Femoral Pulse Pressure Variation Is Not Interchangeable with Radial Pulse Pressure Variation during Living Donor Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Anesthetic Management
2.2. Acquisition of PPV Data
2.3. Statistical Analysis
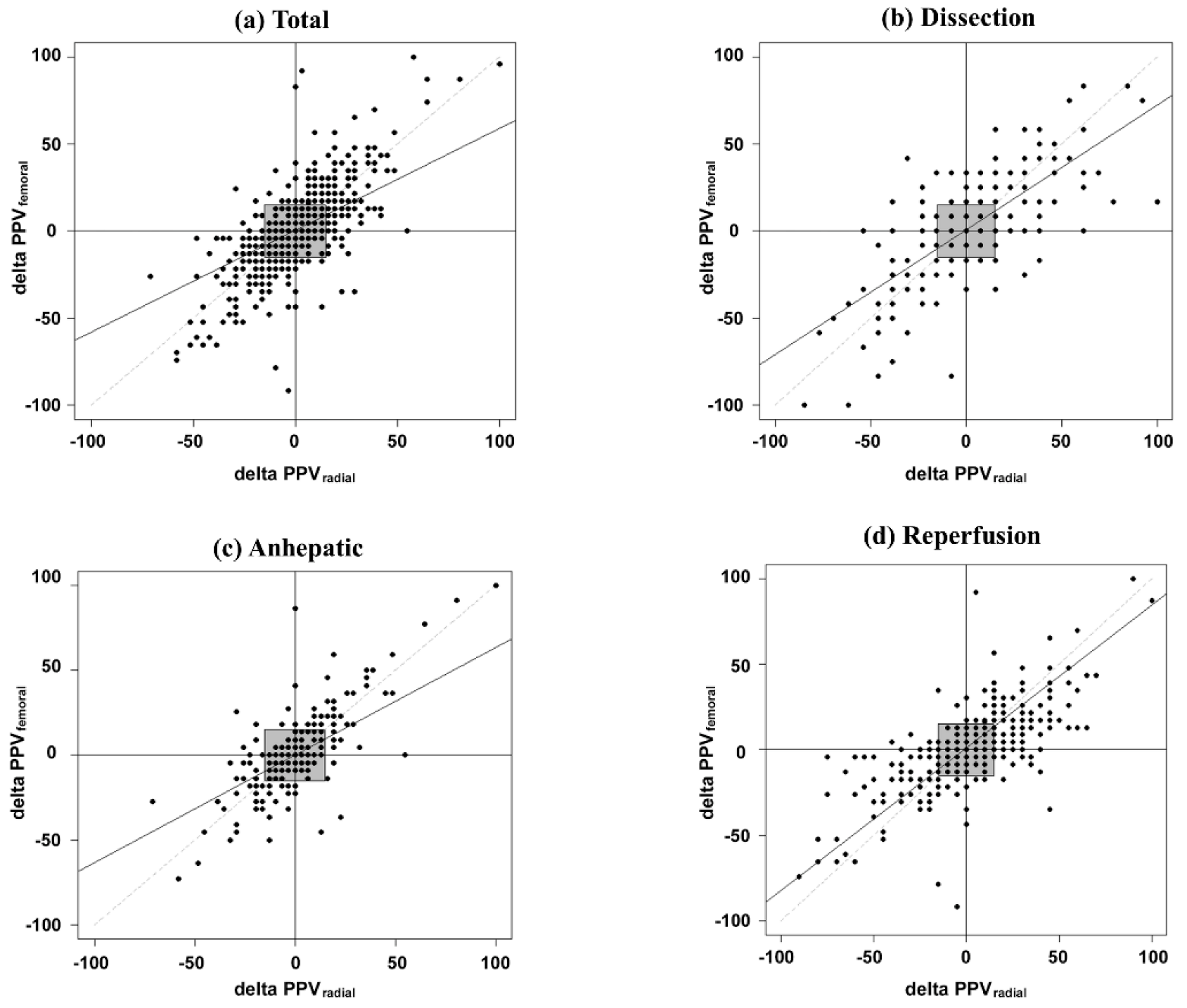
3. Results
3.1. Agreement between PPV-Radial and PPV-Femoral
3.2. Agreement Regarding Volume Depletion between PPV-Radial and PPV-Femoral
3.3. Analysis According to Intravascular Volume Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, R.F.; Glenn, T.K.; Lee, S.S. Cardiac dysfunction in cirrhosis. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.; Henriksen, J.H. Cardiovascular complications of cirrhosis. Gut 2008, 57, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, J.H.; Kim, G.; Ko, J.S.; Choi, S.J.; Kwon, J.H.; Heo, B.Y.; Gwak, M.S. Bioreactance Is Not Interchangeable with Thermodilution for Measuring Cardiac Output during Adult Liver Transplantation. PLoS ONE 2015, 10, e0127981. [Google Scholar] [CrossRef]
- Newby, D.E.; Hayes, P.C. Hyperdynamic circulation in liver cirrhosis: Not peripheral vasodilatation but ‘splanchnic steal’. QJM Int. J. Med. 2002, 95, 827–830. [Google Scholar] [CrossRef]
- Krenn, C.G.; Hoda, R.; Nikolic, A.; Greher, M.; Chevtchik, O.O.; Steltzer, H. Assessment of ventricular contractile function during orthotopic liver transplantation. Transpl. Int. 2004, 17, 101–104. [Google Scholar] [CrossRef]
- Arranz, J.; Soriano, A.; Garcia, I.; Concepción, M.T.; Navarro, J.; Artega, A.; Fiella, X.; Bravi, P.; Barrera, M.; Escribano, S.; et al. Effect of proinflammatory cytokines (IL-6, TNF-alpha, IL-1beta) on hemodynamic performance during orthotopic liver transplantation. Transplant. Proc. 2003, 35, 1884–1887. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kang, Y.; Freeman, J.A.; Fortunato, F.L., Jr.; Pinsky, M.R. Postreperfusion syndrome: Hypotension after reperfusion of the transplanted liver. J. Crit. Care 1993, 8, 154–160. [Google Scholar] [CrossRef]
- Cole, R. Does Central Venous Pressure Predict Fluid Responsiveness? Chest 2008, 134, 1351–1352. [Google Scholar] [CrossRef][Green Version]
- Marik, P.E.; Baram, M.; Vahid, B. Does Central Venous Pressure Predict Fluid Responsiveness?*: A Systematic Review of the Literature and the Tale of Seven Mares. Chest 2008, 134, 172–178. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R. Does the Central Venous Pressure Predict Fluid Responsiveness? An Updated Meta-Analysis and a Plea for Some Common Sense. Crit. Care Med. 2013, 41, 1774–1781. [Google Scholar] [CrossRef]
