The Effects of Percutaneous Coronary Intervention on the Flow in Acute Coronary Syndrome Patients—Geometry in Focus
Abstract
1. Introduction
2. Methods
3. Statistical Methods
4. Results
Evaluation of TFC Δ ≥ 3 vs. Δ < 3
5. Discussion
6. Conclusion
- In ACS with opened culprit arteries, the flow characteristics are similar between the two major forms, referring to a similar patomechanism after the vessel is open.
- Irrespective to the form of ACS, receiving IIb/IIIa receptor inhibitor provided better chance for survival during the six-year follow-up.
- Last, but not least, no STEMI patient died during the follow-up who received IIb/IIIa receptor inhibitor, proving strong potential benefit in this patient cohort.
- These findings prove that using IIb/IIIa inhibitor, in select cases, was safe and effective. For more accurate results and predictions in mortality, alternatively a retroprospective analysis with fitted patient cohorts (same 2/3D parameters and TFC changes, naive or GP IIb/IIIa receptor inhibitor administered) would be of high importance.
7. Limitations
8. Ethical considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Normand, S.-L.T.; Mauri, L.; Kuntz, R.E. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation 2004, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Cilla, M.; Peña, E.; Martínez, M.A.; Kelly, D.J. Comparison of the vulnerability risk for positive versus negative atheroma plaque morphology. J. Biomech. 2013, 46, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol 2006, 47 (Suppl. 8), C13–C18. [Google Scholar] [CrossRef]
- Iannaccone, M.; Vadalà, P.; D’ascenzo, F.; Montefusco, A.; Moretti, C.; D’Amico, M.; Gaita, F. Clinical perspective of optical coherence tomography and intravascular ultrasound in STEMI patients. J. Thorac. Dis. 2016, 8, 754–756. [Google Scholar] [CrossRef][Green Version]
- He, B.; Gai, L.; Gai, J.; Qiao, H.; Zhang, S.; Guan, Z.; Yang, L.; Chen, Y. Correlation between major adverse cardiac events and coronary plaque characteristics. Exp. Clin. Cardiol. 2013, 18, e71–e76. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Üveges, Á.; Jenei, C.; Kiss, T.; Szegedi, Z.; Tar, B.; Szabó, G.T.; Czuriga, D.; Kőszegi, Z. Three-dimensional evaluation of the spatial morphology of stented coronary artery segments in relation to restenosis. Int. J. Cardiovasc. Imaging 2019, 35, 1755–1763. [Google Scholar] [CrossRef]
