Risk Factors and Protective Factors against Ventilator-Associated Pneumonia—A Single-Center Mixed Prospective and Retrospective Cohort Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Setting
2.2. Participants
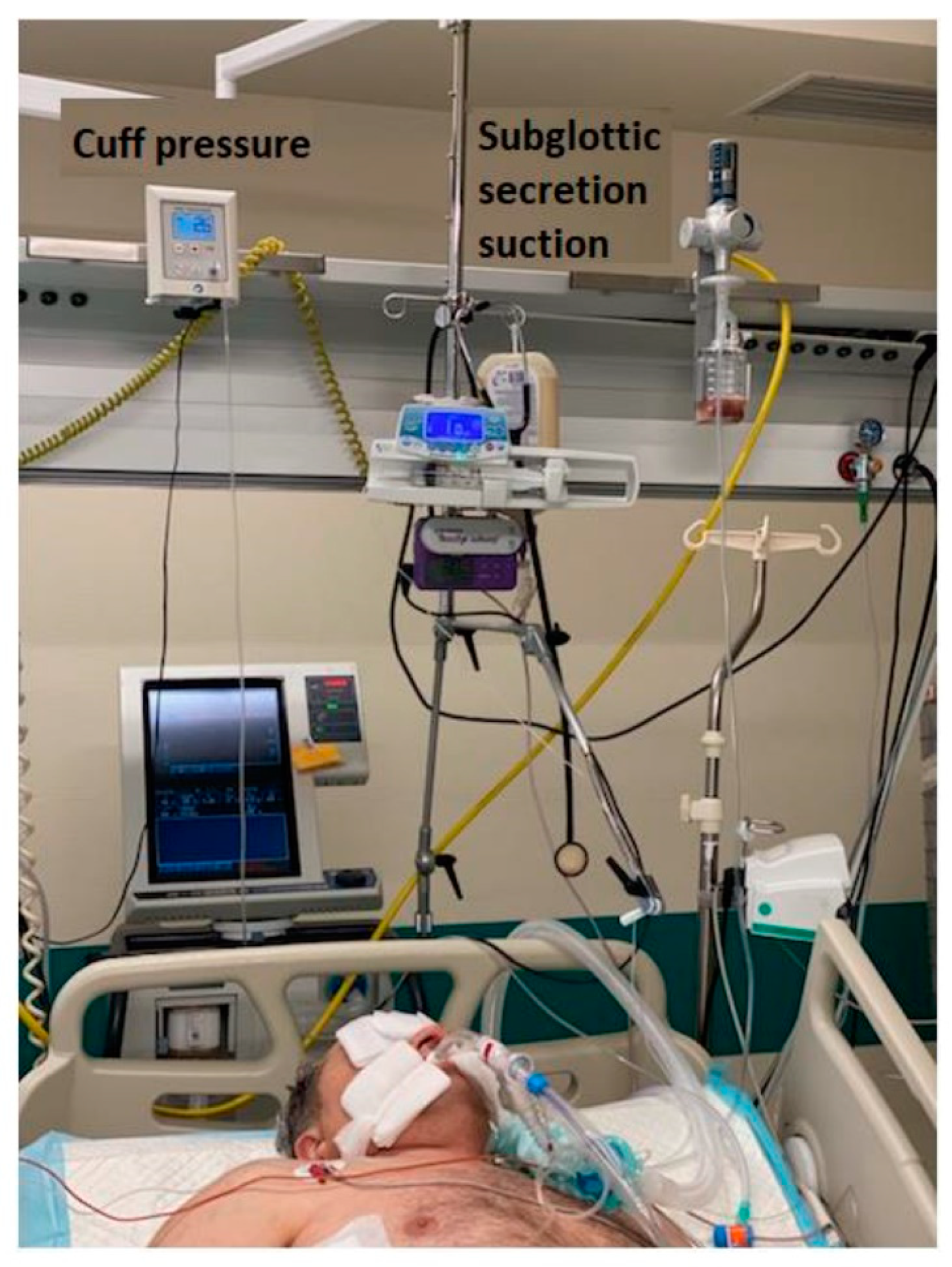
2.3. VAP Prevention Bundle
2.4. Variables
- Body temperature (≥38.5 and <38.9 °C = 1 point; ≥39 and <36 °C = 2 points),
- Procalcitonin (≥0.5 and <1 ng/mL = 1 point; ≥1 ng/mL = 2 points),
- Purulent tracheal secretions = 2 points,
- Positive endotracheal aspirate (>104 colony–forming units/mL) = 2 points,
- Positive infiltrates on chest echograph (sub-pleural echo-poor region or more with tissue-like echo texture) = 2 points,
- Oxygenation PaO2/FiO2 ≤ 240 and absence of acute respiratory distress syndrome = 2 points.
