg/m2 | ||||
| Ideal | 15 (36.58) | 6 (3.4) | 7 (4) | 6 (3.4) |
| Overweight | 18 (43.9) | 10 (5.7) | 21(12) | 21 (12) |
| Obese | 8(19.5) | 15 (8.6) | 30 (17.1) | 59 (33.7) |
| Smoking | 5 (12.2) | 4 (2.3) | 14 (8) | 17 (9.7) |
| Alcohol | 6 (14.6) | 7 (4) | 15 (8.6) | 33 (18.6) |
| Variables | Non-OSA (AHI < 5) | Mild OSA
(AHI 5–14.9) | Moderate OSA (AHI 15–30) | Severe OSA (AHI ≥30) | Significance (p) |
|---|---|---|---|---|---|
| AHI (events/hour) | 1.69 ± 1.34 | 11.79 ± 3.55 | 22.12 ± 4.34 | 59.16 ± 22.15 | 0.00 |
| BMI (kg/m2) | 26.12 ± 2.72 | 31.66 ± 8.32 | 30.52 ± 3.99 | 34.35 ± 8.22 | 0.03 |
| Average oxygen saturation (%) | 95.92 ± 4.15 | 95.66 ± 1.98 | 95.22 ± 2.10 | 92.12 ± 3.44 | 0.00 |
