Abstract
Background: Insulin secretion and glucose tolerance is annually assessed in patients with cystic fibrosis (PwCF) through oral glucose tolerance tests (OGTTs) as a screening measure for cystic fibrosis-related diabetes. We aimed to describe the distribution and provide reference quartiles of OGTT-related variables in the Italian cystic fibrosis population. Methods: Cross-sectional study of PwCF receiving care in three Italian cystic fibrosis centers of excellence, from 2016 to 2020. We performed a modified 2-h OGTT protocol (1.75 g/kg, maximum 75 g), sampling at baseline and at 30-min intervals, analyzing plasma glucose, serum insulin, and C-peptide. The modified OGTT allowed for the modeling of β cell function. For all variables, multivariable quantile regression was performed to estimate the median, the 25th, and 75th percentiles, with age, sex, and pancreatic insufficiency as predictors. Results: We have quantified the deterioration of glucose tolerance and insulin secretion with age according to sex and pancreatic insufficiency, highlighting a deviation from linearity both for patients <10 years and >35 years of age. Conclusions: References of OGTT variables for PwCF provide a necessary tool to not only identify patients at risk for CFRD or other cystic fibrosis-related complications, but also to evaluate the effects of promising pharmacological therapies.
1. Introduction
Cystic fibrosis-related diabetes (CFRD) is the most common comorbidity in patients with cystic fibrosis (PwCF), with a reported prevalence increasing with age, and affecting approximately 40% of adult PwCF [1,2]. Abnormalities of insulin secretion, compromised nutritional status, and more severe lung inflammation are all associated in patients with CFRD [3]. These conditions accelerate the decline in lung function, ultimately leading to lower survival.
In recent years, the introduction of modulator therapies directly targeting the underlying defect of cystic fibrosis changed the natural history of the disease, improving both the nutritional status and pulmonary function of PwCF [4]. While CFTR modulator therapy of any kind does not constitute a cure for CFRD to this day, only limited data are available on long-term effects and early (pre-puberal) administration. Of available CFTR modulator therapies, the ivacaftor monotherapy showed the most promising results [5,6], while the lumacaftor/ivacaftor studies targeting delta F508 homozygous patients showed no significant effects [7,8,9,10,11]. Even the new elexacaftor/tezacaftor/ivacaftor therapy is showing, at best, modest improvement on glucose tolerance in preliminary studies [12,13,14,15,16,17]. Initiating modulator therapies at a young age has the potential to preserve β cells functionality, but the detection of these seemingly small effects needs reference data to compare longitudinal data to the natural history of glucose tolerance and insulin secretory parameters.
CFRD may remain clinically silent for years, with insulin secretion defects beginning earlier than glucose intolerance [18]. The presence of this defect in early ages has severe clinical implication: they are related to the future worsening of glucose tolerance and CFRD [19,20]; they are associated with lung disease in young PwCF with mild to normal pulmonary function [21] independently from hyperglycemia [22]; and insulin is also an important anabolic hormone, and the catabolic effect of insulin insufficiency has important implication on growth [23,24], and is specifically associated with reduced adult height [25].
PwCF undergo annual screening for CFRD with an oral glucose tolerance test (OGTT), starting at ten or even six years of age [26]. Considering that standard OGTT cannot detect insulin secretion defects, we implemented a modified OGTT protocol in three separate and geographically distributed cystic fibrosis Italian centers, expanding the previously studied Milan cohort of PwCF [27]. The modified OGTT protocol included insulin and C-peptide measurement in addition to glucose, allowing for the modeling of parameters describing β cell function, insulin secretion, insulin clearance, and OGTT insulin sensitivity [28,29].
The aim of this study is to describe cross-sectionally at the Italian level the progression of OGTT parameters, β cell function, insulin clearance, and insulin sensitivity up to adulthood, while also providing national references for these parameters.
2. Materials and Methods
2.1. Study Design, Setting, and Participants
This was a cross-sectional study of the Italian cystic fibrosis population that consecutively enrolled patients between 2016 and 2020.
Patients were recruited from three Italian cystic fibrosis centers of excellence, selected to represent the northern, central, and southern geographical areas of Italy: the Cystic Fibrosis Centre of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; the Cystic Fibrosis Unit of the Bambino Gesù Children’s Hospital, Rome, Italy; and the Cystic Fibrosis Referral Center of the University Hospital G. Martino, Messina, Italy.
To be eligible, patients had to be clinically stable in the previous 3 weeks (absence of major clinical events including pulmonary exacerbations, no change in their habitual treatment regimen including introduction of antibiotics or steroids). Exclusion criteria were diagnosis of CFRD, or treatment with insulin or oral hypoglycemic agents in the previous 6 months.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Milan (protocol code 53/19, 26 November 2019). Informed consent was obtained from all subjects involved in the study.
2.2. Variables and Measurements
Outcomes for the main analysis were:
- OGTT parameters: glucose, insulin, and C-peptide (sampled before and at 30, 60, 90, and 120 min of the OGTT), and their area under the curve (AUC).
- β cell function: β cell glucose sensitivity, basal insulin secretion, insulin secretion at a fixed glucose concentration, total insulin secretion.
- insulin clearance: basal and OGTT insulin clearance.
- insulin sensitivity: quantitative insulin-sensitivity check index (QUICKI, for basal insulin sensitivity), and a 2-h oral glucose insulin sensitivity index (2-h OGIS for OGTT insulin sensitivity).
Predictors of the outcomes were age (continuous; in years), sex (categorical; 0 = Female, 1 = Male), and pancreatic sufficiency status (categorical; 0 = pancreatic sufficient, 1 = pancreatic insufficient).
