Incidence and Survival Outcomes of Colorectal Cancer in Long-Term Metformin Users with Diabetes: A Population-Based Cohort Study Using a Common Data Model
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Database
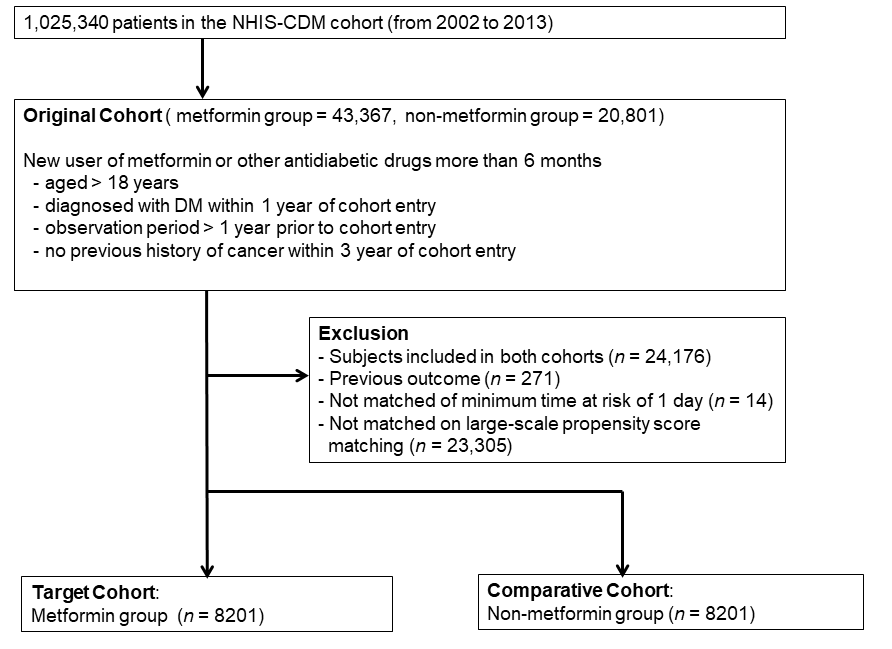
2.2. Cohort Definition
3. Outcomes
4. Main Statistical Analysis
Sensitivity Analyses
5. Results
5.1. Patients Characteristics
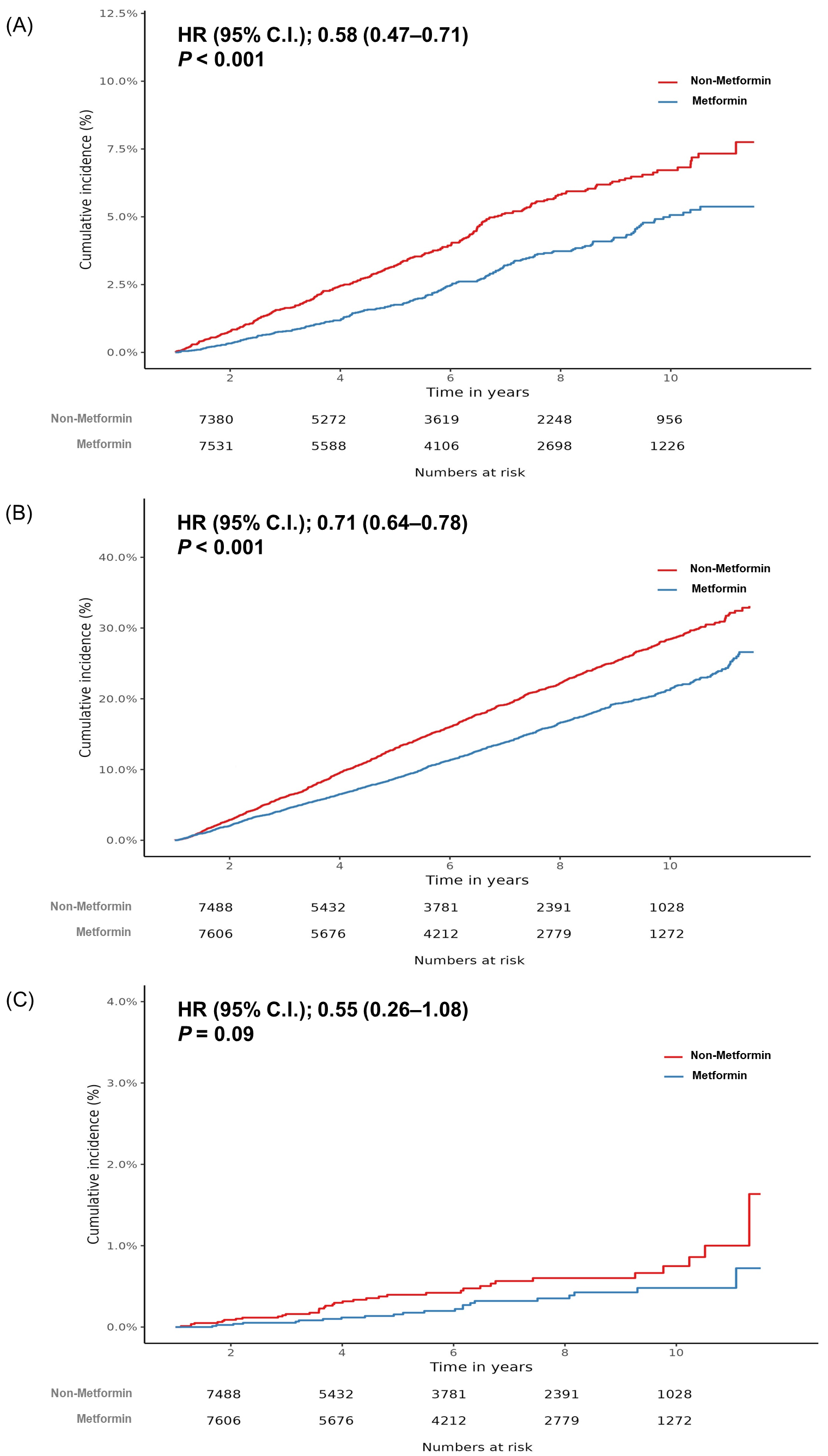
5.2. Incidence of CRC between Metformin Users and Non-Users
5.3. All-Cause and CRC-Related Mortality between Metformin Users and Non-Users
5.4. Sensitivity Analyses of Metformin Users for Over 6 Months
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Giorgi Rossi, P.; Vicentini, M.; Sacchettini, C.; Di Felice, E.; Caroli, S.; Ferrari, F.; Mangone, L.; Pezzarossi, A.; Roncaglia, F.; Campari, C.; et al. Impact of Screening Program on Incidence of Colorectal Cancer: A Cohort Study in Italy. Am. J. Gastroenterol. 2015, 110, 1359–1366. [Google Scholar] [CrossRef]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Whitlock, E.P.; Burda, B.U.; Williams, S.B.; Guirguis-Blake, J.M.; Evans, C.V. Bleeding Risks with Aspirin Use for Primary Prevention in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, D.M.; Yu, O.; Johnson, J. Statin use and cancer risk: A comprehensive review. Expert Opin. Drug Saf. 2010, 9, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Singh, A.G.; Singh, P.P.; Murad, M.H.; Iyer, P.G. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 620–629. [Google Scholar] [CrossRef] [Green Version]
- Clancy, Z.; Keith, S.W.; Rabinowitz, C.; Ceccarelli, M.; Gagne, J.J.; Maio, V. Statins and colorectal cancer risk: A longitudinal study. Cancer Causes Control 2013, 24, 777–782. [Google Scholar] [CrossRef]
- Flick, E.D.; Habel, L.A.; Chan, K.A.; Haque, R.; Quinn, V.P.; Van Den Eeden, S.K.; Sternfeld, B.; Orav, E.J.; Seeger, J.D.; Quesenberry, C.P., Jr.; et al. Statin use and risk of colorectal cancer in a cohort of middle-aged men in the US: A prospective cohort study. Drugs 2009, 69, 1445–1457. [Google Scholar] [CrossRef]