2.5. Outcomes
2.6. Statistics
3. Results
3.1. Demographic and Clinical Information of Patients Admitted to the ICU
3.2. VAP Incidence and VAP Patient Characteristics
3.3. Pathogens in VAP Patients
3.4. Factors Associated with VAP
3.5. Length of Ventilator Use and ICU Length of Stay
3.6. Mortality
4. Discussion
5. Limitations and Strengths of the Study
6. Practical Implications of the Study
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pozuelo-Carrascosa, D.P.; Herráiz-Adillo, Á.; Alvarez-Bueno, C.; Añón, J.M.; Martínez-Vizcaíno, V.; Cavero-Redondo, I. Subglottic secretion drainage for preventing ventilator-associated pneumonia: An overview of systematic reviews and an updated meta-analysis. Eur. Respir. Rev. 2020, 29, 190107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhdari, A.; Shokouhi, S.; Bashar, F.R.; Azimi, A.V.; Shojaei, S.P.; Fathi, M.; Goharani, R.; Sahraei, Z.; Hajiesmaeili, M. Is a Low Incidence Rate of Ventilation Associated Pneumonia Associated with Lower Mortality? A Descriptive Longitudinal Study in Iran. Tanaffos 2018, 17, 110–116. [Google Scholar] [PubMed]
- Sadigov, A.; Mamedova, I.; Mammmadov, K. Ventilator-Associated Pneumonia and In-Hospital Mortality: Which Risk Factors may predict In-Hospital Mortality in Such Patients? J. Lung Health Dis. 2019, 3, 8–12. [Google Scholar] [CrossRef]
- Luckraz, H.; Manga, N.; Senanayake, E.L.; Abdelaziz, M.; Gopal, S.; Charman, S.C.; Giri, R.; Oppong, R.; Andronis, L. Cost of treating ventilator-associated pneumonia post cardiac surgery in the National Health Service: Results from a propensity-matched cohort study. J. Intensive Care Soc. 2018, 19, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathai, A.S.; Phillips, A.; Isaac, R. Ventilator-associated pneumonia: A persistent healthcare problem in Indian Intensive Care Units! Lung India 2016, 33, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Duszynska, W.; Rosenthal, V.D.; Szczesny, A.; Zajaczkowska, K.; Fulek, M.; Tomaszewski, J. Device associated –health care associated infections monitoring, prevention and cost assessment at intensive care unit of University Hospital in Poland (2015–2017). BMC Infect. Dis. 2020, 20, 761. [Google Scholar] [CrossRef]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Huang, Y.; Kang, Y.; Xu, Y.; Ma, X.; Wang, X.; Liu, J.; Wu, D.; Tang, Y.; et al. The Current Epidemiological Landscape of Ventilator-associated Pneumonia in the Intensive Care Unit: A Multicenter Prospective Observational Study in China. Clin. Infect. Dis. 2018, 67, S153–S161. [Google Scholar] [CrossRef]