2.2.1. CFTR Gene Mutation, Clinical Evaluation, Anthropometric and Pulmonary Assessment
CFTR mutations were classified by epidemiological prevalence (F508del homozygous, F508del heterozygous, other) and combining the type of CFTR defect with clinical severity (class I to VI, with decreasing level of severity) [30].
All patients were evaluated before enrollment in the study. The clinician informed the patient of the study procedures and collected informed consent. Clinical records of the following variables were collected: pancreatic insufficiency, intermittent and chronic infections, history of lung transplant, and CFTR modulator therapy.
Anthropometric assessment consisted of measuring weight and height following standard procedures [31]. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. For patients < 20 years, standard deviation scores were calculated based on the Centers for Disease Control and Prevention growth charts for weight, height, and BMI [32]. Patients were classified according to BMI in underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), or obese (BMI ≥ 30 kg/m2), and for patients ≤20 years old according to BMI z-score [33]. Patients were also classified following the Cystic Fibrosis Foundation recommendations: for women BMI ≥ 22, for men BMI ≥ 23, and for people younger than 21 years old ≥ 50th percentile [34].
Spirometry was performed according to the American Thoracic Society and European Respiratory Society guidelines [35]. The forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were expressed as percentage of the reference values [36].
2.2.2. Oral Glucose Tolerance Test and Laboratory Exams
All subjects received a 2-h OGTT (1.75 g/kg, maximum 75 g) sampling at baseline, and at 30-min intervals, the subjects had determinations of plasma glucose, serum insulin, and C-peptide concentrations. Based on plasma glucose concentrations, patients were classified in glucose tolerance categories according to [18].
On the same day, C-reactive protein, and glycated haemoglobin (HbA1C) were also measured.
Plasma glucose was measured on fluoride plasma samples (Gluco-quant; Roche/Hitachi analyser; Roche Diagnostics), and the other analytes were measured by commercial assays (ECLIA-Cobas C6000; Roche Diagnostics).
Based on the plasma glucose concentrations, patients were assigned to one of the following categories of glucose tolerance [18]: normal glucose tolerance, normal glucose tolerance with impaired fasting glucose, indeterminate glucose tolerance, impaired glucose tolerance, cystic fibrosis-related diabetes without fasting hyperglycaemia, and cystic fibrosis-related diabetes with fasting hyperglycaemia.
2.2.3. Modeling of β Cell Function and Other OGTT-Derived Indices
Beta-cell function was assessed by modeling from OGTT glucose and C-peptide, as previously described [27,29,37], using a model that describes the relationship between insulin secretion and glucose concentration. The model expresses insulin secretion as the sum of two components. The first component represents the dependence of insulin secretion on absolute glucose concentration at any time point during the OGTT through a dose-response function relating the two variables. Characteristic parameter of the dose-response is the mean slope over the observed glucose range, denoted as β-cell glucose sensitivity. The dose-response is modulated by a potentiation factor, which accounts for the fact that during an acute stimulation, insulin secretion is higher in the descending phase of hyperglycemia than in the ascending phase at the same glucose concentration. As such, the potentiation factor encompasses several potentiating mechanisms, including prolonged exposure to hyperglycemia, non-glucose substrates, gastrointestinal hormones, and neural modulation. It is set to be a positive function of time and is constrained to average unity during the experiment. In normal subjects, the potentiation factor typically increases from baseline to the end of a 2-h OGTT [29]. To quantify this excursion, we calculated the ratio between the 2-h and the baseline value. This ratio is denoted as potentiation ratio. The second insulin secretion component represents the dependence of insulin secretion on the rate of change of glucose concentration. This component is termed the derivative component and is determined by a single parameter, denoted as rate sensitivity. Rate sensitivity is related to early insulin release [29].
The β cell function parameters derived from the model were β cell glucose sensitivity, i.e., the slope of the relationship between insulin secretion and glucose concentration, and basal and total OGTT insulin secretion.
Basal insulin clearance was calculated as the ratio between basal insulin secretion and concentration, and OGTT insulin clearance as the ratio of total insulin secretion and insulin AUC.
Fasting insulin sensitivity was calculated as QUICKI index [38] and insulin sensitivity during the OGTT as the OGIS index [28].
The total glucose, insulin, and C-peptide excursions during the OGTT were calculated as the glucose AUC using the trapezoidal rule.
2.3. Bias and Study Size
Information bias was mitigated in this study as every PwCF should undergo an annual OGTT screening to early detect glucose intolerance. While it is possible that more severe patients may have been screened more intensively, we specifically excluded OGTT performed in acutely ill patients, according to the inclusion criteria. On the other hand, it is not advisable to conduct an OGTT on diabetic patients, so patients that resulted diabetic after measuring fasting glycemia were not allowed to continue the test. Moreover, the test is less tolerated by young patients, and they may have not completed the test at a higher rate than older patients. Survival bias was a possible source of selection bias that was tested, analyzing the raw trend lines of each outcome variable.
The number of patients tested in the recruiting centers during the study period determined the sample size.
2.4. Quantitative Variables and Statistical Methods
Age was binned with 5-year bins from 10 to 35 years, and patients outside this range were grouped in two extreme groups due to small group sizes.
Most continuous variables were not Gaussian-distributed, and all are reported as median (50th percentile) and interquartile range (IQR; 25th and 75th percentiles). Discrete variables are reported as the number and proportion of subjects with the characteristic of interest.