- Kolozsvári, R.; Tar, B.; Lugosi, P.; Sánta, J.; Béres, Z.; Ungvári, T.; Polgár, P.; Kőszegi, Z. Plaque volume derived from three-dimensional reconstruction of coronary angiography predicts the fractional flow reserve. Int. J. Cardiol. 2012, 160, 140–144. [Google Scholar] [CrossRef]
- Morrow, D.A.; Antman, E.M.; Charlesworth, A.; Cairns, R.; Murphy, S.A.; de Lemos, J.A.; Giugliano, R.P.; McCabe, C.H. Braunwald ETIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000, 102, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Mintz, G.S.; Kim, S.-Y.; Hong, Y.J.; Kim, S.W.; Okabe, T.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Suddath, W.O.; et al. Attenuated plaque detected by intravascular ultrasound: Clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc. Interv. 2009, 2, 65–72. [Google Scholar] [CrossRef] [PubMed]
- French, J.K.; Hyde, T.A.; Straznicky, I.T.; Andrews, J.; Lund, M.; Amos, D.J.; Zambanini, A.; Ellis, C.J.; Webber, B.J.; McLaughlin, S.C.; et al. Relationship between corrected TIMI frame counts at three weeks and late survival after myocardial infarction. J. Am. Coll. Cardiol. 2000, 35, 1516–1524. [Google Scholar] [CrossRef]
- Sahin, M.; Basoglu, T.; Canbaz, F.; Elcik, M.; Kosus, A. The value of the TIMI frame count method in the diagnosis of coronary no-reflow: A comparison with myocardial perfusion, S.P.ECT in patients with acute myocardial infarction. Nucl. Med. Commun. 2002, 23, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Farooq, V.; Serruys, P.W.; Mustafa, A.H.S.; Mamas, M.A.; Malik, N.; Alhous, H.A.; El-Omar, M.; Hendry, C.; Rana, D.N.; Shelton, D.; et al. Forward and back aspiration during ST-elevation myocardial infarction: A feasibility study. EuroIntervention 2016, 11, e1639–e1648. [Google Scholar] [CrossRef]
- Tanaka, A.; Kawarabayashi, T.; Nishibori, Y.; Sano, T.; Nishida, Y.; Fukuda, D.; Shimada, K.; Yoshikawa, J. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation 2002, 105, 2148–2152. [Google Scholar] [CrossRef]