- Wałaszek, M.; Różańska, A.; Wałaszek, M.Z.; Wójkowska-Mach, J.; The Polish Society of Hospital Infections Team. Epidemiology of Ventilator-Associated Pneumonia, microbiological diagnostics and the length of antimicrobial treatment in the Polish Intensive Care Units in the years 2013–2015. BMC Infect. Dis. 2018, 18, 308. [Google Scholar] [CrossRef] [Green Version]
- Sfeir, M.M. Diagnosis of Multidrug-Resistant Pathogens of Pneumonia. Diagnostics 2021, 11, 2287. [Google Scholar] [CrossRef]
- Wu, D.; Wu, C.; Zhang, S.; Zhong, Y. Risk Factors of Ventilator-Associated Pneumonia in Critically III Patients. Front. Pharmacol. 2019, 10, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Lai, C.; Xu, G.; Meng, W.; Zhang, J.; Hou, H.; Pi, H. Risk factors of ventilator-associated pneumonia in elderly patients receiving mechanical ventilation. Clin. Interv. Aging 2019, 14, 1027–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Sul, Y.H.; Kim, S.H.; Ye, J.B.; Lee, J.S.; Choi, H.; Yoon, S.Y.; Choi, J.H. Risk factors for ventilator-associated pneumonia in trauma patients with torso injury: A retrospective single-center study. J. Int. Med. Res. 2021, 49, 3000605211061029. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Mathur, P.; Misra, M.C.; Kumari, M.; Katoch, O.; Hasan, F. Ventilator Associated Pneumonia in Adult Patients Preventive Measures: A Review of the Recent Advances. J. Infect. 2018, 1, 8–12. [Google Scholar] [CrossRef]
- Lerma, F.; García, M.S.; Lorente, L.; Gordo, F.; Añón, J.; Álvarez, J.; Palomar, M.; García, R.; Arias, S.; Vázquez-Calatayud, M.; et al. Guidelines for the prevention of ventilator-associated pneumonia and their implementation. The Spanish “Zero-VAP” bundle. Med. Intensiv. 2014, 38, 226–236. [Google Scholar] [CrossRef]
- Van Der Kooi, T.I.I.; Boshuizen, H.; Wille, J.C.; De Greeff, S.C.; Van Dissel, J.T.; Schoffelen, A.F.; Van Gaalen, R.D. Using flexible methods to determine risk factors for ventilator-associated pneumonia in the Netherlands. PLoS ONE 2019, 14, e0218372. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Lerma, F.; Palomar-Martínez, M.; Sánchez-García, M.; Martínez-Alonso, M.; Rodríguez, J.; Lorente, L.; Arias-Rivera, S.; García, R.; Gordo, F.; Añón, J.M.; et al. Prevention of Ventilator-Associated Pneumonia. Crit. Care Med. 2018, 46, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yang, Y.; Jiao, Y.; Zhang, K.; Hai, Y.; Li, H.; Xing, H.; Xu, B.; Bai, H.; Zhao, Y.; et al. Evaluation of the effects of applying the ventricular care bundle (VCB) method for reducing ventilator-associated pneumonia (VAP) in the intensive care unit of a general Chinese tertiary hospital. Ann. Palliat. Med. 2020, 9, 2853–2861. [Google Scholar] [CrossRef]