- Huber, W.; Umgelter, A.; Reindl, W.; Franzen, M.; Schmidt, C.; von Delius, S.; Geisler, F.; Eckel, F.; Fritsch, R.; Siveke, J.; et al. Volume assessment in patients with necrotizing pancreatitis: A comparison of intrathoracic blood volume index, central venous pressure, and hematocrit, and their correlation to cardiac index and extravascular lung water index. Crit. Care Med. 2008, 36, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Arnal, D.; Garutti, I.; Perez-Peña, J.; Olmedilla, L.; Tzenkov, I.G. Radial to femoral arterial blood pressure differences during liver transplantation. Anaesthesia 2005, 60, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Boldt, J.; Poland, R.; Peterson, A.; Manecke, G.R., Jr. Continuous Arterial Pressure Waveform–Based Cardiac Output Using the FloTrac/Vigileo: A Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2009, 23, 401–406. [Google Scholar] [CrossRef]
- Han, S.; Park, H.-W.; Song, J.H.; Gwak, M.S.; Lee, W.J.; Kim, G.; Lee, S.-K.; Ko, J.S. Association Between Intraoperative Platelet Transfusion and Early Graft Regeneration in Living Donor Liver Transplantation. Ann. Surg. 2016, 264, 1065–1072. [Google Scholar] [CrossRef]
- Song, I.-K.; Kim, E.-H.; Lee, J.-H.; Jang, Y.-E.; Kim, H.-S.; Kim, J.-T. Seldinger vs. modified Seldinger techniques for ultrasound-guided central venous catheterisation in neonates: A randomised controlled trial. Br. J. Anaesth. 2018, 121, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kwon, J.H.; Jung, S.H.; Seo, J.Y.; Jo, Y.J.; Jang, J.S.; Yeon, S.M.; Jung, S.H.; Ko, J.S.; Gwak, M.S.; et al. Perioperative Fresh Red Blood Cell Transfusion May Negatively Affect Recipient Survival After Liver Transplantation. Ann. Surg. 2018, 267, 346–351. [Google Scholar] [CrossRef]
- Michard, F.; Chemla, D.; Richard, C.; Wysocki, M.; Pinsky, M.R.; Lecarpentier, Y.; Teboul, J.-L. Clinical Use of Respiratory Changes in Arterial Pulse Pressure to Monitor the Hemodynamic Effects of PEEP. Am. J. Respir. Crit. Care Med. 1999, 159, 935–939. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Critchley, L.A.H.; Critchley, J.A.J.H. A Meta-Analysis of Studies Using Bias and Precision Statistics to Compare Cardiac Output Measurement Techniques. J. Clin. Monit. Comput. 1999, 15, 85–91. [Google Scholar] [CrossRef]
- Saugel, B.; Grothe, O.; Wagner, J.Y. Tracking Changes in Cardiac Output: Statistical considerations on the 4-quadrant plot and the polar plot methodology. Anesth. Analg. 2015, 121, 514–524. [Google Scholar] [CrossRef]