- Jia, R.; Nie, X.; Li, H.; Zhu, H.; Pu, L.; Li, X.; Han, J.; Yang, D.; Meng, S.; Jin, Z. Impact of attenuated plaques on TIMI grade flow and clinical outcomes of coronary artery disease patients: A systematic review and meta analysis. J. Thorac. Dis. 2016, 8, 527–536. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Daley, W.L.; Dodge, J.T.; Alexander, B.; Marble, S.J.; McCabe, C.H.; Raymond, L.; Fortin, T.; Poole, W.K.; et al. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996, 93, 879–888. [Google Scholar] [CrossRef]
- Vrachatis, A.D.; Alpert, M.A.; Georgulas, V.P.; Nikas, D.J.; Petropoulou, E.N.; Lazaros, G.I.; Michelakakis, N.A.; Karavidis, A.I.; Lakoumentas, J.A.; Stergiou, L.; et al. Comparative efficacy of primary angioplasty w.with stent implantation and thrombolysis in restoring basal coronary artery flow in acute ST segment elevation myocardial infarction: Quantitative assessment using the corrected TIMI frame count. Angiology 2001, 52, 161–166. [Google Scholar] [CrossRef]
- Esen, A.M.; Acar, G.; Esen, O.; Emiroglu, Y.; Akcakoyun, M.; Pala, S.; Karapinar, H.; Kargin, R.; Barutcu, I.; Turkmen, M. The prognostic value of combined fractional flow reserve and TIMI frame count measurements in patients with stable angina pectoris and acute coronary syndrome. J. Interv. Cardiol. 2010, 23, 421–428. [Google Scholar] [CrossRef]
- Abaci, A.; Oguzhan, A.; Eryol, N.K.; Ergin, A. Effect of potential confounding factors on the thrombolysis in myocardial infarction (TIMI) trial frame count and its reproducibility. Circulation 1999, 100, 2219–2223. [Google Scholar] [CrossRef]
- Umman, B.; Nisanci, Y.; Sezer, M.; Umman, S.; Yilmaz, E.; Oflaz, H.; Ozsaruhan, O. The relationship between corrected TIMI frame count and myocardial fractional flow reserve. J. Invasive Cardiol. 2002, 14, 125–128. [Google Scholar] [PubMed]
- Hayıroğlu, M.İ.; Keskin, M.; Uzun, A.O.; Yıldırım, D.İ.; Kaya, A.; Çinier, G.; Bozbeyoğlu, E.; Yıldırımtürk, Ö.; Kozan, Ö.; Pehlivanoğlu, S.; et al. Predictors of In-Hospital Mortality in Patients With, S.T.