- Jadot, L.; Huyghens, L.; De Jaeger, A.; Bourgeois, M.; Biarent, D.; Higuet, A.; De Decker, K.; Laenen, M.V.; Oosterlynck, B.; Ferdinande, P.; et al. Impact of a VAP bundle in Belgian intensive care units. Ann. Intensiv. Care 2018, 8, 65. [Google Scholar] [CrossRef]
- Colombo, S.M.; Palomeque, A.C.; Bassi, G.L. The zero-VAP sophistry and controversies surrounding prevention of ventilator-associated pneumonia. Intensiv. Care Med. 2020, 46, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [Green Version]
- Ghattas, C.; Alsunaid, S.; Pickering, E.M.; Holden, V.K. State of the art: Percutaneous tracheostomy in the intensive care unit. J. Thorac. Dis. 2021, 13, 5261. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Kołpa, M.; Wałaszek, M.; Gniadek, A.; Wolak, Z.; Dobroś, W. Incidence, Microbiological Profile and Risk Factors of Healthcare-Associated Infections in Intensive Care Units: A 10 Year Observation in a Provincial Hospital in Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, C.; Zhang, Y.; Yang, Z.; Wang, J.; Jin, A.; Wang, W.; Chen, R.; Zhan, S. Incidence, temporal trend and factors associated with ventilator-associated pneumonia in mainland China: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 468. [Google Scholar] [CrossRef] [Green Version]
- Altinsoy, S.; Catalca, S.; Sayin, M.M.; Tutuncu, E.E. The risk factors of Ventilator Associated Pneumonia and relationship with type of tracheostomy. Trends Anaesth. Crit. Care 2020, 35, 38–43. [Google Scholar] [CrossRef]
- Dochi, H.; Nojima, M.; Matsumura, M.; Cammack, I.; Furuta, Y. Effect of early tracheostomy in mechanically ventilated patients. Laryngoscope Investig. Otolaryngol. 2019, 4, 292–299. [Google Scholar] [CrossRef]
- Szakmany, T.; Russell, P.; Wilkes, A.R.; Hall, J.E. Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: Meta-analysis of randomized controlled trials. Br. J. Anaesth. 2015, 114, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Adly, A.; Youssef, T.A.; El-Begermy, M.M.; Younis, H.M. Timing of tracheostomy in patients with prolonged endotracheal intubation: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 679–690. [Google Scholar] [CrossRef]
- Chang, Y.; Jeon, K.; Lee, S.-M.; Cho, Y.-J.; Kim, Y.S.; Chong, Y.P.; Hong, S.-B. The Distribution of Multidrug-resistant Microorganisms and Treatment Status of Hospital-acquired Pneumonia/Ventilator-associated Pneumonia in Adult Intensive Care Units: A Prospective Cohort Observational Study. J. Korean Med. Sci. 2021, 36, e251. [Google Scholar] [CrossRef]
