Clinical Effects of the Neutrophil-to-Lymphocyte Ratio/Serum Albumin Ratio in Patients with Gastric Cancer after Gastrectomy
Abstract
1. Introduction
2. Materials and Methods
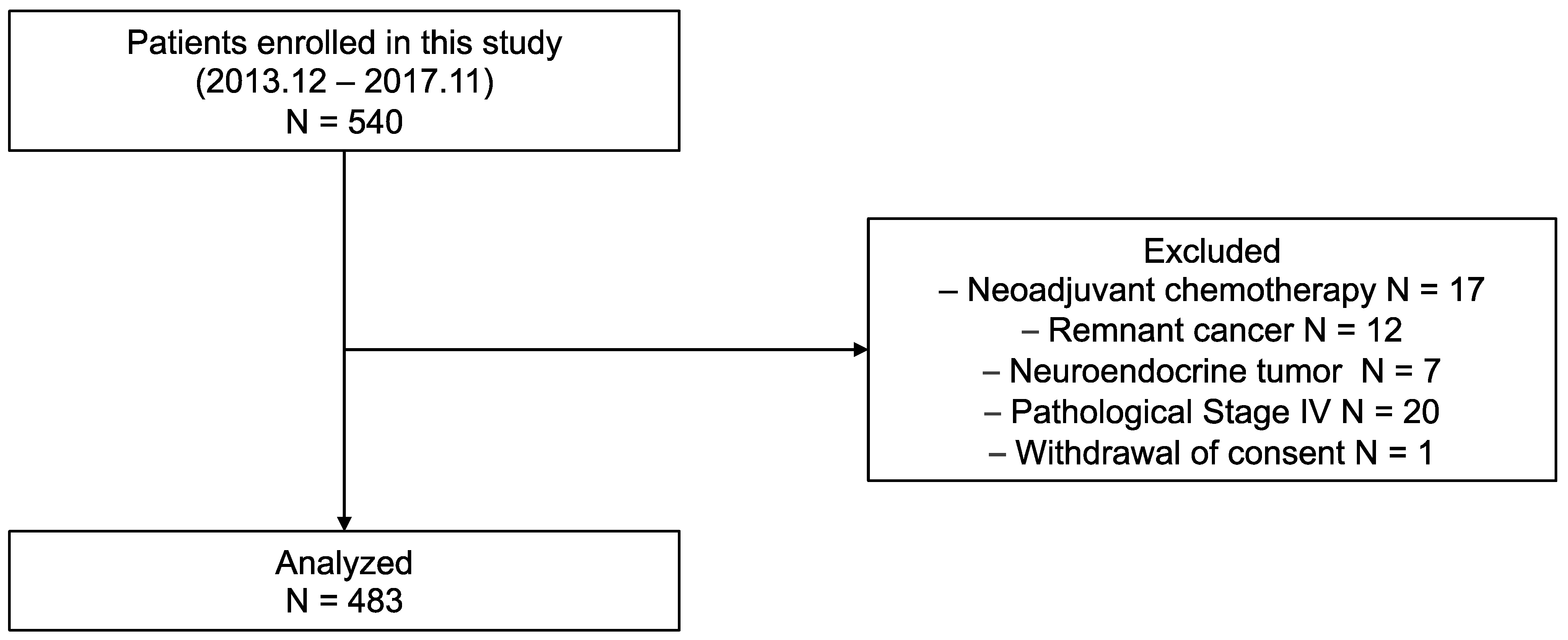
2.1. Patients
2.2. Measurement of the PNI, NLR, and Alb
2.3. Analyzed Parameters
2.4. Statistical Analyses
3. Results
3.1. Association of PNI, NLR, and NLR/Alb with Clinicopathological Factors
3.2. 3Y OS and RFS Rates According to PNI, NLR, and NLR/Alb
3.3. Univariate and Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in Stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kodera, Y.; Kochi, M.; Ichikawa, W.; Kakeji, Y.; Sano, T.; Nagao, N.; Takahashi, M.; Takagane, A.; Watanabe, T.; et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with Stage III gastric cancer: Interim analysis of JACCRO GC-07, a randomized controlled trial. J. Clin. Oncol. 2019, 37, 1296–1304. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, Phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, Phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.-T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2018, 19, 1437–1448. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Wang, Y.; Ji, M.H.; Tong, J.; Yang, J.J.; Xia, H. A machine learning-based predictor for the identification of the recurrence of patients with gastric cancer after operation. Sci. Rep. 2021, 11, 1571. [Google Scholar] [CrossRef]
- Liu, X.; Lei, S.; Wei, Q.; Wang, Y.; Liang, H.; Chen, L. Machine learning-based correlation study between perioperative immunonutritional index and postoperative anastomotic leakage in patients with gastric cancer. Int. J. Med. Sci. 2022, 19, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Wei, Z.J.; Xu, A.M.; Zang, J.H. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? tumor maker retrospective study. Int. J. Surg. 2018, 56, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, S.; Ying, X.; Zhang, L.; Shan, F.; Jia, Y.; Ji, J. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy. Gastric Cancer 2020, 23, 540–549. [Google Scholar] [CrossRef]
- Hirahara, N.; Matsubara, T.; Fujii, Y.; Kaji, S.; Kawabata, Y.; Hyakudomi, R.; Yamamoto, T.; Taniura, T.; Tajima, Y. Comparison of the prognostic value of immunoinflammation-based biomarkers in patients with gastric cancer. Oncotarget 2020, 11, 2625–2635. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhai, S.T.; Lin, H. Role of prognostic nutritional index in patients with gastric cancer: A meta-analysis. Minerva Med. 2016, 107, 322–327. [Google Scholar] [PubMed]
- Takechi, H.; Fujikuni, N.; Tanabe, K.; Hattori, M.; Amano, H.; Noriyuki, T.; Nakahara, M. Using the preoperative prognostic nutritional index as a predictive factor for non-cancer-related death in post-curative resection gastric cancer patients: A retrospective cohort study. BMC Gastroenterol. 2020, 20, 256. [Google Scholar] [CrossRef] [PubMed]