-Segment Elevation Myocardial Infarction Complicated With Cardiogenic Shock. Heart Lung Circ. 2019, 28, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Tricoci, P.; Newby, L.K.; Hasselblad, V.; Kong, D.F.; Giugliano, R.P.; White, H.D.; Théroux, P.; Stone, G.W.; Moliterno, D.J.; Van de Werf, F.; et al. Upstream use of small-molecule glycoprotein iib/iiia inhibitors in patients with non-ST-segment elevation acute coronary syndromes: A systematic overview of randomized clinical trials. Circ. Cardiovasc. Qual Outcomes 2011, 4, 448–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sciahbasi, A.; Biondi-Zoccai, G.; Romagnoli, E.; Valgimigli, M.; Rasoul, S.; van’t Hof, A.; Lioy, E.; Stone, G.W. Routine upstream versus selective downstream administration of glycoprotein IIb/IIIa inhibitors in patients with non-ST-elevation acute coronary syndromes: A meta-analysis of randomized trials. Int. J. Cardiol. 2012, 155, 243–248. [Google Scholar] [CrossRef]
- Howard, J.P.; Jones, D.A.; Gallagher, S.; Rathod, K.; Antoniou, S.; Wright, P.; Knight, C.; Mathur, A.; Weerackody, R.; Wragg, A.; et al. Glycoprotein IIb/IIIa inhibitors use and outcome after percutaneous coronary intervention for non-ST elevation myocardial infarction. Biomed. Res. Int. 2014, 2014, 643981. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Navarese, E.; Marino, P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with, S.T.-segment elevation myocardial infarction treated with primary angioplasty: A meta-regression analysis of randomized trials. Eur. Heart J. 2009, 30, 2705–2713. [Google Scholar] [CrossRef]
- De Luca, G.; Gibson, C.M.; Bellandi, F.; Murphy, S.; Maioli, M.; Noc, M.; Zeymer, U.; Dudek, D.; Arntz, H.R.; Zorman, S. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty (EGYPT) cooperation: An individual patient data meta-analysis. Heart 2008, 94, 1548–1558. [Google Scholar] [CrossRef]
- Bouisset, F.; Ruidavets, J.-B.; Dallongeville, J.; Moitry, M.; Montaye, M.; Biasch, K.; Ferrières, J. Comparison of Short- and Long-Term Prognosis between, S.T.-Elevation and Non-ST-Elevation Myocardial Infarction. J. Clin. Med. 2021, 10, 180. [Google Scholar] [CrossRef]
- Glaser, R.; Glick, H.A.; Herrmann, H.C.; Kimmel, S.E. The role of risk stratification in the decision to provide upstream versus selective glycoprotein IIb/IIIa inhibitors for acute coronary syndromes: A cost-effectiveness analysis. J. Am. Coll. Cardiol. 2006, 47, 529–537. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
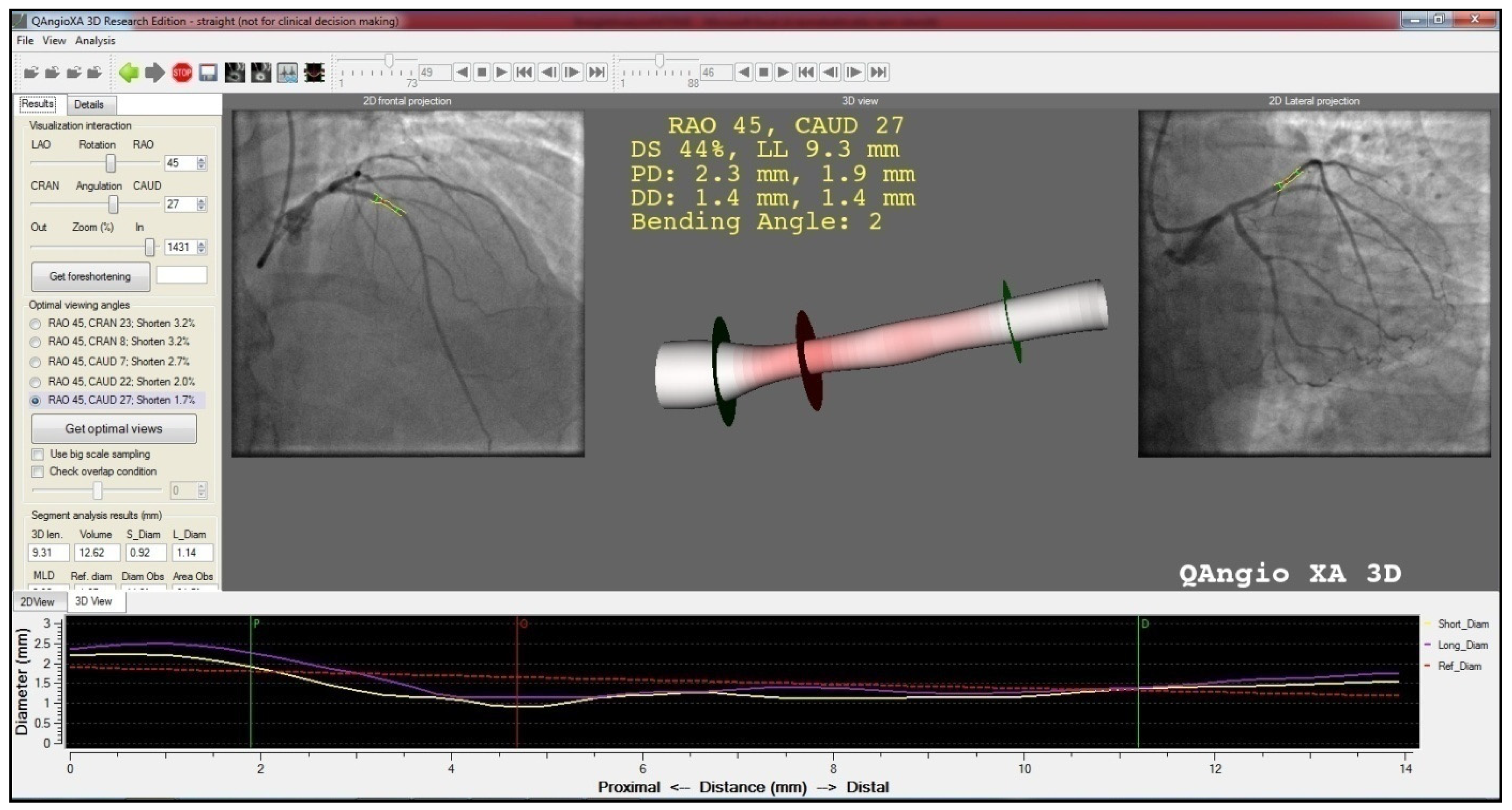