- Mahapatra, A.; Patro, S.; Sarangi, G.; Das, P.; Mohapatra, D.; Paty, B.; Chayani, N. Bacteriological profile of ventilator-associated pneumonia in a tertiary care hospital. Indian J. Pathol. Microbiol. 2018, 61, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Arayasukawat, P.; So-Ngern, A.; Reechaipichitkul, W.; Chumpangern, W.; Arunsurat, I.; Ratanawatkul, P.; Chuennok, W. Microorganisms and clinical outcomes of early- and late-onset ventilator-associated pneumonia at Srinagarind Hospital, a tertiary center in Northeastern Thailand. BMC Pulm. Med. 2021, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.-Y.; Zhou, Y.-Q.; Zhou, M.; Zou, X.-L.; Wang, Y.-H.; Zhang, T.-T. Risk Factors for Mortality Due to Ventilator-Associated Pneumonia in a Chinese Hospital: A Retrospective Study. Med. Sci. Monit. 2019, 25, 7660–7665. [Google Scholar] [CrossRef] [PubMed]
- Dogruel, B.; Dizi, S.; Tosun, M.; Çakar, N.; Edipoglu, I.S. The association between the APACHE-II scores and age groups for predicting mortality in an intensive care unit: A retrospective study. Rom. J. Anaesth. Intensiv. Care 2019, 26, 53–58. [Google Scholar] [CrossRef]
- Karakuzu, Z.; Işçimen, R.; Akalin, H.; Girgin, N.K.; Kahveci, F.; Sinirtas, M. Prognostic Risk Factors in Ventilator-Associated Pneumonia. Med. Sci. Monit. 2018, 24, 1321–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vest, M.T.; Kolm, P.; Bowen, J.; Trabulsi, J.; Lennon, S.L.; Shapero, M.; McGraw, P.; Halbert, J.; Jurkovitz, C. Association Between Enteral Feeding, Weight Status, and Mortality in a Medical Intensive Care Unit. Am. J. Crit. Care 2018, 27, 136–143. [Google Scholar] [CrossRef]
- Weigl, W.; Adamski, J.; Goryński, P.; Kański, A.; Hultström, M. ICU mortality and variables associated with ICU survival in Poland: A nationwide database study. Eur. J. Anaesthesiol. 2018, 35, 949–954. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Xiao, W.; Song, T.; Wang, S. Incidence, Risk Factors, and Outcomes of Ventilator-Associated Pneumonia in Traumatic Brain Injury: A Meta-analysis. Neurocrit. Care 2020, 32, 272–285. [Google Scholar] [CrossRef]
- Gupta, R.; Malik, A.; Rizvi, M.; Ahmed, M.; Singh, A. Epidemiology of multidrug-resistant Gram-negative pathogens isolated from ventilator-associated pneumonia in ICU patients. J. Glob. Antimicrob. Resist. 2017, 9, 47–50. [Google Scholar] [CrossRef]
- Hosamirudsari, H.; Forghanib, S.; Akbarpourc, S. Multi-drug resistant ventilator associated pneumonia: Risk factors and outcomes. Can. J. Infect. Control 2018, 33, 20–24. [Google Scholar]