- Xishan, Z.; Ye, Z.; Feiyan, M.; Liang, X.; Shikai, W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci. Rep. 2020, 10, 17373. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.; Feng, J.-F. A Novel inflammation-based prognostic index for patients with esophageal squamous cell carcinoma: Neutrophil lymphocyte ratio/albumin ratio. Oncotarget 2017, 8, 103535–103542. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J.; Liu, Z.; Tian, Y.; Liu, F. A novel inflammation-based prognostic index for patients with esophageal squamous cell carcinoma: Neutrophil lymphocyte ratio/prealbumin ratio. Medicine 2019, 98, e14562. [Google Scholar] [CrossRef]
- Ueda, K.; Ogasawara, N.; Yonekura, S.; Matsunaga, Y.; Hoshino, R.; Kurose, H.; Chikui, K.; Uemura, K.; Nakiri, M.; Nishihara, K.; et al. The prognostic value of systemic inflammatory markers in advanced renal cell carcinoma patients treated with molecular targeted therapies. Anticancer Res. 2020, 40, 1739–1745. [Google Scholar] [CrossRef]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; McMillan, D.C.; Morrison, D.S.; Fletcher, C.D.; Horgan, P.G.; Clarke, S.J. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br. J. Cancer 2012, 107, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, L.; Daßler-Plenker, J.; Sun, L.; Egeblad, M. Innate immunity and cancer pathophysiology. Annu. Rev. Pathol. 2022, 17, 425–457. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Mizuno, A.; Tanaka, C.; Kobayashi, D.; Fujiwara, M.; Iwata, N.; Hayashi, M.; Yamada, S.; Nakayama, G.; Fujii, T.; et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine 2016, 95, e3781. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.-I.; Kim, Y.-N.; Hong, J.H.; Alshomimi, S.; An, J.Y.; Cheong, J.-H.; Hyung, W.J.; Noh, S.H.; Kim, C.-B. Clinical significance of the prognostic nutritional index for predicting short- and long-term surgical outcomes after gastrectomy: A retrospective analysis of 7781 gastric cancer patients. Medicine 2016, 95, e3539. [Google Scholar] [CrossRef]
- Shimada, H.; Takiguchi, N.; Kainuma, O.; Soda, H.; Ikeda, A.; Cho, A.; Miyazaki, A.; Gunji, H.; Yamamoto, H.; Nagata, M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010, 13, 170–176. [Google Scholar] [CrossRef]
- Wang, S.C.; Chou, J.F.; Strong, V.E.; Brennan, M.F.; Capanu, M.; Coit, D.G. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann. Surg. 2016, 263, 292–297. [Google Scholar] [CrossRef]
- Xin-Ji, Z.; Yong-Gang, L.; Xiao-Jun, S.; Xiao-Wu, C.; Dong, Z.; Da-Jian, Z. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: A meta-analysis. Int. J. Surg. 2015, 21, 84–91. [Google Scholar] [CrossRef]