- Liu, F.; Yan, L.; Wang, Z.; Lu, Y.; Chu, Y.; Li, X.; Liu, Y.; Rui, D.; Nie, S.; Xiang, H. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget 2017, 8, 16017–16026. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Wang, M.; Kang, Y.; Li, B.; Guo, M.; Cheng, Z.; Bi, C. Prognostic role of metformin intake in diabetic patients with colorectal cancer: An updated qualitative evidence of cohort studies. Oncotarget 2017, 8, 26448–26459. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- You, S.C.; Lee, S.; Cho, S.Y.; Park, H.; Jung, S.; Cho, J.; Yoon, D.; Park, R.W. Conversion of National Health Insurance Service-National Sample Cohort (NHIS-NSC) Database into Observational Medical Outcomes Partnership-Common Data Model (OMOP-CDM). Stud. Health Technol. Inform. 2017, 245, 467–470. [Google Scholar] [PubMed]
- Suchard, M.A.; Schuemie, M.J.; Krumholz, H.M.; You, S.C.; Chen, R.; Pratt, N.; Reich, C.G.; Duke, J.; Madigan, D.; Hripcsak, G.; et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: A systematic, multinational, large-scale analysis. Lancet 2019, 394, 1816–1826. [Google Scholar] [CrossRef] [Green Version]
- Vashisht, R.; Jung, K.; Schuler, A.; Banda, J.M.; Park, R.W.; Jin, S.; Li, L.; Dudley, J.T.; Johnson, K.W.; Shervey, M.M.; et al. Association of Hemoglobin A1c Levels with Use of Sulfonylureas, Dipeptidyl Peptidase 4 Inhibitors, and Thiazolidinediones in Patients with Type 2 Diabetes Treated with Metformin: Analysis from the Observational Health Data Sciences and Informatics Initiative. JAMA Netw. Open 2018, 1, e181755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.C.; Jung, S.; Swerdel, J.N.; Ryan, P.B.; Schuemie, M.J.; Suchard, M.A.; Lee, S.; Cho, J.; Hripcsak, G.; Park, R.W.; et al. Comparison of First-Line Dual Combination Treatments in Hypertension: Real-World Evidence from Multinational Heterogeneous Cohorts. Korean Circ. J. 2020, 50, 52–68. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.I.; Park, C.H.; You, S.C.; Kim, J.Y.; Lee, K.J.; Kim, J.; Kim, Y.; Yoo, J.J.; Seo, W.W.; Lee, H.S.; et al. Association between proton pump inhibitor use and gastric cancer: A population-based cohort study using two different types of nationwide databases in Korea. Gut 2021, 70, 2066–2075. [Google Scholar] [CrossRef]
