Type 1A Endoleak after TEVAR in the Aortic Arch: A Review of the Literature
Abstract
1. Introduction
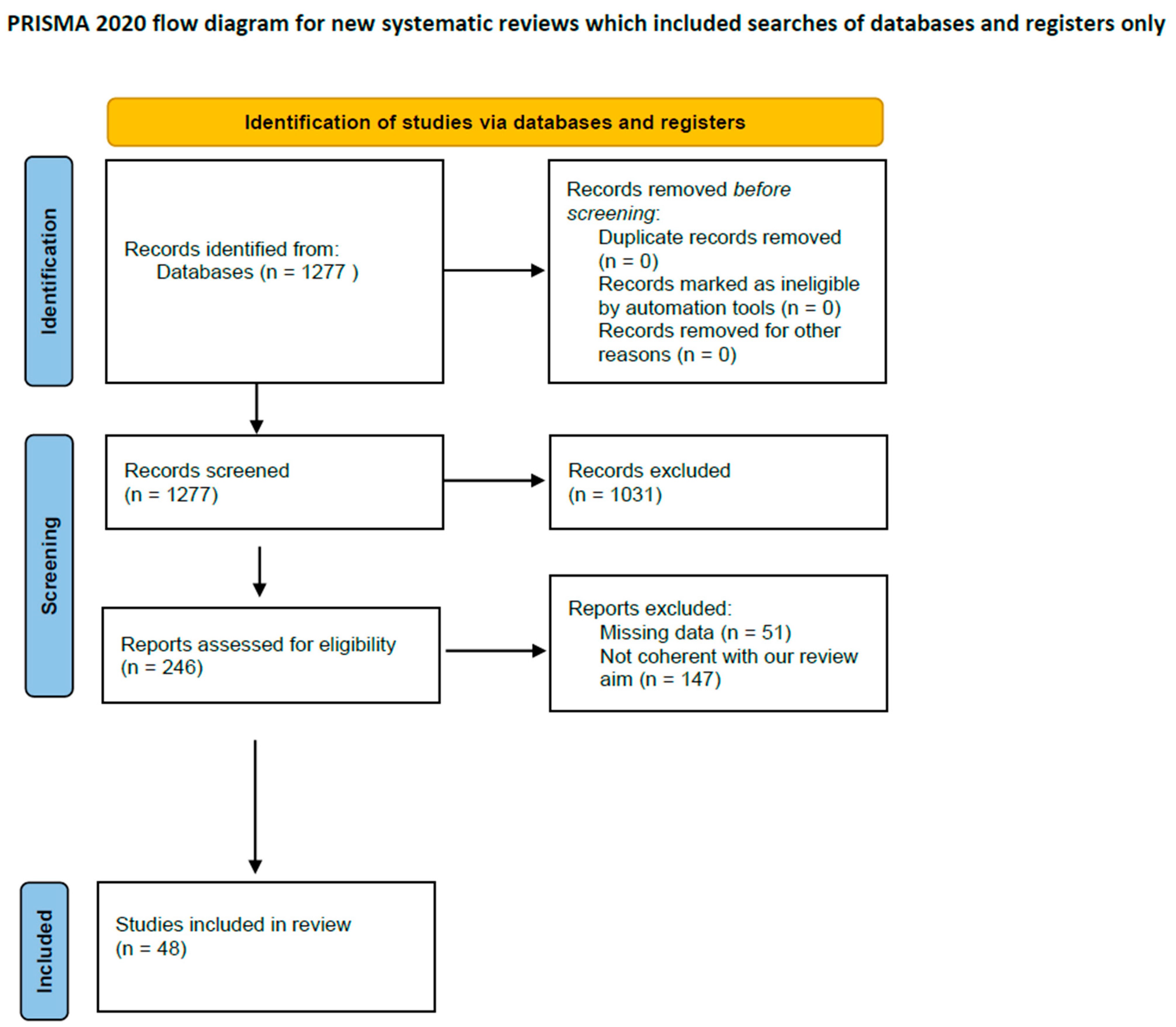
2. Materials and Methods
2.1. Study Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Criteria Appraisal
2.4. Study Selection
3. Results
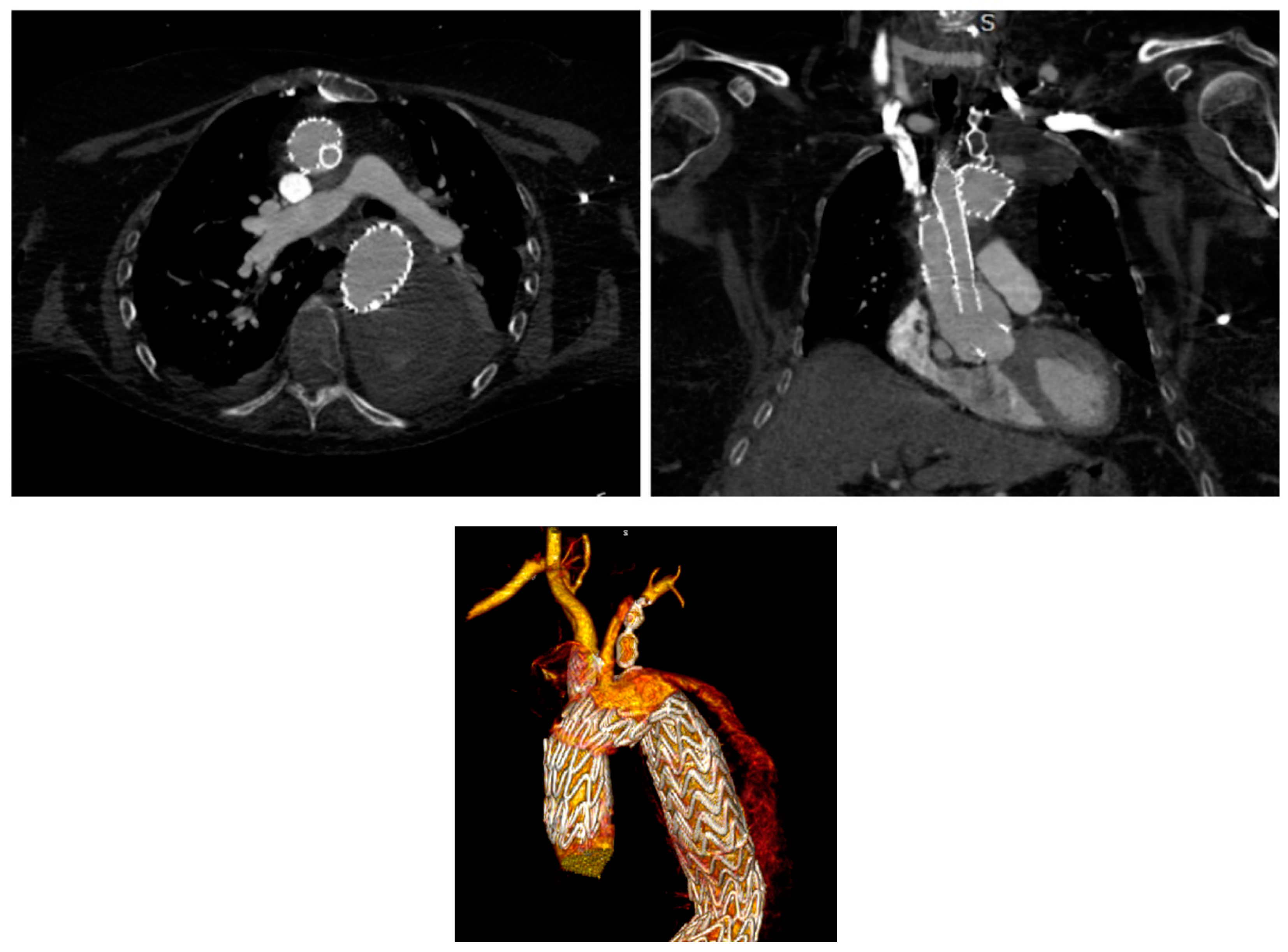
3.1. Chimney Technique
3.2. Fenestrations
3.3. In Situ Fenestration
3.4. Branched Endografts
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furuta, A.; Azuma, T.; Yokoi, Y.; Domoto, S.; Niinami, H. The midterm results of thoracic endovascular aortic repair with a precurved fenestrated endograft in zone 0–1. Eur. J. Cardio-Thorac. Surg. 2020, 58, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ (Clin. Res. Ed.) 2009, 339, b2700. [Google Scholar] [CrossRef]
- Ahmad, W.; Liakopoulos, O.J.; Mylonas, S.; Wegner, M.; Brunkwall, J.; Dorweiler, B. Long-Term Outcomes after Thoracic Endovascular Aortic Repair Using Chimney Grafts for Aortic Arch Pathologies: 10 Years of Single-Center Experience. Ann. Vasc. Surg. 2021, 72, 400–408. [Google Scholar] [CrossRef]
- Bosiers, M.J.; Donas, K.P.; Mangialardi, N.; Torsello, G.; Riambau, V.; Criado, F.J.; Veith, F.J.; Ronchey, S.; Fazzini, S.; Lachat, M. European Multicenter Registry for the Performance of the Chimney/Snorkel Technique in the Treatment of Aortic Arch Pathologic Conditions. Ann. Thorac. Surg. 2016, 101, 2224–2230. [Google Scholar] [CrossRef]
- Huang, W.; Ding, H.; Jiang, M.; Liu, Y.; Huang, C.; Yang, X.; Fan, R.; Luo, J.; Jiang, Z. Outcomes of chimney technique for aortic arch diseases:Asingle-center experiencewith226cases. Clin. Interv. Aging 2019, 14, 1829–1840. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Ohki, T.; Maeda, K.; Shukuzawa, K.; Baba, T.; Tezuka, M.; Omori, M.; Hara, M.; Takizawa, R.; Tachihara, H. Outcomes of Chimney Thoracic Endovascular Aortic Repair for an Aortic Arch Aneurysm. Ann. Vasc. Surg. 2020, 66, 212–219. [Google Scholar] [CrossRef]
- Mangialardi, N.; Serrao, E.; Kasemi, H.; Alberti, V.; Fazzini, S.; Ronchey, S. Clinical Investigation-Chimney Technique for Aortic Arch Pathologies: An 11-Year Single-Center Experience. J. Endovasc. Ther. 2014, 21, 312–323. Available online: www.jevt.org (accessed on 15 May 2022). [CrossRef]
