Prognostic Value of Serum Transferrin Level before Radiotherapy on Radio-Sensitivity and Survival in Patients with Nasopharyngeal Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval of the Study Protocol
2.2. Criteria
2.3. Data Collection
2.4. Detection of TRF
2.5. Treatments
2.6. Assessment of Radio-Sensitivity
2.7. Follow-Up
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-L.; Guo, R.; Li, J.-Y.; Xu, C.; Mao, Y.-P.; Tian, L.; Lin, A.-H.; Sun, Y.; Ma, J.; Tang, L.-L. Nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: Clinical outcomes and patterns of failure among subsets of 8th AJCC stage IVa. Eur. Radiol. 2020, 30, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Ismaila, N.; Chua, M.L.K.; Colevas, A.D.; Haddad, R.; Huang, S.H.; Wee, J.T.S.; Whitley, A.C.; Yi, J.-L.; Yom, S.S.; et al. Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J. Clin. Oncol. 2021, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L.-L.; Li, Y.-Q.; Liu, X.; Liu, Q.; Ma, J. Spontaneous remission of residual post-therapy plasma Epstein-Barr virus DNA and its prognostic implication in nasopharyngeal carcinoma: A large-scale, big-data intelligence platform-based analysis. Int. J. Cancer 2019, 144, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Li, W.; Ma, B.; Lam, W.; Chan, K.; Mo, F.; Ai, Q.; King, A.; Wong, C.; Guo, R.; et al. Integrating postradiotherapy plasma Epstein-Barr virus DNA and TNM stage for risk stratification of nasopharyngeal carcinoma to adjuvant therapy. Ann. Oncol. 2020, 31, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ng, W.T.; Zong, J.F.; Chan, L.L.K.; O’Sullivan, B.; Lin, S.J.; Sze, H.C.K.; Bin Chen, Y.; Choi, H.C.W.; Guo, Q.J.; et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016, 122, 546–558. [Google Scholar] [CrossRef]
- Guo, S.-S.; Liang, Y.-J.; Liu, L.-T.; Chen, Q.-Y.; Wen, Y.-F.; Liu, S.-L.; Sun, X.-S.; Tang, Q.-N.; Li, X.-Y.; Mai, H.-Q.; et al. Increased Angiogenin Expression Correlates with Radiation Resistance and Predicts Poor Survival for Patients with Nasopharyngeal Carcinoma. Front. Pharmacol. 2021, 12, 627935. [Google Scholar] [CrossRef]
- Xue, F.; Ou, D.; Hu, C.; He, X. Local regression and control of T1-2 nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Cancer Med. 2018, 7, 6010–6019. [Google Scholar] [CrossRef]
- Salem, A.; Mistry, H.; Hatton, M.; Locke, I.; Monnet, I.; Blackhall, F.; Faivre-Finn, C. Association of Chemoradiotherapy With Outcomes Among Patients With Stage I to II vs Stage III Small Cell Lung Cancer: Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2019, 5, e185335. [Google Scholar] [CrossRef]