- Jiang, N.; Deng, J.Y.; Ding, X.W.; Ke, B.; Liu, N.; Zhang, R.P.; Liang, H. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J. Gastroenterol. 2014, 20, 10537–10544. [Google Scholar] [CrossRef]
- Aoyama, T.; Nakazono, M.; Segami, K.; Nagasawa, S.; Kano, K.; Yamada, T.; Maezawa, Y.; Hara, K.; Hashimoto, I.; Suematsu, H.; et al. The clinical influence of the C-reactive protein-to-albumin ratio in patients who received curative treatment for gastric cancer. In Vivo 2021, 35, 3475–3482. [Google Scholar] [CrossRef]
- Xu, B.B.; Lu, J.; Zheng, Z.F.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.H.; et al. The predictive value of the preoperative C-reactive protein-albumin ratio for early recurrence and chemotherapy benefit in patients with gastric cancer after radical gastrectomy: Using randomized phase III trial data. Gastric Cancer 2019, 22, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Ichikawa, T.; Yin, C.; Suzuki, A.; Fujikawa, H.; Yasuda, H.; Hiro, J.; et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin. Nutr. 2020, 39, 1209–1217. [Google Scholar] [CrossRef]
- Xiong, J.; Hu, H.; Kang, W.; Liu, H.; Ma, F.; Ma, S.; Li, Y.; Jin, P.; Tian, Y. Prognostic impact of preoperative naples prognostic score in gastric cancer patients undergoing surgery. Front. Surg. 2021, 8, 617744. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, E.; Buoro, V.; Cinausero, M.; Caccialanza, R.; Turri, A.; Fanotto, V.; Basile, D.; Vitale, M.G.; Ermacora, P.; Cardellino, G.G.; et al. Sarcopenia in gastric cancer: When the loss costs too much. Gastric Cancer 2017, 20, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef]
- Zavros, Y.; Merchant, J.L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 451–467. [Google Scholar] [CrossRef]
- Liotti, F.; Marotta, M.; Melillo, R.M.; Prevete, N. The impact of resolution of inflammation on tumor microenvironment: Exploring new ways to control cancer progression. Cancers 2022, 14, 3333. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, J. Neutrophil extracellular traps: New players in cancer research. Front. Immunol. 2022, 13, 937565. [Google Scholar] [CrossRef]
- Li, T.J.; Jiang, Y.M.; Hu, Y.F.; Huang, L.; Yu, J.; Zhao, L.Y.; Deng, H.J.; Mou, T.Y.; Liu, H.; Yang, Y.; et al. Interleukin-17-producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clin. Cancer Res. 2017, 23, 1575–1585. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Chen, J.; Zhang, P.; Ji, R.; Qian, H.; Xu, W.; Zhang, X. Interaction with neutrophils promotes gastric cancer cell migration and invasion by inducing epithelial-mesenchymal transition. Oncol. Rep. 2017, 38, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Zhao, Y.L.; Peng, L.S.; Chen, N.; Chen, W.; Lv, Y.P.; Mao, F.Y.; Zhang, J.Y.; Cheng, P.; Teng, Y.S.; et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017, 66, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, S.; Tanaka, H.; Nishimura, J.; Yamakoshi, Y.; Sakimura, C.; Tamura, T.; Toyokawa, T.; Muguruma, K.; Yashiro, M.; Hirakawa, K.; et al. Gastric cancer cells alter the immunosuppressive function of neutrophils. Oncol. Rep. 2020, 43, 251–259. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Kim, K.; Chie, E.K.; Kim, B.; Lee, K.B.; Jang, J.-Y.; Kim, S.-W.; Oh, D.-Y.; Bang, Y.-J.; et al. High ratio of programmed cell death protein 1 (PD-1)(+)/CD8(+) tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother. Oncol. 2015, 117, 165–170. [Google Scholar] [CrossRef]
- Miura, T.; Yoshizawa, T.; Hirai, H.; Seino, H.; Morohashi, S.; Wu, Y.; Wakiya, T.; Kimura, N.; Kudo, D.; Ishido, K.; et al. Prognostic impact of CD163+ macrophages in tumor stroma and CD8+ T-cells in cancer cell nests in invasive extrahepatic bile duct cancer. Anticancer Res. 2017, 37, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Djenidi, F.; Adam, J.; Goubar, A.; Durgeau, A.; Meurice, G.; de Montpréville, V.; Validire, P.; Besse, B.; Mami-Chouaib, F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015, 194, 3475–3486. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, R.; Jia, J.; He, Y.; He, S. Activation of toll-like receptor 2 enhances peripheral and tumor-infiltrating CD8+ T cell cytotoxicity in patients with gastric cancer. BMC Immunol. 2021, 22, 67. [Google Scholar] [CrossRef]
- Feng, F.; Zheng, G.; Wang, Q.; Liu, S.; Liu, Z.; Xu, G.; Wang, F.; Guo, M.; Lian, X.; Zhang, H. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018, 18, 148. [Google Scholar] [CrossRef]
- Yeun, J.Y.; Kaysen, G.A. Factors influencing serum albumin in dialysis patients. Am. J. Kidney Dis. 1998, 32, S118–S125. [Google Scholar] [CrossRef]
- Oñate-Ocaña, L.F.; Aiello-Crocifoglio, V.; Gallardo-Rincón, D.; Herrera-Goepfert, R.; Brom-Valladares, R.; Carrillo, J.F.; Cervera, E.; Mohar-Betancourt, A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann. Surg. Oncol. 2007, 14, 381–389. [Google Scholar] [CrossRef]
- Mulazzani, G.E.G.; Corti, F.; Della Valle, S.; Di Bartolomeo, M. Nutritional support indications in gastroesophageal cancer patients: From perioperative to palliative systemic therapy. A comprehensive review of the last decade. Nutrients 2021, 13, 2766. [Google Scholar] [CrossRef] [PubMed]