- Voskresensky, I.; Scali, S.T.; Feezor, R.J.; Fatima, J.; Giles, K.A.; Tricarico, R.; Berceli, S.A.; Beck, A.W. Outcomes of thoracic endovascular aortic repair using aortic arch chimney stents in high-risk patients. J. Vasc. Surg. 2017, 66, 9–20.e3. [Google Scholar] [CrossRef]
- Wang, T.; Shu, C.; Li, Q.-M.; Li, M.; Li, X.; He, H.; Dardik, A.; Qiu, J. First experience with the double chimney technique in the treatment of aortic arch diseases. J. Vasc. Surg. 2017, 66, 1018–1027. [Google Scholar] [CrossRef]
- Wang, T.; Shu, C.; Li, M.; Li, Q.M.; Li, X.; Qiu, J.; Fang, K.; Dardik, A.; Yang, C.Z. Thoracic Endovascular Aortic Repair with Single/Double Chimney Technique for Aortic Arch Pathologies. J. Endovasc. Ther. 2017, 24, 383–393. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, J.; Yan, X.; Zhu, G.; Zhou, J.; Li, Z.; Feng, R.; Jing, Z. Outcomes of the Chimney Technique for Endovascular Repair of Aortic Dissection Involving the Arch Branches. Ann. Vasc. Surg. 2019, 58, 238–247.e3. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Jiao, Y.; Zhang, X.; Jiang, J.; Yang, H.; Ma, H. Early- and Mid-term Results of the Chimney Technique in the Repair of Aortic Arch Pathologies. Cardiovasc. Interv. Radiol. 2016, 39, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alonso, L.; Fernández Alonso, S.; Martínez Aguilar, E.; Santamarta Fariña, E.; Alegret Solé, J.; Atienza Pascual, M.; López San Martín, M.; Sánchez Rodríguez, J.M.; Alvarez, A.; Centeno Vallepuga, R. Fenestrated and Scalloped Endovascular Grafts in Zone 0 and Zone 1 for Aortic Arch Disease. Ann. Vasc. Surg. 2020, 69, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Canaud, L.; Ozdemir, B.A.; Chassin-Trubert, L.; Sfeir, J.; Alric, P.; Gandet, T. Homemade Fenestrated Stent-Grafts for Complete Endovascular Repair of Aortic Arch Dissections. J. Endovasc. Ther. 2019, 26, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Canaud, L.; Ozdemir, B.A.; Chassin-Trubert, L.; Sfeir, J.; Alric, P.; Gandet, T. Double homemade fenestrated stent graft for total endovascular aortic arch repair. J. Vasc. Surg. 2019, 70, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Chassin-Trubert, L.; Gandet, T.; Ozdemir, B.A.; Lounes, Y.; Alric, P.; Canaud, L. Aortic Arch Anatomy Pattern in Patients Treated Using Double Homemade Fenestrated Stent-Grafts for Total Endovascular Aortic Arch Repair. J. Endovasc. Ther. 2020, 27, 785–791. [Google Scholar] [CrossRef]