| Oxygen desaturation (events/hour) | 6.09 ± 10.98 | 21.22 ± 20.66 | 23.38 ± 16.23 | 50.04 ± 26.67 | 0.00 |
| Snoring time (minutes) | 22.22 ± 39.61 | 21.99 ± 25.51 | 22.86 ± 16.49 | 31.39 ± 32.51 | 0.25 |
| No. of snores | 35.68 ± 59.78 | 41.49 ± 43.12 | 46.33 ± 39.58 | 86.44 ± 102.88 | 0.00 |
| Symptoms | n (%) |
|---|---|
| Loud snoring | 140 (80) |
| Breathing problem | 98 (56) |
| Day time sleepiness | 112 (64) |
| Family history of snoring | 45 (25.7) |
| Variables | Mean ± SD | r-Value | p-Value |
|---|---|---|---|
| BMI (kg/m2) | 32.17 ± 6.84 | 0.249 | 0.001 |
| Total time analyzed (minutes) | 417.03 ± 34.97 | 0.075 | 0.324 |
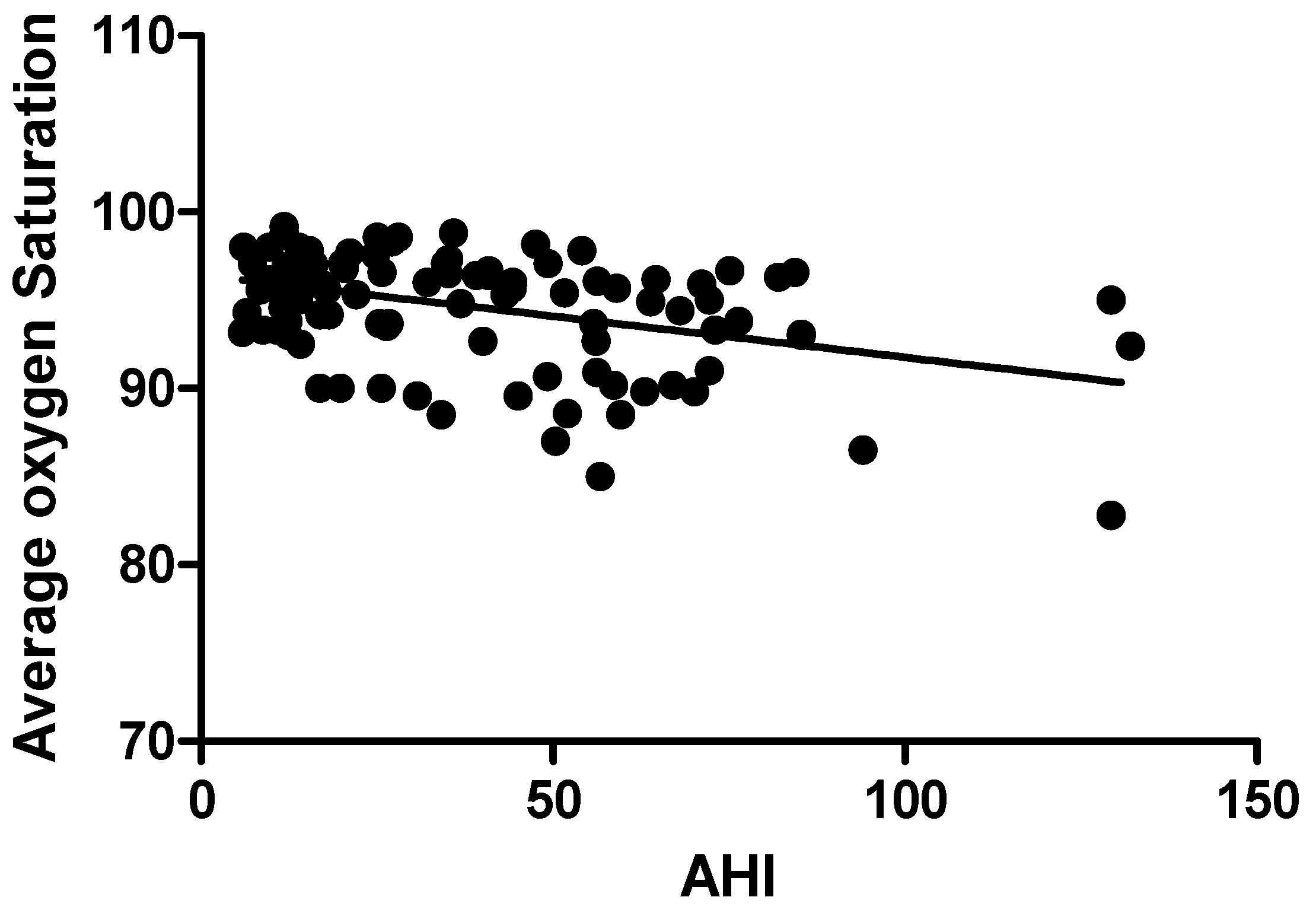
| Average oxygen saturation (%) | 94.65 ± 3.17 | −0.387 | 0.000 |
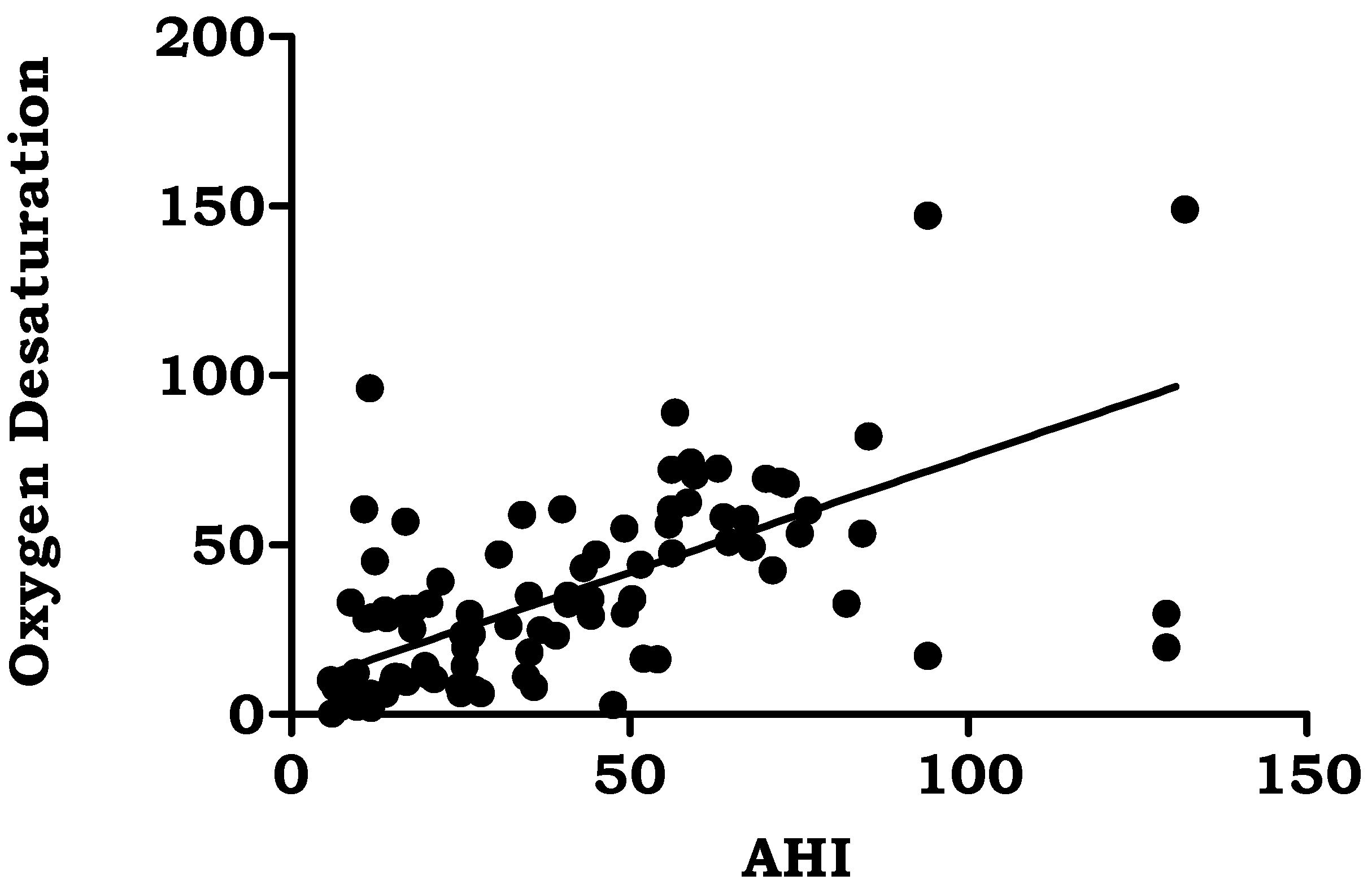
| Oxygen desaturation (events/hour) | 29.29 ± 20.38 | 0.661 | 0.000 |
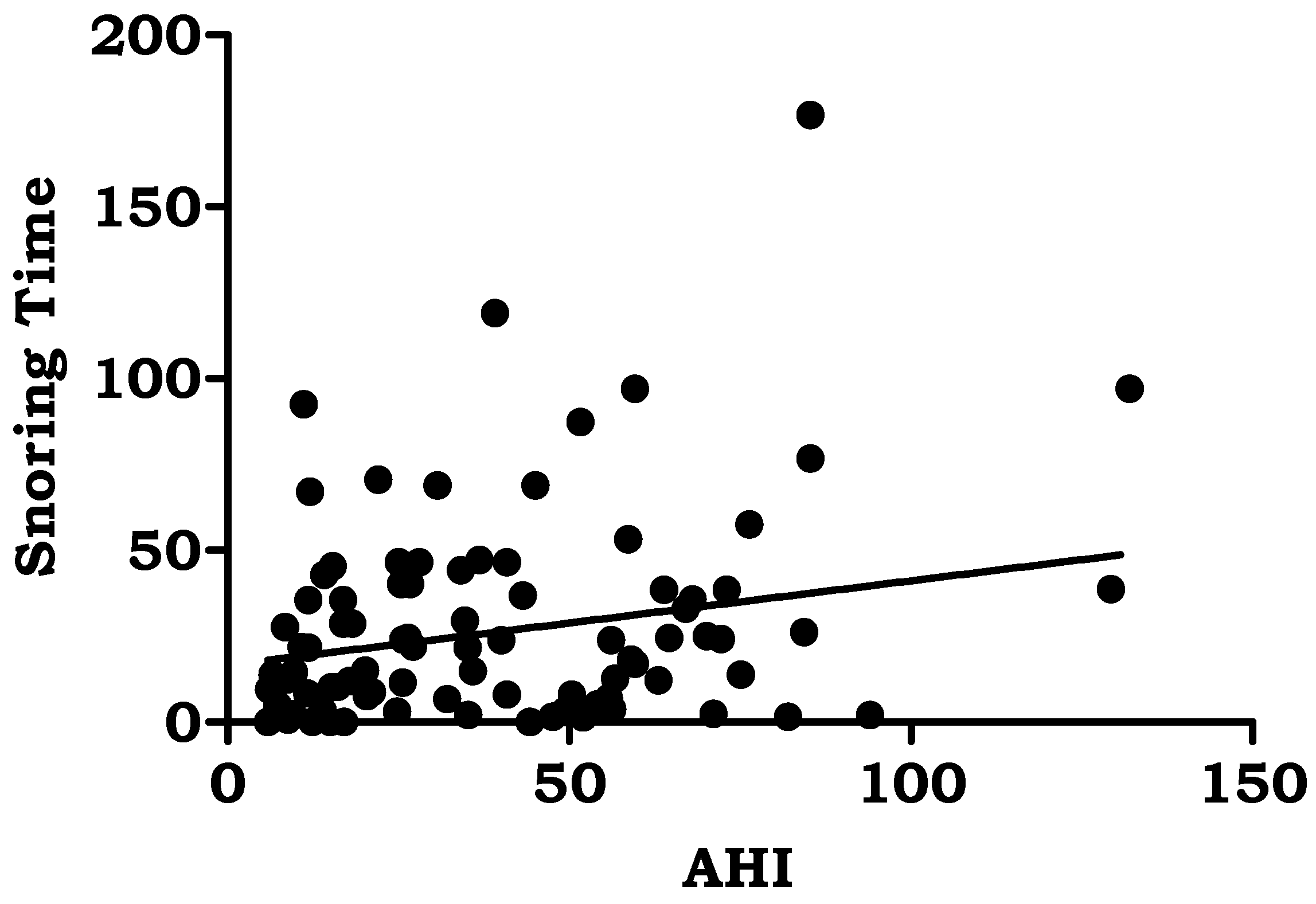
| Snoring time (minutes) | 24.48 ± 25.90 | 0.231 | 0.002 |
| Number of snores | 58.49 ± 62.86 | 0.383 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandasamy, G.; Almeleebia, T. A Prospective Study on Obstructive Sleep Apnea, Clinical Profile and Polysomnographic Variables. J. Pers. Med. 2023, 13, 919. https://doi.org/10.3390/jpm13060919
Kandasamy G, Almeleebia T. A Prospective Study on Obstructive Sleep Apnea, Clinical Profile and Polysomnographic Variables. Journal of Personalized Medicine. 2023; 13(6):919. https://doi.org/10.3390/jpm13060919
Chicago/Turabian StyleKandasamy, Geetha, and Tahani Almeleebia. 2023. "A Prospective Study on Obstructive Sleep Apnea, Clinical Profile and Polysomnographic Variables" Journal of Personalized Medicine 13, no. 6: 919. https://doi.org/10.3390/jpm13060919
APA StyleKandasamy, G., & Almeleebia, T. (2023). A Prospective Study on Obstructive Sleep Apnea, Clinical Profile and Polysomnographic Variables. Journal of Personalized Medicine, 13(6), 919. https://doi.org/10.3390/jpm13060919