The relationship between age groups and outcomes was described through quantile regression to estimate within each age group the median, the lower, and the upper quartile of each outcome, adjusting both for sex and pancreatic insufficiency status.
2.5. Italian Reference Values
To limit the influence of outliers, all continuous variables besides age were winsorized using a tail of 0.01, meaning that values under the 1st percentile were put equal to the 1st percentiles, and values above the 99th percentile were put equal to the 99th percentile [39].
The 25th, 50th and 75th percentiles of each OGTT-related variable were estimated from a quantile regression model employing the variable as outcome and age (continuous, years), sex (discrete, 0 = female; 1 = male) and pancreatic insufficiency (0 = no; 1 = yes) as predictors [40]. Because of missing data for most variables, we fitted quantile regression using multiple imputation by chained equation (MICE), as detailed in the Supplementary Materials.
3. Results
3.1. Participants Characteristics
A total of 369 patients were included in statistical analysis, their characteristics are presented in Table 1. Median (IQR) patient age was 19 (15, 24) years), ranging from 6 to 56 years, and 56% of patients were females. The most frequent CFTR mutation was the F508del (23% were F508del homozygous, and 43% were F508del heterozygous), and 79% of the patients were pancreatic insufficient. While 90% of patients were of normal weight, only 42% were above the BMI target set by the Cystic Fibrosis Foundation. Glucose tolerance was normal in 65% of the patients, while 8.1% resulted affected by CFRD without fasting hyperglycaemia (patients affected by CFRD at basal measurement were not allowed to continue the OGTT).

Table 1.
Patient characteristics.
3.2. Main Results
Table 2 shows patient age distribution and count by age group, stratified by sex and pancreatic insufficiency, while the relationships between outcomes and age groups are shown in Figure 1, Figure 2 and Figure 3.

Table 2.
Patient count and age distribution by age group.
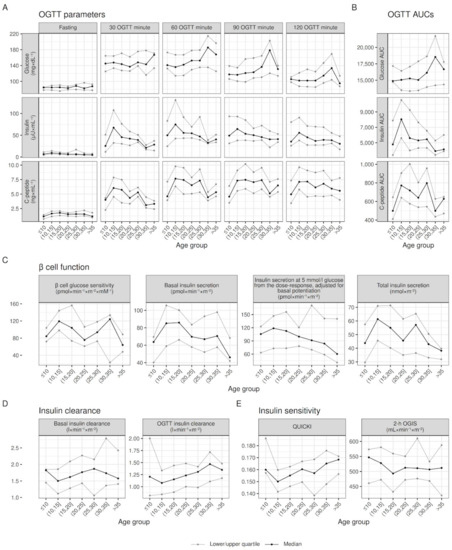
Figure 1.
Within the age-group raw distribution of OGTT parameters, β cell function, insulin clearance, and insulin sensitivity. Quartiles values adjusted for sex and pancreatic insufficiency to account for differences in their prevalence between age groups; lines joining quartiles between age groups do not represent modeling for age.
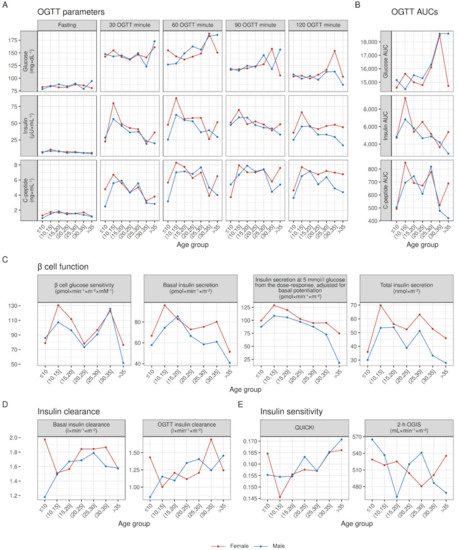
Figure 2.
Within age-group raw medians of OGTT parameters, β cell function, insulin clearance, and insulin sensitivity, stratified by sex. Median values adjusted for pancreatic insufficiency to account for differences in pancreatic insufficiency prevalence between age groups; lines joining medians between age groups do not represent modeling for age.
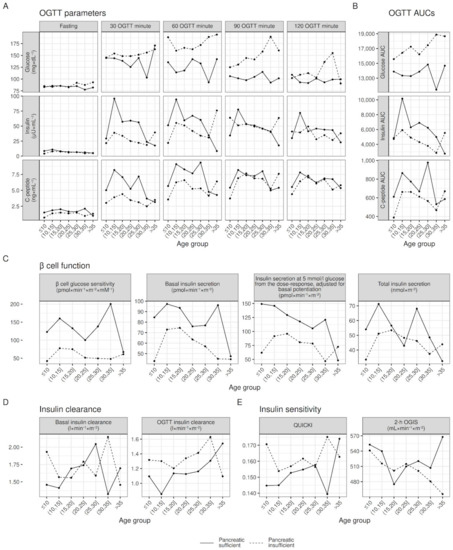
Figure 3.
Within age-group raw medians of OGTT parameters, β cell function, insulin clearance, and insulin sensitivity, stratified by pancreatic insufficiency. Median values adjusted for sex insufficiency to account for differences in sex between age groups; lines joining medians between age groups do not represent modeling for age.