- Iwakoshi, S.; Ichihashi, S.; Itoh, H.; Tabayashi, N.; Sakaguchi, S.; Yoshida, T.; Nakao, Y.; Kichikawa, K. Clinical outcomes of thoracic endovascular aneurysm repair using commercially available fenestrated stent graft (Najuta endograft). J. Vasc. Surg. 2015, 62, 1473–1478. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Maruyama, R.; Ujihira, K.; Nishioka, N.; Hasegawa, K.; Iba, Y.; Hatta, E.; Yamada, A.; Nakanishi, K. Thoracic Endovascular Aortic Repair for Challenging Aortic Arch Diseases Using Fenestrated Stent Grafts From Zone 0. Ann. Thorac. Surg. 2015, 100, 24–33. [Google Scholar] [CrossRef]
- Tan, G.W.L.; Quek, L.; Tan, B.P.; Pua, U. Early Experience and Lessons Learnt with Customized Fenestrated Thoracic Endovascular Aortic Reconstruction for Aortic Arch Pathology in an Asian Population. Cardiovasc. Interv. Radiol. 2018, 41, 544–553. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Law, Y.; Rohlffs, F.; Spanos, K.; Debus, E.S.; Kölbel, T. Fenestrated endovascular repair for diseases involving the aortic arch. J. Vasc. Surg. 2020, 71, 1464–1471. [Google Scholar] [CrossRef]
- Yap, H.Y.; Chong, T.T.; Tay, H.T.; Sung, L.; Lee, Q.S.; Chng, J.K.; Wang, C.C.J.; Tay, K.H.; Choke, E. Fenestrated Endovascular Repair of Zones 1 and 2 Aortic Arch Pathologies. Ann. Vasc. Surg. 2019, 54, 145.e1–145.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, Y.; Cao, H.; Pan, J.; Wang, Q.; Fan, F.; Wang, D. Novel arch fenestrated stent graft for acute Stanford Type A aortic dissection with open antegrade implantation. Interactive Cardiovasc. Thorac. Surg. 2018, 26, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dai, X.; Noiniyom, P.; Luo, Y.; Fan, H.; Feng, Z.; Zhang, Y.; Hu, F. Fenestrated Thoracic Endovascular Aortic Repair Using Physician-Modified Stent Grafts (PMSGs) in Zone 0 and Zone 1 for Aortic Arch Diseases. Cardiovasc. Interv. Radiol. 2019, 42, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ma, C.; Dai, X.; Wang, Z.; Fan, H.; Feng, Z.; Luo, Y.; Zhang, Y.; Hu, F. Outcomes of single physician-modified fenestrated stent grafts for endovascular repair of thoracic aortic lesions involving the distal aortic arch. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, P.; Hua, Z.; Jiao, Z.; Cao, H.; Liu, S.; Zhang, W.W.; Li, Z. Early and midterm outcomes of in situ laser fenestration during thoracic endovascular aortic repair for acute and subacute aortic arch diseases and analysis of its complications. J. Vasc. Surg. 2020, 72, 1524–1533. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Ren, Z.; Hu, R.; Wu, H. Simplified total aortic arch replacement with an in situ stent graft fenestration technique for acute type A aortic dissection. J. Vasc. Surg. 2017, 66, 711–717. [Google Scholar] [CrossRef]
- Katada, Y.; Kondo, S.; Tsuboi, E.; Rokkaku, K.; Irie, Y.; Yokoyama, H. Endovascular Total Arch Repair Using In Situ Fenestration for Arch Aneurysm and Chronic Type A Dissection. Ann. Thorac. Surg. 2016, 101, 625–630. [Google Scholar] [CrossRef]
- Kopp, R.; Katada, Y.; Kondo, S.; Sonesson, B.; Hongo, N.; Tse, L.; Tsilimparis, N.; Crawford, S.; Panneton, J.M.; Kölbel, T.; et al. Multicenter Analysis of Endovascular Aortic Arch In Situ Stent-Graft Fenestrations for Aortic Arch Pathologies. Ann. Vasc. Surg. 2019, 59, 36–47. [Google Scholar] [CrossRef]
- le Houérou, T.; Fabre, D.; Alonso, C.G.; Brenot, P.; Bourkaib, R.; Angel, C.; Amsallem, M.; Haulon, S. In Situ Antegrade Laser Fenestrations During Endovascular Aortic Repair. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 356–362. [Google Scholar] [CrossRef]
- Luo, M.; Fang, K.; Fan, B.; Li, Q.; Li, M.; He, H.; Li, X.; Guo, Y.; Xue, Y.; Zhao, J.; et al. Midterm Results of Retrograde In Situ Needle Fenestration During Thoracic Endovascular Aortic Repair of Aortic Arch Pathologies. J. Endovasc. Ther. 2021, 28, 36–43. [Google Scholar] [CrossRef]
- Redlinger, R.E.; Ahanchi, S.S.; Panneton, J.M. In situ laser fenestration during emergent thoracic endovascular aortic repair is an effective method for left subclavian artery revascularization. J. Vasc. Surg. 2013, 58, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.; Bai, H.; Liu, Y.; Bai, T.; Qiao, Z. In Situ Laser Fenestration for Delayed Left Subclavian Artery Revascularization Following Thoracic Endovascular Aortic Repair of Type B Aortic Dissection. Vasc. Endovasc. Surg. 2021, 55, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Qin, J.; Yin, M.; Liu, G.; Liu, X.; Ye, K.; Wang, R.; Shi, H.; Li, W.; Jiang, M.; et al. In Situ Laser Stent Graft Fenestration of the Left Subclavian Artery during Thoracic Endovascular Repair of Type B Aortic Dissection with Limited Proximal Landing Zones: 5-Year Outcomes. J. Vasc. Interv. Radiol. 2020, 31, 1321–1327. [Google Scholar] [CrossRef]