- Ben Lakhal, H.; M’Rad, A.; Naas, T.; Brahmi, N. Antimicrobial Susceptibility among Pathogens Isolated in Early- versus Late-Onset Ventilator-Associated Pneumonia. Infect. Dis. Rep. 2021, 13, 401–410. [Google Scholar] [CrossRef]
- Robba, C.; Rebora, P.; Banzato, E.; Wiegers, E.J.A.; Stocchetti, N.; Menon, D.K.; Citerio, G. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury Participants and Investigators. Chest 2020, 158, 2292–2303. [Google Scholar] [CrossRef] [PubMed]
- Dougall, L.; Booth, M.; Khoo, E.; Hood, H.; MacGregor, S.; Anderson, J.; Timoshkin, I.; Maclean, M. Continuous monitoring of aerial bioburden within intensive care isolation rooms and identification of high-risk activities. J. Hosp. Infect. 2019, 103, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, O.; Ersoy, Y.; Kuzucu, C.; Gedik, E.; Togal, T.; Yetkin, F. Assessment of the effectiveness of a ventilator associated pneumonia prevention bundle that contains endotracheal tube with subglottic drainage and cuff pressure monitorization. Braz. J. Infect. Dis. 2017, 21, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Wei, L.; Chen, J.; Xie, A.; Li, M.; Bian, L. Is continuous better than intermittent control of tracheal cuff pressure? A meta-analysis. Nurs. Crit. Care 2019, 24, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Ding, J.; Shen, M.; Zhang, H.; Wang, Z. Continuous Versus Intermittent Subglottic Secretion Drainage to Prevent Ventilator-Associated Pneumonia: A Systematic Review. Crit. Care Nurse 2017, 37, e10–e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maertens, B.; Blot, K.; Blot, S. Prevention of Ventilator-Associated and Early Postoperative Pneumonia Through Tapered Endotracheal Tube Cuffs: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2018, 46, 316–323. [Google Scholar] [CrossRef]
- Saito, M.; Maruyama, K.; Mihara, T.; Hoshijima, H.; Hirabayashi, G.; Andoh, T. Comparison of polyurethane tracheal tube cuffs and conventional polyvinyl chloride tube cuff for prevention of ventilator-associated pneumonia: A systematic review with meta-analysis. Medicine 2021, 100, e24906. [Google Scholar] [CrossRef]
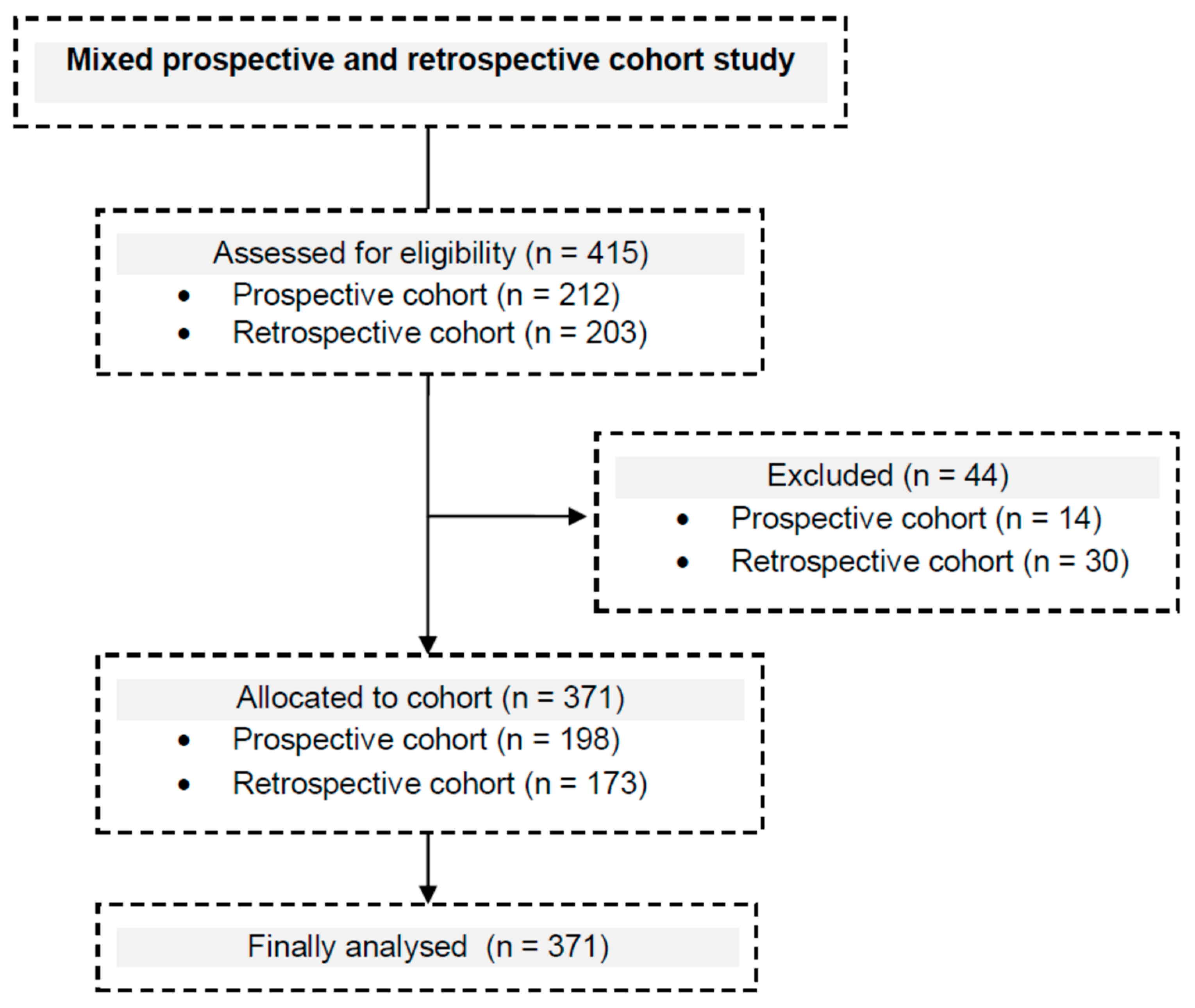
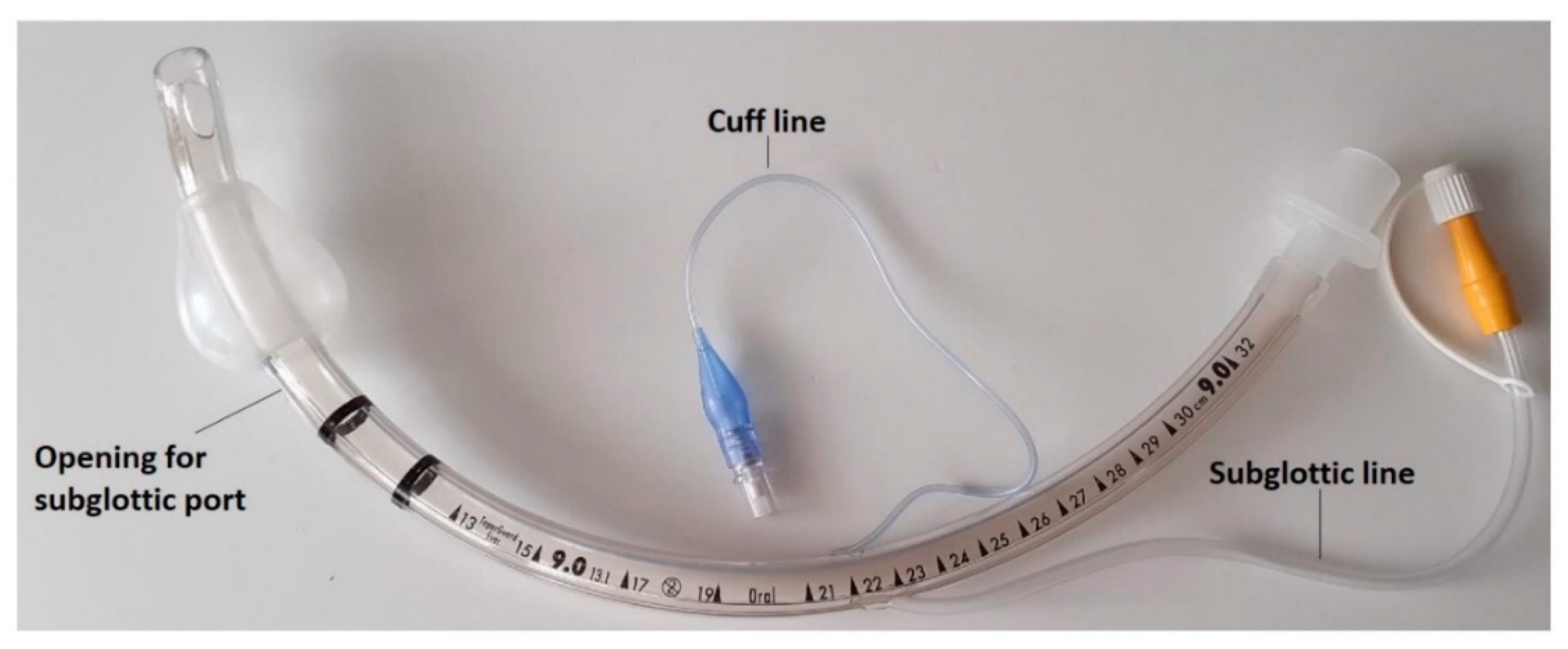
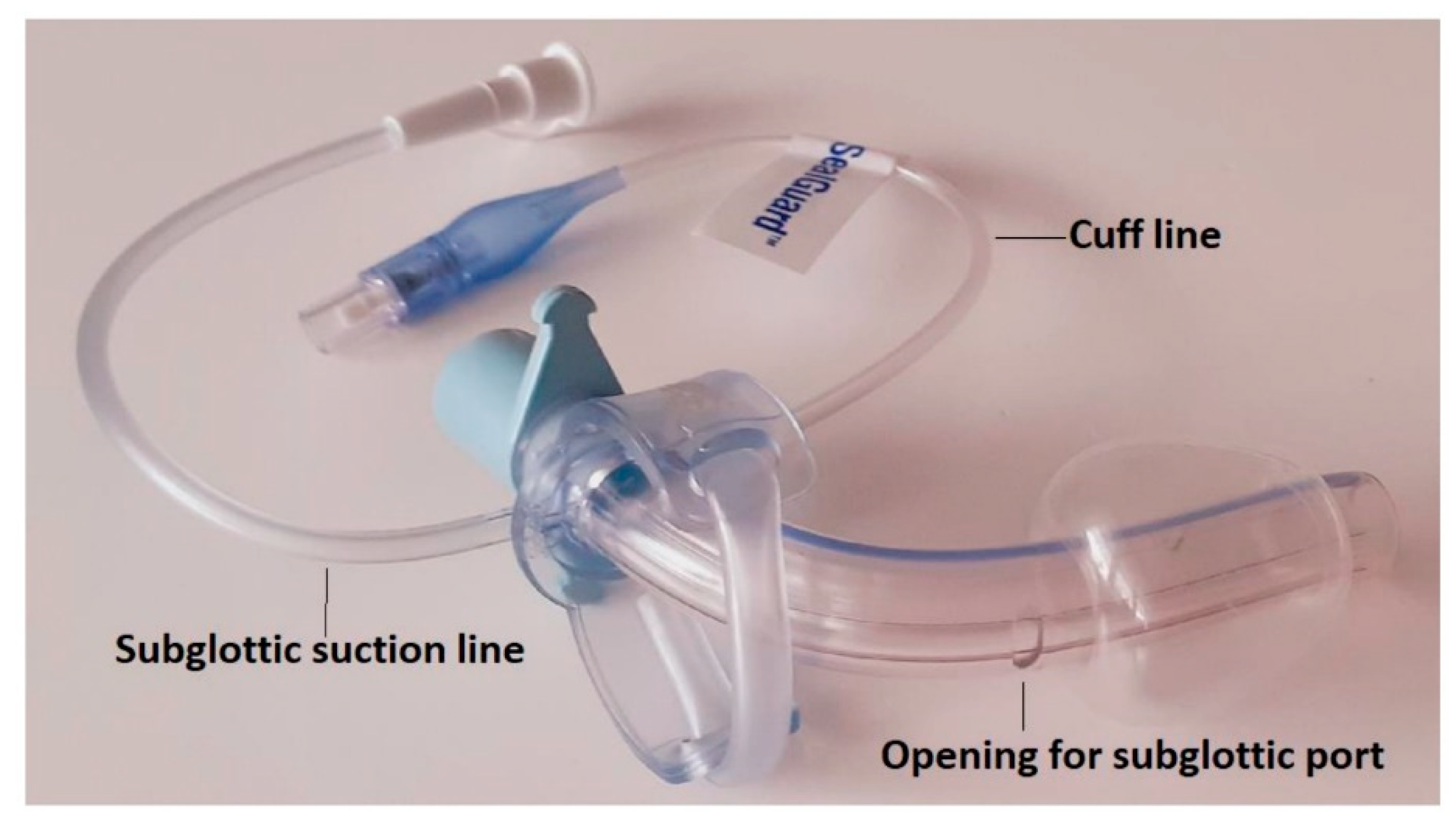
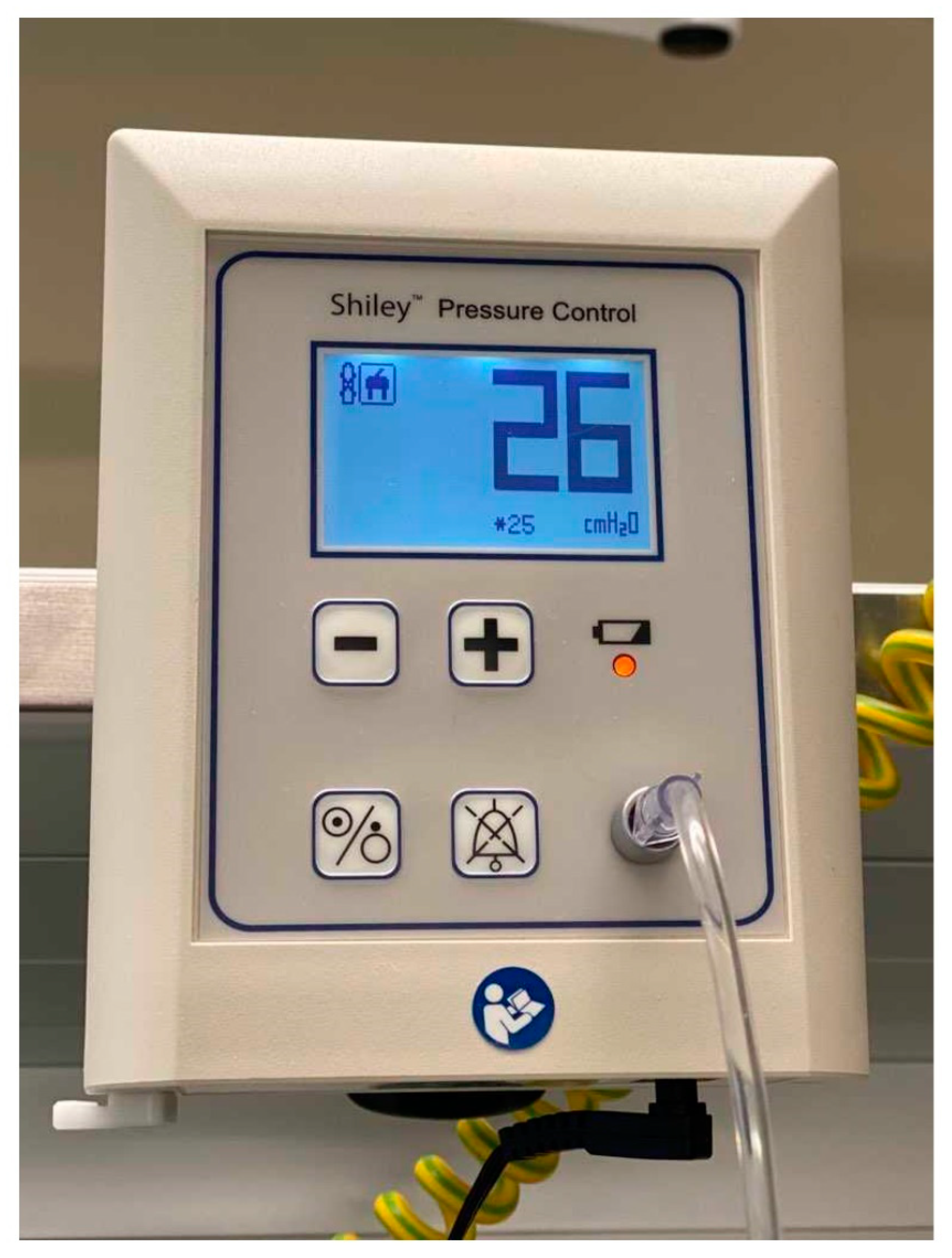

| The Prospective and Retrospective Cohort | |
| |
| The Prospective Cohort | The Retrospective Cohort |
|
|
| Demographic and Clinical Factors | n = 371 |
|---|---|
| Age (years) | 66 [54; 75] |