| STEMI (n = 71) | NSTEMI (n = 73) | p | |
|---|---|---|---|
| Age | 64.90 ± 10.80 | 65.10 ± 9.99 | 0.910 |
| Gender | |||
| -male | 46 (65%) | 51 (70%) | 0.516 |
| -female | 25 (35%) | 22 (30%) | |
| Hypertension | 39 (55%) | 51 (70%) | 0.064 |
| Diabetes | 15 (21%) | 33 (45%) | 0.002 |
| Dyslipidemia | 13 (18%) | 41 (56%) | < 0.001 |
| Smoking | 36 (51%) | 35 (48%) | 0.741 |
| Earlier infarction | 8 (11%) | 27 (37%) | < 0.001 |
| Sodium-heparin in ambulance | 56 (79%) | 16 (22%) | < 0.001 |
| STEMI (n = 71) | NSTEMI (n = 73) | p | |
|---|---|---|---|
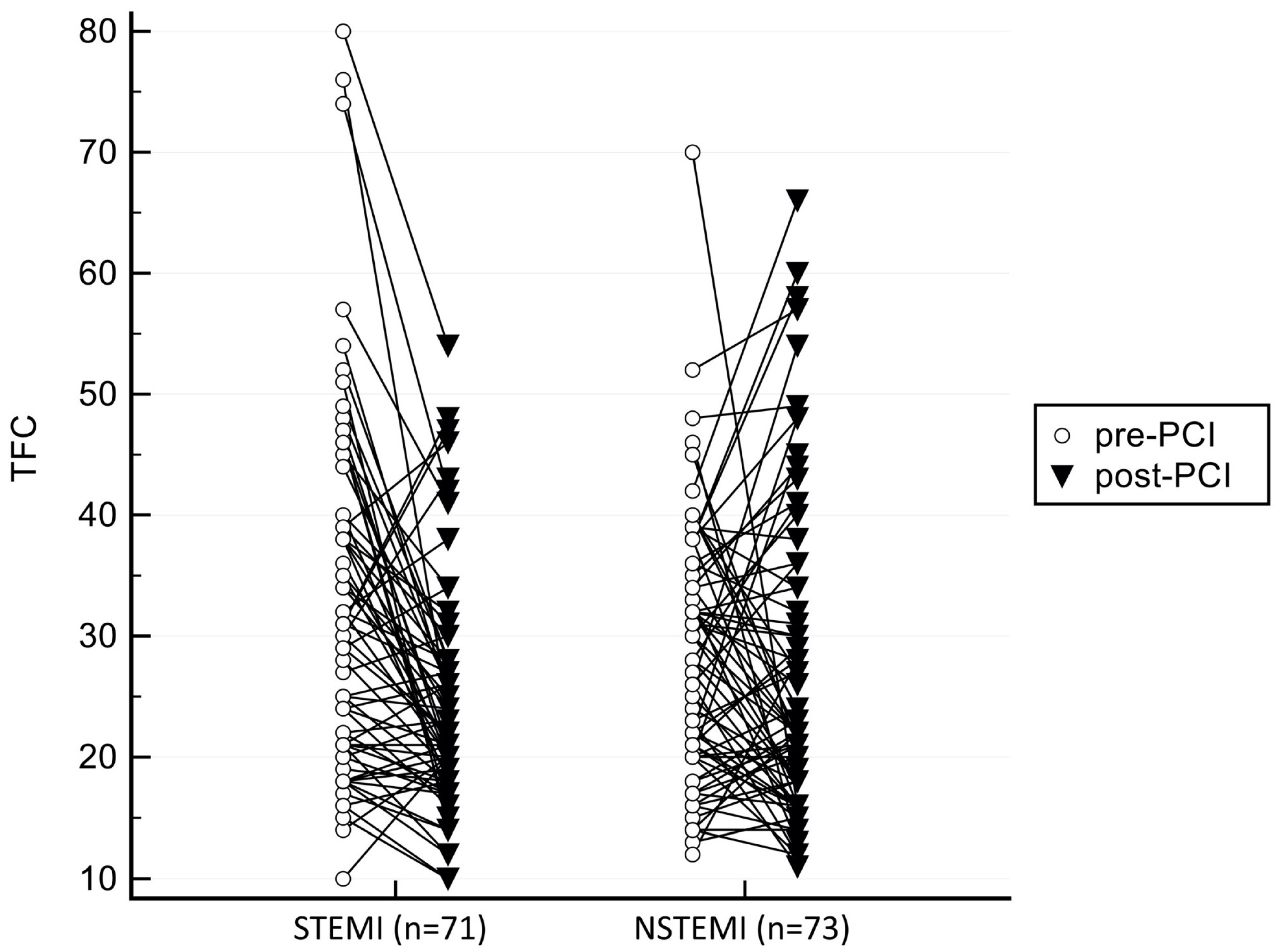
| TFC pre-PCI | 32.42 ± 14.49 | 28.34 ± 10.57 | 0.056 |
| TFC post-PCI | 24.37 ± 9.21 | 26.89 ± 13.16 | 0.184 |
| Significance of ΔTFC | p < 0.001 | p = 0.324 |
| STEMI (n = 71) | NSTEMI (n = 73) | p | |
|---|---|---|---|
| 3D obstruction length (mm) | 18.90 ± 13.64 | 20.54 ± 18.79 | 0.551 |
| Minimal lumen diameter (mm) | 0.92 ± 0.32 | 0.95 ± 0.29 | 0.561 |
| Reference diameter at MLD (mm) | 2.17 ± 0.47 | 2.20 ± 0.52 | 0.726 |
| Percent diameter obstruction at MLD (%) | 56,99 ± 13.05 | 55.87 ± 12.65 | 0.602 |
| Percent area obstruction at MLD (%) | 68.83 ± 14.38 | 64.47 ± 12.65 | 0.106 |
| Minimal lumen area (mm2) | 1.05 ± 0.55 | 1.20 ± 062 | 0.126 |
| Reference area at MLA (mm2) | 3.81 ± 1.72 | 4.02 ± 1.20 | 0.484 |
| Area obstruction at MLA (%) | 70.52 ± 13.3 | 67.50 ± 14.94 | 0.201 |
| Obstruction segment mean diameter (mm) | 1.79 ± 0.35 | 1.89 ± 0.42 | 0.127 |
| Obstruction segment volume (mm3) | 58.29 ± 61.27 | 77.38 ± 117.05 | 0.224 |
| Obstruction segment plaque volume (mm3) | 23.90 ± 27.08 | 24.78 ± 33.84 | 0.863 |
| Obstruction segment reference volume (mm3) | 79.32 ± 81.93 | 97.72 ± 141.50 | 0.343 |
| Obstruction segment mean reference diameter (mm) | 2.17 ± 0.45 | 2.21 ± 0.52 | 0.624 |
| Arch-chord ratio | 1.07 ± 0.09 | 1.10 ± 0.19 | 0.180 |
| Lesion eccentricity index | 0.27 ± 0.15 | 0.23 ± 0.12 | 0.106 |
| Δ ≥ 3 (n = 38) | Δ < 3 (n = 106) | p | |
|---|---|---|---|
| 3D obstruction length (mm) | 18.98 ± 19.45 | 20.00 ± 15.28 | 0.744 |
| Minimal lumen diameter (MLD) (mm) | 1.06 ± 0.311 | 0.89 ± 0.29 | 0.002 |
| Reference diameter at MLD (mm) | 2.21 ± 0.510 | 2.17 ± 0.49 | 0.031 |