Visual inspection of Figure 1 shows increasing glucose values in the second hour of the OGTT, and decreasing insulin and C-peptide values, going from younger to older ages (see Panel A and B). This was paired with decreasing β cell glucose sensitivity and insulin secretion (see Panel C), increasing insulin clearance (see panel D) and greater fasting insulin sensitivity (see Panel E). Despite these overall trends, most variables showed a deviation from linearity at one or both extreme age groups (≤10 and >35 years). Indeed, contrary to the overall trend highlighted in Figure 1, secretory parameters (C-peptide at all time points, β cell glucose sensitivity, basal and total insulin secretion) of patients ≤10 were significantly lower than in patients (10,15] years old (see Table 3). On the other hand, patients >35 years of age showed similar, if not improved (see C-peptide and β cell glucose sensitivity in Table 3) than younger patients, instead of continuing the deteriorating trend.

Table 3.
Differences between patients in the extreme and adjacent age groups, controlling for differences in sex and pancreatic insufficiency status.
3.3. Italian Reference Values
To produce reference values of the OGTT parameter, patient selection was performed to obtain a sample that was of uniform composition and sufficiently sized on the age scale. We did not have a sufficiently sized sample to flexibly model the deviation occurring at extreme age groups (≤10 and >35) and thus, the analysis was limited to central age groups as they showed a more linear trend. We chose to limit the sample size to post-puberal patients (≥13 years old for females and ≥15 years for males) for three reasons: (1) exploratory analysis identified a peak in secretory parameters in the age group that includes puberty; (2) an increase in secretory parameters occurring with puberty seems compatible with other changes occurring during puberty (i.e. greater insulin resistance [41]); and (3) the sample was not sufficiently sized before puberty to model a slope change and the sample generally exhibited a linear trend for all variables, excluding patient before puberty (Figure S3 in the Supplementary Materials show linear trends of OGTT-related parameters stratified by sex and puberty status). Patients >35 years old were also excluded from the analysis for similar reasons: (1) visual inspection, and Table 3 showed at least no deterioration, and in the case of β cell glucose sensitivity, an improvement of insulin secretory parameters after the 30–35 years age group; (2) these changes are compatible with a survival bias; and (3) the sample was not sufficiently sized to model age groups >35 years.
Figure 4 shows point estimates from quantile regression of all OGTT-related variables, stratified by sex. Most variables show an either increasing or decreasing trend with age: glucose tolerance (see raw glucose values in the second hour of the OGTT and glucose AUC) degrades with age, but it does not seem related to increasing insulin resistance, as insulin sensitivity (both fasting as QUICKI or during OGTT as OGIS) is stable throughout the age range; insulin secretion (expressed by raw insulin and C-peptide values, and modeled basal and total insulin secretion) generally degrades with age, accordingly with decreasing β cell glucose sensitivity and increasing insulin clearance. We deem all changes going from puberty to 35 years of age clinically significant, except for fasting glucose, glucose values in the first hour of OGTT, and insulin sensitivity parameters, that have shown minimal changes. Figures S4–S8 in the Supplementary Materials show point estimates and 95% confidence intervals from quantile regression of all OGTT-related variables stratified by sex, while Figure S9 shows β-cell glucose sensitivity stratified by age groups and glucose tolerance categories.
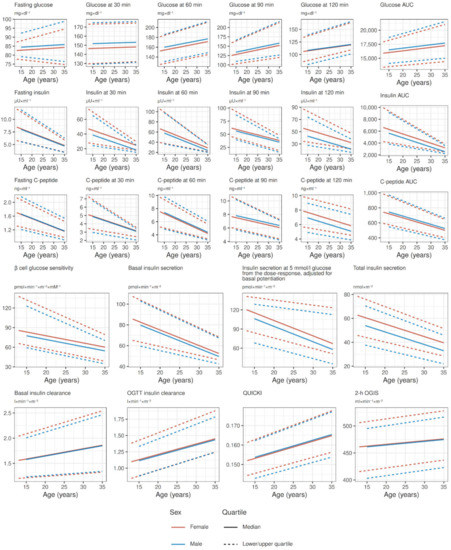
Figure 4.
Reference values of OGTT-related variables for both sexes in the age range between puberal age (>13 years for females, >15 years for males) and 35 years, derived from point estimates of quantile regression.
4. Discussion
In this study we present a description of the distribution and Italian reference quartiles for OGTT parameters and their AUCs, β cell function, insulin clearance, and insulin sensitivity. For all variables, we described the distribution from pre-puberal age to old adults accounting for differences in sex and pancreatic insufficiency between age groups, while we produced reference values for post-puberal patients and young adults that are age- and sex-specifically adjusted for pancreatic insufficiency. Our data confirmed an approximately linear degradation of glucose tolerance and insulin secretion during adulthood of PwCF and up until 35 years of age, with trend deviations occurring both in younger and older patients (these age groups were therefore excluded from the modeling of reference quartiles). We provide suggestive findings in the two extreme age groups: our data and the comparison with fasting insulin reference data for the general population (see Table S1, Figures S1 and S2 in the Supplementary Materials) suggest that a peak in insulin secretion occurs approximately across puberty, both in PwCF and in the general population. On the other hand, patients >35 years of age show better glucose tolerance and insulin secretion than younger peers, seemingly identifying survivors with preserved pancreatic function.