- Busk, M.; Horsman, M.R.; Overgaard, J.; Jakobsen, S. Dual-tracer PET of viable tumor volume and hypoxia for identification of necrosis-containing radio-resistant Sub-volumes. Acta Oncol. 2019, 58, 1476–1482. [Google Scholar] [CrossRef]
- Harrison, L.; Blackwell, K. Hypoxia and anemia: Factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist 2004, 9 (Suppl. S5), 31–40. [Google Scholar] [CrossRef]
- Stüben, G.; Pöttgen, C.; Knühmann, K.; Schmid, K.; Stuschke, M.; Thews, O.; Vaupel, P. Erythropoietin restores the anemia-induced reduction in radio-sensitivity of experimental human tumors in nude mice. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Wozny, A.-S.; Gauthier, A.; Alphonse, G.; Malésys, C.; Varoclier, V.; Beuve, M.; Brichart-Vernos, D.; Magné, N.; Vial, N.; Ardail, D.; et al. Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation. Cancers 2021, 13, 3833. [Google Scholar] [CrossRef] [PubMed]
- Melsens, E.; De Vlieghere, E.; Descamps, B.; Vanhove, C.; Kersemans, K.; De Vos, F.; Goethals, I.; Brans, B.; De Wever, O.; Ceelen, W.; et al. Hypoxia imaging with (18)F-FAZA PET/CT predicts radiotherapy response in esophageal adenocarcinoma xenografts. Radiat. Oncol. 2018, 13, 39. [Google Scholar] [CrossRef]
- Chen, H.; Luo, M.; Wang, X.; Liang, T.; Huang, C.; Huang, C.; Wei, L. Inhibition of PAD4 enhances radio-sensitivity and inhibits aggressive phenotypes of nasopharyngeal carcinoma cells. Cell Mol. Biol. Lett. 2021, 26, 9. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yan, M.; Zheng, J.; Li, R.; Lin, J.; Xu, A.; Liang, Y.; Zheng, R.; Yuan, Y. miR-483-5p decreases the radio-sensitivity of nasopharyngeal carcinoma cells by targeting DAPK1. Lab. Investig. 2019, 99, 602–611. [Google Scholar] [CrossRef]
- He, Y.; Jing, Y.; Wei, F.; Tang, Y.; Yang, L.; Luo, J.; Yang, P.; Ni, Q.; Pang, J.; Liao, Q.; et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, J.; Liu, J.; Wu, G.; Yu, Y.; Zhu, H.; Liu, J. LncRNA ANCR promotes proliferation and radiation resistance of nasopharyngeal carcinoma by inhibiting PTEN expression. OncoTargets Ther. 2018, 11, 8399–8408. [Google Scholar] [CrossRef]