| Percent diameter obstruction at MLD (%) | 51.23 ± 13.04 | 58.8 ± 12.27 | 0.003 |
| Percent area obstruction at MLD (%) | 59.76 ± 19.05 | 69.08 ± 14.29 | 0.002 |
| Minimal lumen area (mm2) | 1.36 ± 0.66 | 1.04 ± 0.54 | 0.003 |
| Reference area at MLA (mm2) | 4.05 ± 1.86 | 3.87 ± 1,87 | 0.617 |
| Area obstruction at MLA (%) | 63.42 ± 15.70 | 70.99 ± 12.99 | 0.004 |
| Obstruction segment mean diameter (mm) | 1.91 ± 0.40 | 1.82 ± 0.39 | 0.220 |
| Obstruction segment volume (mm3) | 73.89 ± 131.56 | 65.84 ± 76.85 | 0.652 |
| Obstruction segment plaque volume (mm3) | 21.01 ± 27.33 | 25.54 ± 31.72 | 0.435 |
| Obstruction segment reference volume (mm3) | 90.88 ± 150.34 | 87.84 ± 101.75 | 0.890 |
| Obstruction segment mean reference diameter (mm) | 2.20 ± 0.47 | 2.19 ± 0.49 | 0.868 |
| Arch-chord ratio | 1.10 ± 0.17 | 1.08 ± 0.14 | 0.553 |
| Lesion eccentricity index | 0.23 ± 0.13 | 0.26 ± 0.14 | 0.331 |
| Δ ≥ 3 (n = 12) | Δ < 3 (n = 59) | p | |
|---|---|---|---|
| 3D obstruction length (mm) | 14.18 ± 6.69 | 19.86 ± 14.51 | 0.421 |
| Minimal lumen diameter (mm) | 1.01 ± 0.29 | 0.90 ± 0.32 | 0.262 |
| Reference diameter at MLD (mm) | 2.16 ± 0.43 | 2.17 ± 0.48 | 0.812 |
| Percent diameter obstruction at MLD (%) | 52.67 ± 10.80 | 57.86 ± 13.38 | 0.220 |
| Percent area obstruction at MLD (%) | 68.93 ± 12.22 | 68.81 ± 14.87 | 0.794 |
| Minimal lumen area (mm2) | 1.09 ± 0.54 | 1.04 ± 0.56 | 0.667 |
| Reference area at MLA (mm2) | 3.80 ± 1.44 | 3.81 ± 1.78 | 0.724 |
| Area obstruction at MLA (%) | 69.39 ± 12.22 | 70.74 ± 13.39 | 0.629 |
| Obstruction segment mean diameter (mm) | 1.78 ± 0.27 | 1.79 ± 0.37 | 0.896 |
| Obstruction segment volume (mm3) | 37.87 ± 19.25 | 62.45 ± 66.01 | 0.560 |
| Obstruction segment plaque volume (mm3) | 18.11 ± 13.79 | 25.07 ± 29.00 | 0.471 |
| Obstruction segment reference volume (mm3) | 54.22 ± 30.65 | 84.42 ± 88.13 | 0.443 |
| Obstruction segment mean reference diameter (mm) | 2.15 ± 0.36 | 2.18 ± 0.47 | 0.939 |
| Arch-chord ratio | 1.05 ± 0.05 | 1.07 ± 0.10 | 0.454 |
| Lesion eccentricity index | 0.31 ± 0.14 | 0.26 ± 0.16 | 0.200 |
| Δ ≥ 3 (n = 26) | Δ < 3 (n = 47) | p | |
|---|---|---|---|
| 3D obstruction length (mm) | 21.19 ± 22.89 | 20.17 ± 10.35 | 0.826 |
| Minimal lumen diameter (MLD) (mm) | 1.07 ± 0.32 | 0.87 ± 0.24 | 0.007 |