- Critchley, L.A.; Lee, A.; Ho, A.M.-H. A Critical Review of the Ability of Continuous Cardiac Output Monitors to Measure Trends in Cardiac Output. Anesth. Analg. 2010, 111, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Hadian, M.; Severyn, D.A.; Pinsky, M.R. The effects of vasoactive drugs on pulse pressure and stroke volume variation in postoperative ventilated patients. J. Crit. Care 2011, 26, 328.e1–328.e8. [Google Scholar] [CrossRef] [PubMed]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Mutchler, S.M.; Straub, A.C. Compartmentalized nitric oxide signaling in the resistance vasculature. Nitric Oxide 2015, 49, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Weinberg, L.; Pearce, B.; Scurrah, N.; Story, D.; Pillai, P.; McCall, P.R.; McNicol, L.P.; Peyton, P.J. Agreement between radial and femoral arterial blood pressure measurements during orthotopic liver transplantation. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2015, 17, 101–107. [Google Scholar]
- Bouchard-Dechêne, V.; Couture, P.; Su, A.; Deschamps, A.; Lamarche, Y.; Desjardins, G.; Levesque, S.; Denault, A.Y. Risk Factors for Radial-to-Femoral Artery Pressure Gradient in Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2018, 32, 692–698. [Google Scholar] [CrossRef]
- Remington, J.W. Contour changes of the aortic pulse during propagation. Am. J. Physiol. Leg. Content 1960, 199, 331–334. [Google Scholar] [CrossRef]
- Kanazawa, M.; Fukuyama, H.; Kinefuchi, Y.; Takiguchi, M.; Suzuki, T. Relationship between Aortic-to-radial Arterial Pressure Gradient after Cardiopulmonary Bypass and Changes in Arterial Elasticity. Anesthesiology 2003, 99, 48–53. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Groszmann, R.J. The hyperdynamic circulation of chronic liver diseases: From the patient to the molecule. Hepatology 2006, 43, S121–S131. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Byrns, R.E.; Wood, K.S. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ. Res. 1987, 60, 82–92. [Google Scholar] [CrossRef]
- Dart, A.M.; Kingwell, A.B. Pulse pressure—A review of mechanisms and clinical relevance. J. Am. Coll. Cardiol. 2001, 37, 975–984. [Google Scholar] [CrossRef]


| Variables | Descriptive Statistics |
|---|---|
| Donor factor | |
| Gender (male, %) | 36 (71) |
| Age (years) | 33 [25–48] |
| Body mass index (kg/m2) | 24 [21–26] |
| Graft-to-recipient body weight ratio | 1.06 [0.87–1.20] |
| Macrosteatosis | 15 (29) |
| Recipient factor | |
| Gender (male) | 38 (75) |
| Age (years) | 57 [51–62] |
| Body mass index (kg/m2) | 24.3 [21.7–27.2] |
| MELD score | 10 (8–19) |
| Etiology | |
| Viral | 33 (65) |
| Alcoholic | 8 (16) |
| Biliary | 6 (12) |
| Cryptogenic | 4 (8) |
| Preoperative laboratory findings | |
| White blood cell (×103/μL) | 4.06 [3.02–5.02] |
| Hemoglobin (g/dL) | 12.0 [9.7–13.6] |
| Hematocrit (%) | 35.3 [28.7–40.7] |
| Platelet (×103/μL) | 100 [51.0–122.0] |
| Prothrombin time (INR) | 1.27 [1.07–1.62] |
| Albumin (g/dL) | 3.4 [2.9–4.2] |