- Huang, W.; Shi, G.; Yong, Z.; Li, J.; Qiu, J.; Cao, Y.; Zhao, Y.; Yuan, L. Downregulation of RKIP promotes radioresistance of nasopharyngeal carcinoma by activating NRF2/NQO1 axis via downregulating miR-450b-5p. Cell Death Dis. 2020, 11, 504. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, Y.; Tu, Y.; Zhang, G.; Zeng, X.; Lin, J.; Ai, M.; Mao, Z.; Zheng, R.; Yuan, Y. microRNA-124 inhibits stem-like properties and enhances radio-sensitivity in nasopharyngeal carcinoma cells via direct repression of expression of JAMA. J. Cell Mol. Med. 2020, 24, 9533–9544. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, J.-W.; Liu, G.-Y.; Wang, F.-G.; Ju, Z.-S.; Zhou, D.; Wang, R.-Y. MicroRNA-372 enhances radio-sensitivity while inhibiting cell invasion and metastasis in nasopharyngeal carcinoma through activating the PBK-dependent p53 signaling pathway. Cancer Med. 2019, 8, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xiao, W.; Tian, Y.; Lan, Y.; Zhang, C.; Bai, L. MicroRNA-195-3p inhibits cyclin dependent kinase 1 to induce radio-sensitivity in nasopharyngeal carcinoma. Bioengineered 2021, 12, 7325–7334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Ren, H.; Lin, F.; Pan, Z.; Wu, L.; Yang, N. Evaluation of the Effect of Nutritional Intervention on Patients with Nasopharyngeal Carcinoma. J. Healthc. Eng. 2022, 2022, 2531671. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Onda, S.; Taniai, T.; Hamura, R.; Kumamoto, T.; Shirai, Y.; Yasuda, J.; Haruki, K.; Shiozaki, H.; Gocho, T.; et al. Transferrin predicts outcome in patients who underwent liver resection for colorectal liver metastases. Jpn. J. Clin. Oncol. 2021, 51, 1400–1406. [Google Scholar] [CrossRef]
- Sawayama, H.; Miyamoto, Y.; Hiyoshi, Y.; Shimokawa, M.; Kato, R.; Akiyama, T.; Sakamoto, Y.; Daitoku, N.; Yoshida, N.; Baba, H. Preoperative transferrin level is a novel prognostic marker for colorectal cancer. Ann. Gastroenterol. Surg. 2021, 5, 243–251. [Google Scholar] [CrossRef]
- Yamane, T.; Sawayama, H.; Yoshida, N.; Morinaga, T.; Akiyama, T.; Eto, K.; Harada, K.; Ogawa, K.; Iwatsuki, M.; Iwagami, S.; et al. Preoperative transferrin level is a novel indicator of short- and long-term outcomes after esophageal cancer surgery. Int. J. Clin. Oncol. 2021, 27, 131–140. [Google Scholar] [CrossRef]
- Hong, J.; Yao, Y.; Zhang, Y.; Tang, T.; Zhang, H.; Bao, D.; Chen, Y.; Pan, J. Value of magnetic resonance diffusion-weighted imaging for the prediction of radio-sensitivity in nasopharyngeal carcinoma. Otolaryngol. Head Neck Surg. 2013, 149, 707–713. [Google Scholar] [CrossRef]