- Chen, L.W.; Lu, L.; Dai, X.F.; Wu, X.J.; Zhang, G.C.; Yang, G.F.; Dong, Y. Total arch repair with open triple-branched stent graft placement for acute type A aortic dissection: Experience with 122 patients. J. Thorac. Cardiovasc. Surg. 2014, 148, 521–528. [Google Scholar] [CrossRef]
- Clough, R.E.; Martin-Gonzalez, T.; van Calster, K.; Hertault, A.; Spear, R.; Azzaoui, R.; Sobocinski, J.; Haulon, S. Endovascular Repair of Thoracoabdominal and Arch Aneurysms in Patients with Connective Tissue Disease Using Branched and Fenestrated Devices. Ann. Vasc. Surg. 2017, 44, 158–163. [Google Scholar] [CrossRef]
- Czerny, M.; Berger, T.; Kondov, S.; Siepe, M.; Saint Lebes, B.; Mokrane, F.; Rousseau, H.; Lescan, M.; Schlensak, C.; Andic, M.; et al. Results of endovascular aortic arch repair using the Relay Branch system. Eur. J. Cardio-Thorac. Surg. 2021, 60, 662–668. [Google Scholar] [CrossRef]
- Dai, X.F.; Chen, L.W.; Wu, X.J.; Dong, Y.; Wang, Q.M. Total Aortic Arch Reconstruction with Triple-Branched Stent Graft or Hemiarch Replacement for Acute Debakey Type i Aortic Dissection: Five-Years Experience with 93 Patients. J. Card. Surg. 2015, 30, 749–755. [Google Scholar] [CrossRef]
- Ferrer, C.; Coscarella, C.; Cao, P. Endovascular repair of aortic arch disease with double inner branched thoracic stent graft: The Bolton perspective. J. Cardiovasc. Surg. 2018, 59, 547–553. [Google Scholar] [CrossRef]
- Ferrer, C.; Cao, P.; Coscarella, C.; Ferri, M.; Lovato, L.; Camparini, S.; di Marzo, L.; Giudice, R.; Pogany, G.; de Gregorio, C.; et al. iTalian RegIstry of doUble inner branch stent graft for arch PatHology (the TRIUmPH Registry). J. Vasc. Surg. 2019, 70, 672–682.e1. [Google Scholar] [CrossRef]
- Kawajiri, H.; Tenorio, E.R.; Khasawneh, M.A.; Pochettino, A.; Mendes, B.C.; Marcondes, G.B.; Lima, G.B.B.; Oderich, G.S. Staged total arch replacement, followed by fenestrated-branched endovascular aortic repair, for patients with mega aortic syndrome. J. Vasc. Surg. 2021, 73, 1488–1497.e1. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Song, C.; Zhang, H.; Xia, S.; Li, H.; Jing, Z.; Lu, Q. Long-Term Outcomes of Thoracic Endovascular Repair for Aortic Arch Dissection Using Customized Single-Branched Fenestrated Stent-Graft. Vasc. Endovasc. Surg. 2021, 55, 577–585. [Google Scholar] [CrossRef]