- Tian, Y.; Schuemie, M.J.; Suchard, M.A. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int. J. Epidemiol. 2018, 47, 2005–2014. [Google Scholar] [CrossRef]
- Higurashi, T.; Hosono, K.; Takahashi, H.; Komiya, Y.; Umezawa, S.; Sakai, E.; Uchiyama, T.; Taniguchi, L.; Hata, Y.; Uchiyama, S.; et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016, 17, 475–483. [Google Scholar] [CrossRef]
- Home, P.D.; Kahn, S.E.; Jones, N.P.; Noronha, D.; Beck-Nielsen, H.; Viberti, G.; Group, A.S.; Committee, R.S. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010, 53, 1838–1845. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Diabetes, metformin use, and colon cancer: A population-based cohort study in Taiwan. Eur. J. Endocrinol. 2012, 167, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilidis, K.K.; Capothanassi, D.; Allen, N.E.; Rizos, E.C.; Lopez, D.S.; Van Veldhoven, K.; Sacerdote, C.; Ashby, D.; Vineis, P.; Tzoulaki, I.; et al. Metformin does not affect cancer risk: A cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care 2014, 37, 2522–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, C.R.; Hassabo, H.M.; Bhadkamkar, N.A.; Wen, S.; Baladandayuthapani, V.; Kee, B.K.; Eng, C.; Hassan, M.M. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br. J. Cancer 2012, 106, 1374–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, T.I.; Jeon, S.M.; Hong, S.P.; Cheon, J.H.; Kim, W.H. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int. J. Cancer 2012, 131, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Mc Menamin, U.C.; Murray, L.J.; Hughes, C.M.; Cardwell, C.R. Metformin use and survival after colorectal cancer: A population-based cohort study. Int. J. Cancer 2016, 138, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, P.; Reineke, L.C.; Knutsen, E.; Chen, M.; Pichler, M.; Ling, H.; Calin, G.A. Metformin blocks MYC protein synthesis in colorectal cancer via mTOR-4EBP-eIF4E and MNK1-eIF4G-eIF4E signaling. Mol. Oncol. 