- Shigeta, S.; Toyoshima, M.; Kitatani, K.; Ishibashi, M.; Usui, T.; Yaegashi, N. Transferrin facilitates the formation of DNA double-strand breaks via transferrin receptor 1: The possible involvement of transferrin in carcinogenesis of high-grade serous ovarian cancer. Oncogene 2016, 35, 3577–3586. [Google Scholar] [CrossRef]
- Park, E.; Chung, S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; You, J.H.; Shin, D.; Roh, J.-L. Inhibition of Glutaredoxin 5 predisposes Cisplatin-resistant Head and Neck Cancer Cells to Ferroptosis. Theranostics 2020, 10, 7775–7786. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Dai, R.; Xu, J.; Feng, H. Transferrin receptor-1 and VEGF are prognostic factors for osteosarcoma. J. Orthop. Surg. Res. 2019, 14, 296. [Google Scholar] [CrossRef]
- Zhao, Q.; Ji, J.; Cai, Q.; Wang, C.; Shi, M.; Zhou, C.; Zhu, Z.; Zhang, J. Low expression of transferrin receptor 2 predict poor prognosis in gastric cancer patients. Kaohsiung J. Med. Sci. 2020, 36, 1014–1020. [Google Scholar] [CrossRef]
- Deng, J.; He, Y.; Sun, X.-S.; Li, J.-M.; Xin, M.-Z.; Li, W.-Q.; Li, Z.-X.; Nie, S.; Wang, C.; Li, Y.-Z.; et al. Construction of a comprehensive nutritional index and its correlation with quality of life and survival in patients with nasopharyngeal carcinoma undergoing IMRT: A prospective study. Oral. Oncol. 2019, 98, 62–68. [Google Scholar] [CrossRef]
- Ho, Y.-W.; Yeh, K.-Y.; Hsueh, S.-W.; Hung, C.-Y.; Lu, C.-H.; Tsang, N.-M.; Wang, H.-M.; Hung, Y.-S.; Chou, W.-C. Impact of early nutrition counseling in head and neck cancer patients with normal nutritional status. Support. Care Cancer 2021, 29, 2777–2785. [Google Scholar] [CrossRef]
- Hong, J.-S.; Hua, Y.-J.; Su, L.; Zhang, H.-R.; Lv, W.-L.; Chen, X.-Y.; Tian, J.; Zhang, W.-J. Modified-Nutrition Index is a Significant Prognostic Factor for the Overall Survival of the Nasopharyngeal Carcinoma Patients who Undergo Intensity-modulated Radiotherapy. Nutr. Cancer 2017, 69, 1011–1018. [Google Scholar] [CrossRef]
- Kono, T.; Sakamoto, K.; Shinden, S.; Ogawa, K. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin. Nutr. 2017, 36, 1681–1685. [Google Scholar] [CrossRef]
- Li, G.; Gao, J.; Liu, Z.-G.; Tao, Y.-L.; Xu, B.-Q.; Tu, Z.-W.; Zhang, X.-P.; Zeng, M.; Xia, Y.-F. Influence of pretreatment ideal body weight percentile and albumin on prognosis of nasopharyngeal carcinoma: Long-term outcomes of 512 patients from a single institution. Head Neck 2014, 36, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.C.; Knuiman, M.W.; Trinder, D.; Divitini, M.L.; Olynyk, J.K. Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am. J. Clin. Nutr. 2016, 104, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Hu, G.-Q.; Zhang, N.; Zhu, X.-D.; Yang, K.-Y.; Jin, F.; Shi, M.; Chen, Y.-P.; Hu, W.-H.; et al. Final Overall Survival Analysis of Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma: A Multicenter, Randomized Phase III Trial. J. Clin. Oncol. 2022, 40, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Huang, S.H.; Taylor, R.; Su, J.; Xu, W.; Hansen, A.R.; Jang, R.; Bayley, A.; Hosni, A.; Giuliani, M.; et al. Impact of cumulative cisplatin dose and adjuvant chemotherapy in locally-advanced nasopharyngeal carcinoma treated with definitive chemoradiotherapy. Oral. Oncol. 2020, 105, 104666. [Google Scholar] [CrossRef] [PubMed]
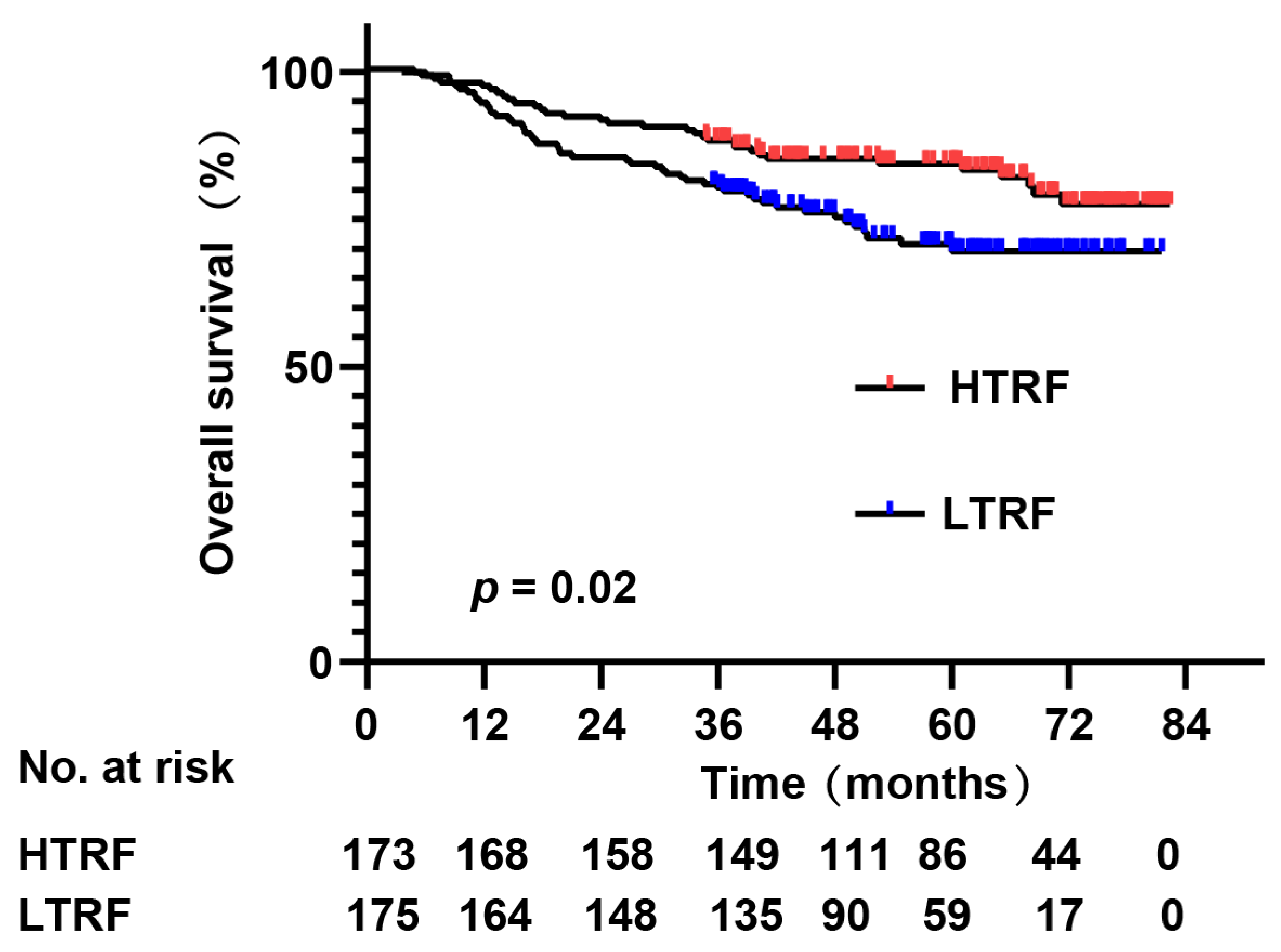
| Variables | LTRF (≤2.16 g/L) | HTRF (>2.16 g/L) | χ2 | p |
|---|---|---|---|---|
| Gender | ||||
| Male | 143 | 105 | 18.772 | 0.001 |
| Female | 32 | 68 | ||
| Age(years) | ||||
| ≤40 | 40 | 43 | 0.191 | 0.662 |
| >40 | 135 | 130 | ||
| Education | ||||
| Illiteracy | 67 | 64 | 0.062 | 0.804 |
| Not illiteracy | 108 | 109 | ||
| Chronic disease | ||||
| No | 139 | 127 | 1.749 | 0.186 |
| Yes | 46 | 36 | ||
| Histology type * | ||||
| I + II | 38 | 42 | 0.323 | 0.570 |
| III | 137 | 131 | ||
| AJCC 8th T classification | ||||
| T1–T2 | 51 | 60 | 1.229 | 0.268 |
| T3–T4 | 124 | 113 | ||
| AJCC 8th N classification | ||||
| N0–N1 | 97 | 79 | 3.318 | 0.069 |
| N2–N3 | 78 | 94 | ||
| AJCC 8th TNM classification | ||||
| I–III | 85 | 90 | 2.276 | 0.131 |
| IV | 98 | 75 | ||
| Chemotherapy | ||||
| No | 19 | 27 | 1.711 | 0.191 |
| Yes | 156 | 146 | ||
| Absorbed dose of GTVnx (Gy) | ||||
| ≤70 | 142 | 135 | 0.518 | 0.472 |
| >70 | 32 | 38 |
| Variables | Assign | B | SE | Wald | p | OR (95%CI) |
|---|---|---|---|---|---|---|
| Gender | Male | |||||
| Female | 0.305 | 0.281 | 1.059 | 0.278 | 1.356 (0.782–2.352) | |
| Age (years) | ≤40 | |||||
| >40 | 0.190 | 0.294 | 0.419 | 0.517 | 1.209 (0.680–2.150) | |
| Education | Illiteracy | |||||
| Not illiteracy | 0.450 | 0.266 | 2.860 | 0.091 | 1.568 (0.931–2.641) | |
| Chronic disease | No | |||||
| Yes | 0.446 | 0.311 | 2.056 | 0.152 | 1.561 (0.849–2.871) | |
| Histology type * | I + II | |||||
| III | 0.233 | 0.291 | 0.639 | 0.424 | 1.262 (0.713–2.234) | |
| AJCC 8th T classification | T1–T2 | |||||
| T3–T4 | 0.110 | 0.261 | 0.176 | 0.675 | 1.116 (0.669–1.862) | |
| Chemotherapy | No | |||||
| Yes | 0.168 | 0.362 | 0.216 | 0.642 | 1.183 (0.582–2.406) | |