- Patel, H.J.; Dake, M.D.; Bavaria, J.E.; Singh, M.J.; Filinger, M.; Fischbein, M.P.; Williams, D.M.; Matsumura, J.S.; Oderich, G. Branched Endovascular Therapy of the Distal Aortic Arch: Preliminary Results of the Feasibility Multicenter Trial of the Gore Thoracic Branch Endoprosthesis. Ann. Thorac. Surg. 2016, 102, 1190–1198. [Google Scholar] [CrossRef]
- Tazaki, J.; Inoue, K.; Higami, H.; Higashitani, N.; Toma, M.; Saito, N.; Kawatou, M.; Kimura, T. Thoracic endovascular aortic repair with branched Inoue Stent Graft for arch aortic aneurysms. J. Vasc. Surg. 2017, 66, 1340–1348.e5. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Detter, C.; Heidemann, F.; Spanos, K.; Rohlffs, F.; von Kodolitsch, Y.; Debus, S.E.; Kölbel, T. Branched endografts in the aortic arch following open repair for DeBakey Type i aortic dissection. Eur. J. Cardio-Thorac. Surg. 2018, 54, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Detter, C.; Law, Y.; Rohlffs, F.; Heidemann, F.; Brickwedel, J.; von Kodolitsch, Y.; Debus, E.S.; Kölbel, T. Single-center experience with an inner branched arch endograft. J. Vasc. Surg. 2019, 69, 977–985.e1. [Google Scholar] [CrossRef] [PubMed]
- Verscheure, D.; Haulon, S.; Tsilimparis, N.; Resch, T.; Wanhainen, A.; Mani, K.; Dias, N.; Sobocinski, J.; Eagleton, M.; Ferreira, M.; et al. Endovascular treatment of post type a chronic aortic arch dissection with a branched endograft early results from a retrospective International multicenter study. Ann. Surg. 2021, 273, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Rylski, B.; Morlock, J.; Schröfel, H.; Beyersdorf, F.; Lebes, B.S.; Meyrignac, O.; Mokrane, F.; Lescan, M.; Schlensak, C.; et al. Orthotopic branched endovascular aortic arch repair in patients who cannot undergo classical surgery. Eur. J. Cardio-Thorac. Surg. 2018, 53, 1007–1012. [Google Scholar] [CrossRef]
- Konstantinou, N.; Kölbel, T.; Debus, E.S.; Rohlffs, F.; Tsilimparis, N. Fenestrated versus debranching thoracic endovascular aortic repair for endovascular treatment of distal aortic arch and descending aortic lesions. J. Vasc. Surg. 2021, 73, 1915–1924. [Google Scholar] [CrossRef]