Puberty. Puberty seems associated with an increase in insulin secretory parameters. Our exploratory analysis highlighted a peak in C-peptide values, and modeled β cell glucose sensitivity, basal and total insulin secretion. The dynamics of insulin secretion across puberty are poorly studied even in the general population, although available evidence seems to confirm our data. Insulin secretion in puberty was studied cross-sectionally by [42] in 23 subjects using the hyperglycemic clamp technique; they found that adolescents display greater insulin and C-peptide responses when compared to both pre-adolescents and adults, even though glucose responses were similar for all groups. The authors of [43] also studied insulin secretory capacity using the hyperglycemic clamp in 133 subjects cross-sectionally, and 24–27 subjects longitudinally, both analyses showing an increase of insulin secretory capacity across puberty and a decrease later in adulthood. Moreover, combined general-population reference data for fasting insulin available in the Supplementary Materials [44,45,46,47] show an increasing trend before puberty and a decreasing trend thereafter. Superimposing cystic fibrosis quartiles of fasting insulin produced in this study to combine reference quartiles for the general population shows that at least fasting insulin, that is reflection of insulin secretion, seems generally preserved in young PwCF, although a faster degeneration with age is evident in PwCF, as expected.
Older patients. PwCF >35 years old show a better glucose tolerance and insulin secretion than younger patients. We provide two possible explanations for this phenomenon: (1) we only included patients without CFRD, as OGTT is not feasible in those patients. As CFRD prevalence increases with age [26], and it represents the final stage of progressive glucose tolerance and insulin secretion degradation, patients with lower glucose tolerance and lower insulin secretion are progressively excluded in our analysis (selection bias); and (2) as CFRD is associated with lower survival, PwCF that live longer tend to show better pancreatic function (survival bias). In this cross-sectional analysis, patients with better pancreatic function are present in all age groups, but the combination of the aforementioned factors cause a deviation from linearity in the extreme age group of patients >35 years old; therefore, they were excluded from the analysis. Older CFRD-free PwCF may represent a model to better understand the mechanism behind CFRD occurrence.
References values in post-puberal patients. We previously published OGTT-related variable quartiles from a smaller sample recruited at a single center [27]. In this study, we can confirm the relationship between OGTT-variables and age while introducing novel findings: (1) glucose tolerance deteriorates with age seemingly starting after puberty, in particular in the second hour of the OGTT; (2) glucose tolerance derangements are seemingly due to reduced insulin secretion, as highlighted by reduced raw insulin values, both fasting and particularly during OGTT, but perhaps more importantly as highlighted by a reduction of the β cell response to an increase in glucose concentration that delay the insulin response, causing glucose elevation in the second half of the OGTT; (3) insulin sensitivity does not deteriorate with age, reinforcing the hypothesis that glucose derangements recorded in PwCF are caused by an insulin secretory defect and not by an increased insulin resistance; (4) contributing to insulin secretory defects, both basal and OGTT insulin clearance appear to increase with age, although this may be in fact due to lower circulating and secreted insulin levels that do not saturate the systemic (mostly hepatic and renal) abilities to clear insulin; and (5) β cell dysfunction highlighted by reduced β cell glucose sensitivity is closely related to pancreatic dysfunction.
Sex differences. In comparison with [27], sex-related differences shown in this study seem reduced. Here we accounted for differences in pancreatic insufficiency, and, perhaps more importantly, we included only post-puberal patients, as younger patients displayed a puberty-related increment in insulin secretion that we could not model reliably. It is plausible that the inclusion of pre-puberal patients in our previous work displaced the overall linear trends of studied parameters. In contrast, when accounting for age differences in puberty incidence, the two sexes show similar trends.
Pancreatic insufficiency. Pancreatic sufficiency status was the greatest determinant of glucose tolerance and insulin secretion. There is a known correlation and plausible biological mechanism linking pancreatic insufficiency and β cell function, with pancreatic insufficient patients showing lower glucose tolerance and lower insulin secretion [48,49]. In PwCF with pancreatic insufficiency, exocrine pancreas is replaced in large amounts by fibrotic and/or fatty tissue, while islet mass is relatively conserved [50]. Emerging theories have suggested that a crosstalk between pancreatic ductal epithelial cells and beta cells may be a main contributor to beta cell dysfunction in PwCF, as CFTR is expressed in ducts and diseased ducts may influence β cell function through exocrine-derived proinflammatory factors [51]. In this study, we included pancreatic sufficiency status as a covariate to adjust for difference in pancreatic insufficiency prevalence in calculated quartiles, but we do not provide separate references for pancreatic sufficient and insufficient patients, as outcomes related to glucose tolerance and insulin secretion are likely to occur independently from pancreatic insufficiency when considering the endocrine pancreatic function.
The study has some limitations. As has been noted, the sample size and age distribution prevented to acceptably model patients in the extreme age groups, and so they were excluded from the analysis. No gold-standard methods were used to measure insulin secretion and sensitivity, although we consider these reasonable constraints of a relatively large study. A potential limitation of the modeling of β cell function employed in this study is the use of the C-peptide kinetic model by [52], as previously reported [27]. On the other hand, this study improved on the previous work [27] by avoiding the trajectories bias produced by including puberal and pre-puberal patients in the analysis, by improving sample size with greater recruitment of patients from multiple sites, and finally, by including the contribution of pancreatic sufficiency status in the developed quantiles.