| Reference diameter at MLD (mm) | 2.24 ± 0.55 | 2.17 ± 0.50 | 0.600 |
| Percent diameter obstruction at MLD (%) | 50.57 ± 14.11 | 58.80 ± 10.85 | 0.007 |
| Percent area obstruction at MLD (%) | 55.52 ± 20.31 | 69.42 ± 13.68 | 0.001 |
| Minimal lumen area (mm2) | 1.49 ± 0.68 | 1.04 ± 0.52 | 0.002 |
| Reference area at MLA (mm2) | 4.16 ± 2.03 | 3.95 ± 2.00 | 0.663 |
| Area obstruction at MLA (%) | 60.66 ± 16.55 | 71.29 ± 12.61 | 0.003 |
| Obstruction segment mean diameter (mm) | 1.97 ± 0.44 | 1.85 ± 0.41 | 0.250 |
| Obstruction segment volume (mm3) | 90.51 ± 156.66 | 70.11 ± 89.19 | 0.480 |
| Obstruction segment plaque volume (mm3) | 22.35 ± 31.87 | 26.13 ± 35.14 | 0.651 |
| Obstruction segment reference volume (mm3) | 107.80 ± 179.15 | 92.14 ± 117.50 | 0.654 |
| Obstruction segment mean reference diameter (mm) | 2.23 ± 0.51 | 2.20 ± 0.53 | 0.851 |
| Arch-chord ratio | 1.12 ± 0.20 | 1.09 ± 0.18 | 0.508 |
| Lesion eccentricity index | 0.20 ± 0.10 | 0.25 ± 0.13 | 0.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racz, A.O.; Racz, I.; Szabo, G.T.; Uveges, A.; Koszegi, Z.; Penczu, B.; Kolozsvari, R. The Effects of Percutaneous Coronary Intervention on the Flow in Acute Coronary Syndrome Patients—Geometry in Focus. J. Pers. Med. 2022, 12, 1264. https://doi.org/10.3390/jpm12081264
Racz AO, Racz I, Szabo GT, Uveges A, Koszegi Z, Penczu B, Kolozsvari R. The Effects of Percutaneous Coronary Intervention on the Flow in Acute Coronary Syndrome Patients—Geometry in Focus. Journal of Personalized Medicine. 2022; 12(8):1264. https://doi.org/10.3390/jpm12081264
Chicago/Turabian StyleRacz, Agnes Orsolya, Ildiko Racz, Gabor Tamas Szabo, Aron Uveges, Zsolt Koszegi, Bence Penczu, and Rudolf Kolozsvari. 2022. "The Effects of Percutaneous Coronary Intervention on the Flow in Acute Coronary Syndrome Patients—Geometry in Focus" Journal of Personalized Medicine 12, no. 8: 1264. https://doi.org/10.3390/jpm12081264
APA StyleRacz, A. O., Racz, I., Szabo, G. T., Uveges, A., Koszegi, Z., Penczu, B., & Kolozsvari, R. (2022). The Effects of Percutaneous Coronary Intervention on the Flow in Acute Coronary Syndrome Patients—Geometry in Focus. Journal of Personalized Medicine, 12(8), 1264. https://doi.org/10.3390/jpm12081264