| Body mass index (kg/m2) | 26 [23; 29] |
| APACHE II scores admission | 23 [19; 28] |
| Estimated risk of death admission (%) | 46 [32.2; 67.2] |
| Antibiotics prior to VAP | 72 (19.4) |
| Sex | |
| Female | 123 (33.1) |
| Male | 248 (66.8) |
| Admission category | |
| Neurosurgical | 180 (48.5) |
| Cardiovascular | 77 (20.7) |
| General surgical | 63 (17.0) |
| Non-cardiac internal medicine | 51 (13.7) |
| Comorbidities | |
| Chronic heart failure | 73 (19.7) |
| Chronic renal failure | 35 (9.4) |
| Chronic liver failure | 9 (2.4) |
| Chronic obstructive pulmonary disease | 40 (10.8) |
| Gastric and duodenal ulcer | 16 (4.3) |
| Diabetes mellitus | 89 (24.0) |
| Immunosuppression | 17 (4.5) |
| Smoking status | 82 (22.1) |
| ICU stay | |
| Continuous control of cuff pressure and subglottic secretion suction used together | 198 (53.4) |
| MDR pathogen in the culture of the lower respiratory secretions | 42 (11.3) |
| Septic shock | 70 (18.9) |
| Tracheotomy | 113 (30.4) |
| Enteral nutrition | 322 (86.8) |
| Length of ventilator use (day) | 8 [3; 17] |
| ICU length of stay (day) | 10 [4; 22] |
| ICU mortality | 166 (44.7) |
| Pathogens | VAP (n = 52) | Non-VAP (n = 319) | p Value |
|---|---|---|---|
| Multidrug-resistant bacteria | 21 (40.4) | 21 (6.6) | <0.001 |
| Gram-positive bacteria | |||
| Methicyllin-resistant Staphylococcus aureus | 2 (3.8) | 4 (1.2) | 0.17 |
| Methicillin-sensitive Staphylococcus aureus | 4 (7.7) | 18 (5.6) | 0.53 |
| Staphylococcus epidermidis | 4 (7.7) | 18 (5.6) | 0.53 |
| Vancomycin-resistant enterococcus | 1 (1.9) | 1 (0.3) | 0.26 |
| Vancomycin-sensitive enterococcus | 1 (1.9) | - | - |
| Gram-negative bacteria | |||
| Acinetobacter baumanii | 8 (15.4) | 10 (3.3) | <0.001 |
| Pseumonas aeruginosa | 8 (15.4) | 1 (0.3) | <0.001 |
| Klebsiella pneumoniae (Enterobacteriaceae) | 15 (28.8) | 26 (8.1) | <0.001 |
| Other Enterobacteriaceae species | 15 (28.8) | 37 (11.6) | <0.001 |
| Haemophilus influenzae | 2 (3.8) | 14 (4.4) | 0.61 |
| Stenotrophomonas maltofila | 3 (5.8) | 15 (4.7) | 0.73 |
| Candida albicans | 1 (1.9) | 3 (0.9) | 0.45 |
| Factors | B | SE | Wald | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Simple logistic regression | ||||||
| Admission category—neurosurgical | 0.31 | 0.15 | 4.02 | 1.36 | 1.01–1.84 | 0.04 |
| Urinary tract infection | 0.51 | 0.17 | 8.40 | 1.66 | 1.18–2.33 | 0.004 |
| Enteral nutrition | 0.73 | 0.37 | 3.93 | 2.08 | 1.01–4.28 | 0.048 |
| Tracheotomy | 0.98 | 0.16 | 35.63 | 2.66 | 1.93–3.67 | <0.001 |
| Multidrug-resistant bacteria 1 | 1.13 | 0.18 | 39.14 | 3.10 | 2.17–4.42 | <0.001 |