In conclusion, we provide a description of the distribution and Italian reference quartiles for OGTT-related variables. We have already shown how these parameters are likely to predict overt CFRD [19], how the deterioration of these parameters is linked with lung function [22] and reduced adult stature [25], and finally, how these parameters are seemingly unaffected by the lumacaftor/ivacaftor combination therapy when administered in post-puberal PwCF [11]. These references provide a necessary tool to not only identify PwCF at risk for CFRD or other cystic fibrosis-related complications, but also to evaluate the effects of promising pharmacological therapies. As administration in early ages of new therapies has the greatest potential to provide significant improvements in glucose tolerance and insulin secretion, a better characterization of the natural history of these parameters during puberty is strongly needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13030469/s1, Figure S1: Centiles of fasting insulin available in the literature, with dashed interpolated lines between studies. Figure S2: Comparison between cystic fibrosis and general population quartiles of fasting insulin. Figure S3: Within sex linear trends before and after puberty of OGTT parameters, beta cell function, insulin clearance, and insulin sensitivity. Figure S4: Point estimates and 95% confidence intervals from quantile regression of OGTT parameters. Figure S5: Point estimates and 95% confidence intervals from quantile regression of OGTT AUCs. Figure S6: Point estimates and 95% confidence intervals from quantile regression of beta cell function parameters Figure S7: Point estimates and 95% confidence intervals from quantile regression of insulin clearance parameters. Figure S8: Point estimates and 95% confidence intervals from quantile regression of insulin sensitivity indices. Figure S9: Beta cell glucose sensitivity comparison (Wilcoxon test) across glucose tolerance category, stratified by age groups. Table S1: Sample characteristics of studies providing reference values for fasting insulin [44,45,46,47,53,54,55,56,57,58].
Author Contributions
Conceptualization, A.B. (Alberto Battezzati); Methodology, C.C., V.L., M.L. and A.M.; Formal Analysis, G.B.; Investigation, A.B. (Arianna Bisogno), S.B., F.C., F.A. (Federico Alghisi), M.A.C.; Data Curation, A.F.; Writing—Original Draft Preparation, A.F.; Writing—Review and Editing, all authors; Supervision, A.B. (Alberto Battezzati), C.C., V.L. and M.L.; Project Administration, A.B. (Alberto Battezzati); Funding Acquisition, A.B. (Alberto Battezzati). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Italian Cystic Fibrosis Research Foundation FFC#16/2005, FFC#21/2013, FFC#20/2016, and FFC#24/2019.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Milan (protocol code 53/19, 26 November 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon reasonable request due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lanng, S.; Hansen, A.; Thorsteinsson, B.; Koch, C. Glucose tolerance in patients with cystic fibrosis: Five year prospective study. BMJ 1995, 311, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Dunitz, J.; Nathan, B.; Saeed, A.; Holme, B.; Thomas, W. Cystic fibrosisrelated diabetes: Current trends in prevalence, incidence, and mortality. Diabetes Care 2009, 32, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Sandouk, Z.; Khan, F.; Khare, S.; Moran, A. Cystic fibrosis related diabetes (CFRD) prognosis. J. Clin. Transl. Endocrinol. 2021, 26, 100278. [Google Scholar] [CrossRef] [PubMed]
- Merjaneh, L.; Hasan, S.; Kasim, N.; Ode, K.L. The role of modulators in cystic fibrosis related diabetes. J. Clin. Transl. Endocrinol. 2022, 27, 100286. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Leon, D.D.D.; Sheikh, S.; Camburn, D.; Kubrak, C.; Peleckis, A.J.; Stefanovski, D.; Hadjiliadis, D.; Rickels, M.R.; Rubenstein, R.C. Islet hormone and incretin secretion in cystic fibrosis after four months of ivacaftor therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 342–351. [Google Scholar] [CrossRef]
- Volkova, N.; Moy, K.; Evans, J.; Campbell, D.; Tian, S.; Simard, C.; Higgins, M.; Konstan, M.W.; Sawicki, G.S.; Elbert, A.; et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J. Cyst. Fibros. 2020, 19, 68–79. [Google Scholar] [CrossRef]
- Thomassen, J.C.; Mueller, M.I.; Alcazar, M.A.A.; Rietschel, E.; Koningsbruggen-Rietschel, S. Effect of lumacaftor/ivacaftor on glucose metabolism and insulin secretion in Phe508del homozygous cystic fibrosis patients. J. Cyst. Fibros. 2018, 17, 271–275. [Google Scholar] [CrossRef]
- Li, A.; Vigers, T.; Pyle, L.; Zemanick, E.; Nadeau, K.; Sagel, S.D.; Chan, C.L. Continuous glucose monitoring in youth with cystic fibrosis treated with lumacaftor-ivacaftor. J. Cyst. Fibros. 2019, 18, 144–149. [Google Scholar] [CrossRef]
- Misgault, B.; Chatron, E.; Reynaud, Q.; Touzet, S.; Abely, M.; Melly, L.; Dominique, S.; Troussier, F.; Ronsin-Pradel, O.; Gerardin, M.; et al. Effect of one-year lumacaftorivacaftor treatment on glucose tolerance abnormalities in cystic fibrosis patients. J. Cyst. Fibros. 2020, 19, 712–716. [Google Scholar] [CrossRef]