| Absorbed dose of GTVnx (Gy) | <70 | |||||
| ≥70 | 0.197 | 0.293 | 0.453 | 0.501 | 1.218 (0.686–2.163) | |
| Transferrin before IMRT | LTRF | |||||
| HTRF | 0.532 | 0.249 | 4.549 | 0.033 | 1.702 (1.044–2.775) |
| Variables | Assign | B | SE | Wald | p | HR (95%CI) |
|---|---|---|---|---|---|---|
| Gender | Male | |||||
| Female | 0.119 | 0.306 | 0.151 | 0.697 | 1.126 (0.619–2.051) | |
| Age(years) | ≤40 | |||||
| >40 | 0.213 | 0.228 | 0.548 | 0.459 | 1.238 (0.704–2.176) | |
| Education level | Illiteracy | |||||
| Not illiteracy | 0.563 | 0.264 | 4.535 | 0.033 | 1.756 (1.046–2.949) | |
| With chronic disease | No | |||||
| Yes | 0.476 | 0.258 | 3.421 | 0.064 | 1.610 (0.972–2.667) | |
| Histology type * | I + II | |||||
| III | 0.121 | 0.266 | 0.206 | 0.650 | 1.128 (0.670–1.899) | |
| AJCC 8th T classification | T1–T2 | |||||
| T3–T4 | 1.024 | 0.318 | 10.369 | 0.001 | 2.784 (1.493–5.192) | |
| Chemotherapy | No | |||||
| Yes | 0.210 | 0.328 | 0.410 | 0.522 | 1.234 (0.649–2.346) | |
| Absorbed dose of GTVnx (Gy) | <70 | |||||
| ≥70 | 0.285 | 0.260 | 1.195 | 0.274 | 1.329 (0.798–2.214) | |
| Transferrin before IMRT | LTRF | |||||
| HTRF | 0.534 | 0.240 | 4.945 | 0.026 | 1.706 (1.065–2.731) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.; Su, L.; Lin, Q.; Pan, X.; Li, X.; Zhou, W.; Zhang, W.; Hong, J. Prognostic Value of Serum Transferrin Level before Radiotherapy on Radio-Sensitivity and Survival in Patients with Nasopharyngeal Carcinoma. J. Pers. Med. 2023, 13, 511. https://doi.org/10.3390/jpm13030511
Zhan Y, Su L, Lin Q, Pan X, Li X, Zhou W, Zhang W, Hong J. Prognostic Value of Serum Transferrin Level before Radiotherapy on Radio-Sensitivity and Survival in Patients with Nasopharyngeal Carcinoma. Journal of Personalized Medicine. 2023; 13(3):511. https://doi.org/10.3390/jpm13030511
Chicago/Turabian StyleZhan, Yuping, Li Su, Qiaojing Lin, Xiaoxian Pan, Xiaoxia Li, Weitong Zhou, Weijian Zhang, and Jinsheng Hong. 2023. "Prognostic Value of Serum Transferrin Level before Radiotherapy on Radio-Sensitivity and Survival in Patients with Nasopharyngeal Carcinoma" Journal of Personalized Medicine 13, no. 3: 511. https://doi.org/10.3390/jpm13030511
APA StyleZhan, Y., Su, L., Lin, Q., Pan, X., Li, X., Zhou, W., Zhang, W., & Hong, J. (2023). Prognostic Value of Serum Transferrin Level before Radiotherapy on Radio-Sensitivity and Survival in Patients with Nasopharyngeal Carcinoma. Journal of Personalized Medicine, 13(3), 511. https://doi.org/10.3390/jpm13030511