- Tsuburaya, A.; Mizusawa, J.; Tanaka, Y.; Fukushima, N.; Nashimoto, A.; Sasako, M.; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br. J. Surg. 2014, 101, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Terashima, M.; Mizusawa, J.; Katayama, H.; Nakamura, K.; Katai, H.; Yoshikawa, T.; Ito, S.; Kaji, M.; Kimura, Y.; et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): An open-label, phase 3, randomized controlled trial. Gastric Cancer 2021, 24, 492–502. [Google Scholar] [CrossRef] [PubMed]

| Variables | All Patients (n = 483) | NLR/Alb | p-Value | |
|---|---|---|---|---|
| Low (n = 139) | High (n = 344) | |||
| Age (years) | <65 | 60 (43.2) | 114 (33.1) | 0.05 |
| ≥65 | 79 (56.8) | 230 (66.9) | ||
| Sex | Male | 103 (74.1) | 217 (63.1) | 0.03 |
| Female | 36 (25.9) | 127 (36.9) | ||
| BMI (kg/m2) | <18.5 | 9 (6.5) | 40 (11.6) | 0.17 |
| ≥18.5, <25.0 | 94 (67.6) | 231 (67.2) | ||
| ≥25 | 36 (25.9) | 73 (21.2) | ||
| Preoperative CRP level | (Mean SD) | 0.12 (0.19) | 0.22 (0.44) | 0.01 |
| Operation | Not TG | 109 (78.4) | 253 (73.5) | 0.30 |
| TG | 30 (21.6) | 91 (26.5) | ||
| Tumor size (mm) | ≤30 | 78 (56.1) | 169 (49.1) | 0.19 |
| >30 | 61 (43.9) | 175 (50.9) | ||
| Histological type | Well/moderately | 74 (53.2) | 175 (50.9) | 0.69 |
| Poorly | 65 (46.8) | 169 (49.1) | ||
| Lymphatic invasion | − | 101 (72.7) | 232 (67.4) | 0.28 |
| + | 38 (27.3) | 112 (32.6) | ||
| Venous invasion | − | 83 (59.7) | 197 (57.3) | 0.68 |
| + | 56 (40.3) | 147 (42.7) | ||
| pStage | I | 100 (71.9) | 231 (67.2) | 0.33 |
| II/III | 39 (28.1) | 113 (32.8) | ||
| Surgical complications | − | 116 (83.5) | 290 (84.3) | 0.89 |
| + | 23 (16.5) | 54 (15.7) | ||
| Hospitalization | (Mean SD) | 9.81 (7.48) | 10.03 (8.94) | 0.80 |
| Factors | Univariate | p-Value | Multivariate | p-Value | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Age (years) | <65 | 1 | 1 | ||||
| ≥65 | 2.99 | 1.33–6.73 | 0.01 | 1.98 | 0.87–4.49 | 0.10 | |
| Sex | Male | 1 | |||||
| Female | 0.66 | 0.33–1.32 | 0.24 | ||||
| BMI (kg/m2) | <18.5 | 1 | |||||
| ≥18.5, <25.0 | 0.60 | 0.26–1.37 | 0.22 | ||||
| ≥25 | 0.42 | 0.15–1.21 | 0.11 | ||||
| Operation | Not TG | 1 | 1 | ||||
| TG | 2.52 | 1.38–4.60 | 0.003 | 1.37 | 0.74–2.54 | 0.32 | |
| Tumor size (mm) | ≤30 | 1 | |||||
| >30 | 1.80 | 0.97–3.33 | 0.06 | ||||
| Histological type | Well/moderately | 1 | |||||
| Poorly | 1.50 | 0.82–2.75 | 0.19 | ||||
| Lymphatic invasion | − | 1 | 1 | ||||
| + | 3.21 | 1.75–5.87 | <0.001 | 1.02 | 0.52–2.02 | 0.95 | |
| Venous invasion | − | 1 | 1 | ||||
| + | 7.62 | 3.39–17.13 | <0.001 | 3.81 | 1.50–9.68 | 0.005 | |
| pStage | I | 1 | 1 | ||||
| II/III | 6.09 | 3.13–11.87 | <0.001 | 2.65 | 1.21–5.81 | 0.02 | |
| Surgical complications | − | 1 | |||||
| + | 0.59 | 0.21–1.67 | 0.32 | ||||
| Preoperative CRP | Low | 1 | 1 | ||||
| High | 1.71 | 1.01–2.89 | 0.045 | 1.38 | 0.74–2.55 | 0.31 | |
| Preoperative NLR/Alb | Low | 1 | 1 | ||||