- XiaoHui, M.; Li, W.; Wei, G.; XiaoPing, L.; Xin, J.; Hongpeng, Z.; Lijun, W. Comparison of supra-arch in situ fenestration and chimney techniques for aortic dissection involving the left subclavian artery. Vascular 2019, 27, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Visceral aortic patch aneurysm after thoracoabdominal aortic repair: Conventional vs. hybrid treatment. J. Vasc. Surg. 2008, 48, 1083–1091. [CrossRef] [PubMed][Green Version]
- Melissano, G.; Tshomba, Y.; Bertoglio, L.; Rinaldi, E.; Chiesa, R. Analysis of stroke after TEVAR involving the aortic arch. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 269–275. [Google Scholar] [CrossRef]
- Tshomba, Y.; Bertoglio, L.; Marone, E.M.; Logaldo, D.; Maisano, F.; Chiesa, R. Retrograde type A dissection after endovascular repair of a “zone 0” nondissecting aortic arch aneurysm. Ann. Vasc. Surg. 2010, 24, 952.e1–952.e7. [Google Scholar] [CrossRef]

| Author | Study Cohort | Endoleaks |
|---|---|---|
| Chimney | ||
| Ahmad et al. (2020) [3] | 54 | 8 (14.8%) |
| Bosiers et al. (2016) [4] | 95 | 10 (10.5%) |
| Huang et al. (2019) [5] | 226 | 37 (16.3%) |
| Kanaoka et al. (2018) [6] | 55 | 22 (40%) |
| Mangialardi et al. (2014) [7] | 26 | 6 (23%) |
| Voskresensky et al. (2017) [8] | 27 | 2 (7.4%) |
| Wang et al. (2017) [9] | 122 | 13 (10.6%) |
| Wang et al. (2017) [10] | 23 | 3 (13%) |
| Zhao et al. (2019) [11] | 234 | 75 (32%) |
| Zou et al. (2015) [12] | 35 | 5 (14.2%) |
| Total | 897 | 181 (20.1%) |
| Fenestrated | ||
| Canaud et al. (2019) [14] | 35 | 0 |
| Canaud et al. (2019) [15] | 17 | 0 |
| Chassin-Traubert et al. (2020) [16] | 50 | 1 (2%) |
| Fernandez-Alonso et al. (2020) [13] | 14 | 3 (21.4%) |
| Furuta et al. (2020) [1] | 205 | 7 (3.4%) |
| Iwakoshi et al. (2015) [17] | 32 | 3 (9.3%) |
| Kurimoto et al. (2015) [18] | 37 | 12 (32.4%) |
| Tan et al. (2020) [19] | 7 | 0 |
| Tsilimparis et al. (2020) [20] | 44 | 0 |
| Yap et al. (2018) [21] | 5 | 0 |
| Zhou et al. (2017) [22] | 42 | 0 |
| Zhu et al. (2018) [23] | 10 | 0 |
| Zhu et al. (2020) [24] | 58 | 2 (3.4%) |
| Total | 556 | 28 (5%) |
| Branched | ||
| Chen et al. (2013) [34] | 122 | 12 (9.8%) |
| Clough et al. (2017) [35] | 30 | 0 |
| Czerny et al. (2017) [47] | 15 | 1 (6.6%) |
| Czerny et al. (2021) [36] | 43 | 2 (4.6%) |
| Dai et al. (2015) [37] | 93 | 0 |