- Moheet, A.; Beisang, D.; Zhang, L.; Sagel, S.D.; VanDalfsen, J.M.; Heltshe, S.L.; Frederick, C.; Mann, M.; Antos, N.; Billings, J.; et al. Lumacaftor/ivacaftor therapy fails to increase insulin secretion in F508del/F508del CF patients. J. Cyst. Fibros. 2021, 20, 333–338. [Google Scholar] [CrossRef]
- Colombo, C.; Foppiani, A.; Bisogno, A.; Gambazza, S.; Daccò, V.; Nazzari, E.; Leone, A.; Giana, A.; Mari, A.; Battezzati, A. Lumacaftor/ivacaftor in cystic fibrosis: Effects on glucose metabolism and insulin secretion. J. Endocrinol. Investig. 2021, 44, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Begnel, L.; Wallendorf, M.; Litvin, M. Effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Crow, H.; Bengtson, C.; Shi, X.; Graves, L.; Anabtawi, A. CGM patterns in adults with cystic fibrosis-related diabetes before and after elexacaftor-tezacaftor-ivacaftor therapy. J. Clin. Transl. Endocrinol. 2022, 30, 100307. [Google Scholar] [CrossRef] [PubMed]
- Scully, K.J.; Marchetti, P.; Sawicki, G.S.; Uluer, A.; Cernadas, M.; Cagnina, R.E.; Kennedy, J.C.; Putman, M.S. The effect of elexacaftor/tezacaftor/ivacaftor (ETI) on glycemia in adults with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 258–263. [Google Scholar] [CrossRef]
- Nielsen, B.; Olsen, M.; Mathiesen, I.; Pressler, T.; Jensen-Fangel, S.; Ritz, C.; Almdal, T.; Faurholt-Jepsen, D. 6 decline in HbA1c during the first year on elexacaftor/tezacaftor/ivacaftor in the danish cystic fibrosis population. J. Cyst. Fibros. 2022, 21, S4. [Google Scholar] [CrossRef]
- Stalvey, M.; Walega, R.; Rowe, S.; Nichols, D.; Stefanovski, D.; Kelly, A. 15 promise: Glucose excursion and insulin secretion after 12 to 18 months of highly effective modulator therapy. J. Cyst. Fibros. 2022, 21, S11–S12. [Google Scholar] [CrossRef]
- Chan, C.L.; Granados, A.; Moheet, A.; Singh, S.; Vigers, T.; Arbeláez, A.M.; Yi, Y.; Hu, S.; Norris, A.W.; Ode, K.L. Glycemia and -cell function before and after elexacaftor/tezacaftor/ivacaftor in youth and adults with cystic fibrosis. J. Clin. Transl. Endocrinol. 2022, 30, 100311. [Google Scholar] [CrossRef]
- Moran, A.; Pillay, K.; Becker, D.; Granados, A.; Hameed, S.; Acerini, C.L. ISPAD clinical practice consensus guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 64–74. [Google Scholar] [CrossRef]
- Battezzati, A.; Battezzati, P.; Costantini, D.; Zazzeron, L.; Russo, M.; Daccò, V.; Motta, V.; Colombo, C. Cystic Fibrosis Related Diabetes Is Anticipated By Reduced Insulin Secretion During Ogtt. J. Cyst. Fibros. 2008, 7, S22–S23. [Google Scholar] [CrossRef]
- Potter, K.J.; Boudreau, V.; Bonhoure, A.; Tremblay, F.; Lavoie, A.; Carricart, M.; Senior, P.A.; Rabasa-Lhoret, R. Insulinogenic index and early phase insulin secretion predict increased risk of worsening glucose tolerance and of cystic fibrosis-related diabetes. J. Cyst. Fibros. 2022. [Google Scholar] [CrossRef]
- Milla, C.E.; Warwick, W.J.; Moran, A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am. J. Respir. Crit. Care Med. 2000, 162, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Alicandro, G.; Gambazza, S.; Mileto, P.; Mari, A.; Grespan, E.; Nazzari, E.; Russo, M.C.; Battezzati, A. Ventilation inhomogeneity is associated with OGTT-derived insulin secretory defects in cystic fibrosis. Pediatr. Pulmonol. 2018, 54, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.S.; Bridges, N.A.; Prasad, S.A.; Francis, J.; Carr, S.B.; Suri, R.; Balfour-Lynn, I. Growth in children with cystic fibrosis-related diabetes. Pediatr. Pulmonol. 2009, 44, 1223–1225. [Google Scholar] [CrossRef]
- Ripa, P.; Robertson, I.; Cowley, D.; Harris, M.; Masters, I.B.; Cotterill, A.M. The relationship between insulin secretion, the insulin-like growth factor axis and growth in children with cystic fibrosis. Clin. Endocrinol. 2002, 56, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Battezzati, A.; Foppiani, A.; Alicandro, G.; Bisogno, A.; Biffi, A.; Bedogni, G.; Bertoli, S.; De Carlo, G.; Nazzari, E.; Colombo, C. Prepuberal insulin secretory indices are long-term predictors of short adult stature in cystic fibrosis. Endocr. Connect. 2022, 11, e220056. [Google Scholar] [CrossRef]
- Khare, S.; Desimone, M.; Kasim, N.; Chan, C.L. Cystic fibrosis-related diabetes: Prevalence, screening, and diagnosis. J. Clin. Transl. Endocrinol. 2022, 27, 100290. [Google Scholar] [CrossRef]
- Battezzati, A.; Bedogni, G.; Zazzeron, L.; Mari, A.; Battezzati, P.M.; Alicandro, G.; Bertoli, S.; Colombo, C. Age- and sex-dependent distribution of OGTT-related variables in a population of cystic fibrosis patients. J. Clin. Endocrinol. Metab. 2015, 100, 2963–2971. [Google Scholar] [CrossRef]
- Mari, A.; Pacini, G.; Murphy, E.; Ludvik, B.; Nolan, J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001, 24, 539–548. [Google Scholar] [CrossRef]
- Mari, A.; Ferrannini, E. β-cell function assessment from modelling of oral tests: An effective approach. Diabetes Obes. Metab. 2008, 10, 77–87. [Google Scholar] [CrossRef]
- Marson, F.A.L.; Bertuzzo, C.S.; Ribeiro, J.D. Classification of CFTR mutation classes. Lancet Respir. Med. 2016, 4, e37–e38. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F. Anthropometric Standardization Reference Manual; Human Kinetics: Champaign, IL, USA, 1988. [Google Scholar]
- Center for Disease Control and Prevention. CDC Growth Charts: United States 2000. Available online: http://www.cdc.gov/growthcharts/ (accessed on 1 February 2023).