| Total bilirubin (mg/dL) | 1.6 [0.7–5.3] |
| Creatinine (mg/dL) | 0.75 [0.63–0.96] |
| Glucose (mg/dL) | 115 [90–173] |
| Sodium (mmol/L) | 138 [134–140] |
| Intraoperative | |
| Total anesthesia time (min) | 494 [465–559] |
| Operation time (min) | 401 [363–460] |
| Cold ischemia time (min) | 73 [63–84] |
| Warm ischemia time (min) | 37 [34–47] |
| Fluid administration and transfusion | |
| Crystaloid (mL) | 4850 [4050–6400] |
| 5% albumin (mL) | 890 [760–1100] |
| Synthetic colloid (mL) | 1000 [500–1000] |
| Red blood cell (unit) | 0 (0–2) |
| Fresh frozen plasma (unit) | 0 (0–2) |
| Single donor platelet (unit) | 0 (0–0) |
| Cryoprecipitate (unit) | 0 (0–0) |
| Phase | k, Kappa (95%CI) | Agreement, n (%) | Disagreement, n (%) |
|---|---|---|---|
| Overall | 0.641 (0.614, 0.669) | ||
| Non-depleted | 1312 (70) | 56 (3) | |
| Depleted | 319 (17) | 191 (10) | |
| Dissection | 0.607 (0.491, 0.722) | ||
| Non-depleted | 474 (87) | 4 (1) | |
| Depleted | 32 (6) | 32 (6) | |
| Anhepatic | 0.771 (0.712, 0.830) | ||
| Non-depleted | 289 (59) | 23 (5) | |
| Depleted | 151 (31) | 29 (6) | |
| Reperfusion | 0.510 (0.447, 0.572) | ||
| Non-depleted | 539 (64) | 25 (3) | |
| Depleted | 138 (16) | 139 (17) |
| Phase | Sensitivity | Specificity | Accuracy | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|
| Overall | 85 | 87 | 87 | 63 | 95 |
| Dissection | 89 | 93 | 93 | 50 | 99 |
| Anhepatic | 87 | 91 | 89 | 84 | 93 |
| Reperfusion | 85 | 80 | 81 | 50 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Ahn, J.H.; Han, S.; Ko, J.S.; Gwak, M.S.; Kim, G.S. Femoral Pulse Pressure Variation Is Not Interchangeable with Radial Pulse Pressure Variation during Living Donor Liver Transplantation. J. Pers. Med. 2022, 12, 1352. https://doi.org/10.3390/jpm12081352
Kim D, Ahn JH, Han S, Ko JS, Gwak MS, Kim GS. Femoral Pulse Pressure Variation Is Not Interchangeable with Radial Pulse Pressure Variation during Living Donor Liver Transplantation. Journal of Personalized Medicine. 2022; 12(8):1352. https://doi.org/10.3390/jpm12081352
Chicago/Turabian StyleKim, Doyeon, Jin Hee Ahn, Sangbin Han, Justin Sangwook Ko, Mi Sook Gwak, and Gaab Soo Kim. 2022. "Femoral Pulse Pressure Variation Is Not Interchangeable with Radial Pulse Pressure Variation during Living Donor Liver Transplantation" Journal of Personalized Medicine 12, no. 8: 1352. https://doi.org/10.3390/jpm12081352
APA StyleKim, D., Ahn, J. H., Han, S., Ko, J. S., Gwak, M. S., & Kim, G. S. (2022). Femoral Pulse Pressure Variation Is Not Interchangeable with Radial Pulse Pressure Variation during Living Donor Liver Transplantation. Journal of Personalized Medicine, 12(8), 1352. https://doi.org/10.3390/jpm12081352