| Ferrer et al. (2018) [38] | 7 | 0 |
| Ferrer et al. (2019) [39] | 24 | 0 |
| Kawajiri et al. (2018) [40] | 11 | 2 (18.1%) |
| Li et al. (2021) [41] | 16 | 1 (6.2%) |
| Patel et al. (2016) [42] | 22 | 4 (18.1%) |
| Tazaki et al. (2017) [43] | 217 | 9 (4.14%) |
| Tsilimparis et al. (2017) [44] | 20 | 1 (5%) |
| Tsilimparis et al. (2018) [45] | 54 | 2 (3.7%) |
| Verscheure et al. (2019) [46] | 70 | 2 (2.8%) |
| Total | 744 | 36 (4.8%) |
| In situ fenestration | ||
| Hu et al. (2017) [26] | 107 | 0 |
| Katada et al. (2015) [27] | 7 | 0 |
| Kopp et al. (2018) [28] | 25 | 1 (4%) |
| Le Houreou et al. (2018) [29] | 16 | 0 |
| LiChong et al. (2020) [25] | 148 | 7 (4.7%) |
| Luo et al. (2020) [30] | 50 | 0 |
| Redlinger et al. (2013) [31] | 22 | 0 |
| Wang et al. (2021) [32] | 5 | 0 |
| Zhao et al. (2020) [33] | 130 | 4 (3%) |
| Total | 510 | 12 (2.3%) |
| Comparative studies | ||
| Konstantinou et al. (2020) [48] | 36 (19 Fenestrated, 17 Branched) | 1 type (Branched) |
| XiaoHui et al. (2018) [49] | 85 (67 Chimney, 18 In situ fenestration) | 1 type 1 (Chimney) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scurto, L.; Peluso, N.; Pascucci, F.; Sica, S.; De Nigris, F.; Filipponi, M.; Minelli, F.; Donati, T.; Tinelli, G.; Tshomba, Y. Type 1A Endoleak after TEVAR in the Aortic Arch: A Review of the Literature. J. Pers. Med. 2022, 12, 1279. https://doi.org/10.3390/jpm12081279
Scurto L, Peluso N, Pascucci F, Sica S, De Nigris F, Filipponi M, Minelli F, Donati T, Tinelli G, Tshomba Y. Type 1A Endoleak after TEVAR in the Aortic Arch: A Review of the Literature. Journal of Personalized Medicine. 2022; 12(8):1279. https://doi.org/10.3390/jpm12081279
Chicago/Turabian StyleScurto, Lucia, Nicolò Peluso, Federico Pascucci, Simona Sica, Francesca De Nigris, Marco Filipponi, Fabrizio Minelli, Tommaso Donati, Giovanni Tinelli, and Yamume Tshomba. 2022. "Type 1A Endoleak after TEVAR in the Aortic Arch: A Review of the Literature" Journal of Personalized Medicine 12, no. 8: 1279. https://doi.org/10.3390/jpm12081279
APA StyleScurto, L., Peluso, N., Pascucci, F., Sica, S., De Nigris, F., Filipponi, M., Minelli, F., Donati, T., Tinelli, G., & Tshomba, Y. (2022). Type 1A Endoleak after TEVAR in the Aortic Arch: A Review of the Literature. Journal of Personalized Medicine, 12(8), 1279. https://doi.org/10.3390/jpm12081279