| ICU length of stay >5 days | 1.47 | 0.36 | 16.31 | 4.37 | 2.13–8.93 | <0.001 |
| Body mass index | −0.08 | 0.03 | 6.27 | 0.92 | 0.86–0.98 | 0.01 |
| Continuous control of cuff pressure and subglottic secretion suction used together | −0.40 | 0.15 | 6.65 | 0.67 | 0.50–0.91 | 0.01 |
| Multivariable logistic regression model 2 | ||||||
| Tracheotomy | 0.47 | 0.19 | 6.23 | 1.60 | 1.10–2.31 | 0.01 |
| Multidrug-resistant bacteria 1 | 1.0 | 0.20 | 24.14 | 2.73 | 1.83–4.07 | <0.001 |
| ICU length of stay >5 days | 1.2 | 0.39 | 9.27 | 3.32 | 1.53–7.19 | 0.002 |
| Continuous control of cuff pressure and subglottic secretion suction used together | −0.50 | 0.18 | 7.55 | 0.61 | 0.43–0.87 | 0.006 |
| Factors | B | SE | Wald | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Simple logistic regression | ||||||
| Age | 0.04 | 0.01 | 26.37 | 1.04 | 1.02–1.06 | <0.0001 |
| APACHE II admission | 0.16 | 0.02 | 63.03 | 1.18 | 1.13–1.23 | <0.001 |
| Smoking status | 0.29 | 0.13 | 5.41 | 1.34 | 1.05–1.72 | 0.02 |
| Enteral nutrition | −0.59 | 0.16 | 12.84 | 0.55 | 0.40–0.76 | 0.0003 |
| Multivariable logistic regression model 1 | ||||||
| Age | 0.02 | 0.01 | 4.91 | 1.02 | 1.00–1.04 | 0.03 |
| APACHE II admission | 0.14 | 0.02 | 43.6 | 1.16 | 1.12–1.23 | <0.001 |
| Enteral nutrition | −0.46 | 0.20 | 5.43 | 0.63 | 0.43–0.93 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, J.; Tomaszek, L.; Mazurek, H.; Mędrzycka-Dąbrowska, W. Risk Factors and Protective Factors against Ventilator-Associated Pneumonia—A Single-Center Mixed Prospective and Retrospective Cohort Study. J. Pers. Med. 2022, 12, 597. https://doi.org/10.3390/jpm12040597
Pawlik J, Tomaszek L, Mazurek H, Mędrzycka-Dąbrowska W. Risk Factors and Protective Factors against Ventilator-Associated Pneumonia—A Single-Center Mixed Prospective and Retrospective Cohort Study. Journal of Personalized Medicine. 2022; 12(4):597. https://doi.org/10.3390/jpm12040597
Chicago/Turabian StylePawlik, Jarosław, Lucyna Tomaszek, Henryk Mazurek, and Wioletta Mędrzycka-Dąbrowska. 2022. "Risk Factors and Protective Factors against Ventilator-Associated Pneumonia—A Single-Center Mixed Prospective and Retrospective Cohort Study" Journal of Personalized Medicine 12, no. 4: 597. https://doi.org/10.3390/jpm12040597
APA StylePawlik, J., Tomaszek, L., Mazurek, H., & Mędrzycka-Dąbrowska, W. (2022). Risk Factors and Protective Factors against Ventilator-Associated Pneumonia—A Single-Center Mixed Prospective and Retrospective Cohort Study. Journal of Personalized Medicine, 12(4), 597. https://doi.org/10.3390/jpm12040597