- Center for Disease Control and Prevention. Use and Interpretation of the WHO and CDC Growth Charts for Children from Birth to 20 Years in the United States 2014. Available online: https://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/growthchart.pdf (accessed on 1 February 2023).
- Cystic Fibrosis Foundation. Nutritional Basics 2022. Available online: https://www.cff.org/managing-cf/nutritional-basics (accessed on 1 February 2023).
- Miller, M.R. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 395-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Battezzati, A.; Mari, A.; Zazzeron, L.; Alicandro, G.; Claut, L.; Battezzati, P.M.; Colombo, C. Identification of insulin secretory defects and insulin resistance during oral glucose tolerance test in a cohort of cystic fibrosis patients. Eur. J. Endocrinol. 2011, 165, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J. The future of data analysis. Ann. Math. Statist. 1962, 33, 1–67. [Google Scholar] [CrossRef]
- Koenker, R.; Chernozhukov, V.; He, X.; Peng, L. Handbook of Quantile Regression; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Kelsey, M.M.; Zeitler, P.S. Insulin resistance of puberty. Curr. Diabetes Rep. 2016, 16, 64. [Google Scholar] [CrossRef]
- Caprio, S.; Plewe, G.; Diamond, M.P.; Simonson, D.C.; Boulware, S.D.; Sherwin, R.S.; Tamborlane, W.V. Increased insulin secretion in puberty: A compensatory response to reductions in insulin sensitivity. J. Pediatr. 1989, 114, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Marwitz, S.E.; Gaines, M.V.; Brady, S.M.; Mi, S.J.; Broadney, M.M.; Yanovski, S.Z.; Hubbard, V.S.; Yanovski, J.A. Cross-sectional and longitudinal examination of insulin sensitivity and secretion across puberty among non-hispanic black and white children. Endocrinol. Metab. 2020, 35, 847–857. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; Imperatore, G.; Cook, S. Age, sex, and ethnic variations in serum insulin concentrations among u.s. youth. Diabetes Care 2006, 29, 2605–2611. [Google Scholar] [CrossRef]
- Koester-Weber, T.; Valtueña, J.; Breidenassel, C.; Beghin, L.; Plada, M.; Moreno, S.; Huybrechts, I.; Palacios, G.; Gómez-Martínez, S.; Albers, U.; et al. Reference values for leptin, cortisol, insulin and glucose, among european adolescents and their association with adiposity: The HELENA study. Nutr. Hosp. 2014, 30, 1181–1190. [Google Scholar]
- Peplies, J.; on behalf of the IDEFICS Consortium; Jiménez-Pavón, D.; Savva, S.C.; Buck, C.; Günther, K.; Fraterman, A.; Russo, P.; Iacoviello, L.; Veidebaum, T.; et al. Percentiles of fasting serum insulin, glucose, HbA1c and HOMA-IR in pre-pubertal normal weight european children from the IDEFICS cohort. Int. J. Obes. 2014, 38, S39–S47. [Google Scholar] [CrossRef]
- Tohidi, M.; Ghasemi, A.; Hadaegh, F.; Derakhshan, A.; Chary, A.; Azizi, F. Age- and sex-specific reference values for fasting serum insulin levels and insulin resistance/sensitivity indices in healthy iranian adults: Tehran lipid and glucose study. Clin. Biochem. 2014, 47, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Battistoni, A.; Gambari, R.; Pompella, A.; Bragonzi, A.; Pilolli, F.; Iuliano, L.; Piroddi, M.; Dechecchi, M.C.; Cabrini, G. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 690–713. [Google Scholar] [CrossRef]
- Moheet, A.; Moran, A. CF-related diabetes: Containing the metabolic miscreant of cystic fibrosis. Pediatr. Pulmonol. 2017, 52, S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Norris, A.W.; Hull, R.L. A tale of two pancreases: Exocrine pathology and endocrine dysfunction. Diabetologia 2020, 63, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Mun, K.S.; Arora, K.; Huang, Y.; Yang, F.; Yarlagadda, S.; Ramananda, Y.; Abu-El-Haija, M.; Palermo, J.J.; Appakalai, B.N.; Nathan, J.D.; et al. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat. Commun. 2019, 10, 3124. [Google Scholar] [CrossRef]
- Cauter, E.V.; Mestrez, F.; Sturis, J.; Polonsky, K.S. Estimation of insulin secretion rates from c-peptide levels: Comparison of individual and standard kinetic parameters for c-peptide clearance. Diabetes 1992, 41, 368–377. [Google Scholar] [CrossRef]
- Klein, D. MIMRGNS: Stata Module to Run Margins after mi Estimate (Statistical Software Components); Boston College Department of Economics: Chestnut Hill, MA, USA, 2014. [Google Scholar]
- Morris, T.P.; White, I.R.; Carpenter, J.R.; Stanworth, S.J.; Royston, P. Combining fractional polynomial model building with multiple imputation. Stat. Med. 2015, 34, 3298–3317. [Google Scholar] [CrossRef]
- Stern, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338. [Google Scholar] [CrossRef]
- Van Buuren, S. Flexible Imputation of Missing Data. CRC Press: Boca Raton, FL, USA, 2015; Available online: https://stefvanbuuren.name/fimd/ (accessed on 1 February 2023).
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 20, 377–399. [Google Scholar] [CrossRef]
- Williams, R. Using the Margins Command to Estimate and Interpret Adjusted Predictions and Marginal Effects. Stat. J. 2012, 12, 308–331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).