| High | 5.58 | 1.73–18.05 | 0.004 | 4.13 | 1.26–13.55 | 0.02 | |
| Factors | Univariate | p-Value | Multivariate | p-Value | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Age (years) | <65 | 1 | 1 | ||||
| ≥65 | 2.12 | 1.15–3.91 | 0.02 | 1.45 | 0.77–2.72 | 0.25 | |
| Sex | Male | 1 | |||||
| Female | 1.05 | 0.62–1.79 | 0.85 | ||||
| BMI (kg/m2) | <18.5 | 1 | |||||
| ≥18.5, <25.0 | 0.51 | 0.25–1.02 | 0.29 | ||||
| ≥25 | 0.58 | 0.26–1.30 | 0.18 | ||||
| Operation | Not TG | 1 | 1 | ||||
| TG | 1.97 | 1.17–3.32 | 0.01 | 1.19 | 0.69–2.05 | 0.53 | |
| Tumor size (mm) | ≤30 | 1 | |||||
| ≥30 | 2.34 | 1.36–4.03 | 0.002 | 0.87 | 0.47–1.60 | 0.65 | |
| Histological type | Well/moderately | 1 | |||||
| Poorly | 1.63 | 0.97–2.74 | 0.06 | ||||
| Lymphatic invasion | − | 1 | 1 | ||||
| + | 4.56 | 2.68–7.75 | <0.001 | 2.13 | 1.03–4.42 | 0.04 | |
| Venous invasion | − | 1 | 1 | ||||
| + | 5.51 | 2.98–10.18 | <0.001 | 1.79 | 0.98–3.25 | 0.06 | |
| pStage | I | 1 | 1 | ||||
| II/III | 6.87 | 3.88–12.19 | <0.001 | 3.48 | 1.68–7.20 | <0.001 | |
| Surgical complications | − | 1 | |||||
| + | 1.20 | 0.62–2.30 | 0.59 | ||||
| Preoperative CRP | Low | 1 | |||||
| High | 1.66 | 0.98–2.81 | 0.06 | ||||
| Preoperative NLR/Alb | Low | 1 | 1 | ||||
| High | 3.81 | 1.64–8.86 | 0.002 | 3.16 | 1.34–7.45 | 0.009 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onuma, S.; Hashimoto, I.; Suematsu, H.; Nagasawa, S.; Kanematsu, K.; Aoyama, T.; Yamada, T.; Rino, Y.; Ogata, T.; Oshima, T. Clinical Effects of the Neutrophil-to-Lymphocyte Ratio/Serum Albumin Ratio in Patients with Gastric Cancer after Gastrectomy. J. Pers. Med. 2023, 13, 432. https://doi.org/10.3390/jpm13030432
Onuma S, Hashimoto I, Suematsu H, Nagasawa S, Kanematsu K, Aoyama T, Yamada T, Rino Y, Ogata T, Oshima T. Clinical Effects of the Neutrophil-to-Lymphocyte Ratio/Serum Albumin Ratio in Patients with Gastric Cancer after Gastrectomy. Journal of Personalized Medicine. 2023; 13(3):432. https://doi.org/10.3390/jpm13030432
Chicago/Turabian StyleOnuma, Shizune, Itaru Hashimoto, Hideaki Suematsu, Shinsuke Nagasawa, Kyohei Kanematsu, Toru Aoyama, Takanobu Yamada, Yasushi Rino, Takashi Ogata, and Takashi Oshima. 2023. "Clinical Effects of the Neutrophil-to-Lymphocyte Ratio/Serum Albumin Ratio in Patients with Gastric Cancer after Gastrectomy" Journal of Personalized Medicine 13, no. 3: 432. https://doi.org/10.3390/jpm13030432
APA StyleOnuma, S., Hashimoto, I., Suematsu, H., Nagasawa, S., Kanematsu, K., Aoyama, T., Yamada, T., Rino, Y., Ogata, T., & Oshima, T. (2023). Clinical Effects of the Neutrophil-to-Lymphocyte Ratio/Serum Albumin Ratio in Patients with Gastric Cancer after Gastrectomy. Journal of Personalized Medicine, 13(3), 432. https://doi.org/10.3390/jpm13030432