2018, 12, 1856–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amable, G.; Martinez-Leon, E.; Picco, M.E.; Di Siervi, N.; Davio, C.; Rozengurt, E.; Rey, O. Metformin inhibits beta-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int. J. Biochem. Cell Biol. 2019, 112, 88–94. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, D.; Kee, S.H. Metformin-activated AMPK regulates beta-catenin to reduce cell proliferation in colon carcinoma RKO cells. Oncol. Lett. 2019, 17, 2695–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, Y. Metformin attenuates cells stemness and epithelialmesenchymal transition in colorectal cancer cells by inhibiting the Wnt3a/betacatenin pathway. Mol. Med. Rep. 2019, 19, 1203–1209. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Ma, Z.; Liu, X.; Zhou, W.; He, S.; Xu, X.; Ren, G.; Xu, G.; Tian, K. Metformin prevents DMH-induced colorectal cancer in diabetic rats by reversing the warburg effect. Cancer Med. 2015, 4, 1730–1741. [Google Scholar] [CrossRef]
- Yoshida, K.; Solomon, D.H.; Kim, S.C. Active-comparator design and new-user design in observational studies. Nat. Rev. Rheumatol. 2015, 11, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Hripcsak, G.; Duke, J.D.; Shah, N.H.; Reich, C.G.; Huser, V.; Schuemie, M.J.; Suchard, M.A.; Park, R.W.; Wong, I.C.; Rijnbeek, P.R.; et al. Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers. Stud. Health Technol. Inform. 2015, 216, 574–578. [Google Scholar]
| Characteristic | Before PS Adjustment | After PS Adjustment | ||||
|---|---|---|---|---|---|---|
| Metformin (n = 31,071) | Non-Metformin (n = 8636) | SMD | Metformin (n = 8201) | Non-Metformin (n = 8201) | SMD | |
| Age group (years, %) | ||||||
| 45–49 | 11 | 8.2 | 0.09 | 8.5 | 8.6 | 0 |
| 50–54 | 14.8 | 10.4 | 0.13 | 10.4 | 10.6 | −0.01 |
| 55–59 | 14.6 | 12 | 0.08 | 12.5 | 12.3 | 0.01 |
| 60–64 | 14.7 | 15.2 | −0.01 | 14.8 | 15.1 | −0.01 |
| 65–69 | 13.1 | 16 | −0.08 | 15.7 | 15.9 | −0.01 |
| 70–74 | 9.7 | 13.4 | −0.11 | 13.4 | 13.3 | 0 |
| 75–79 | 5.8 | 9.7 | −0.15 | 9.5 | 9.3 | 0.01 |
| Sex: female, % | 46.6 | 48.4 | −0.04 | 49.1 | 48.5 | 0.01 |
| Cigarette smoker, % | 6.3 | 5.2 | 0.05 | 5.2 | 5.3 | −0.01 |
| Alcoholics, % | 12.9 | 10.2 | 0.08 | 9.6 | 10.6 | −0.03 |
| Medical history, % | ||||||
| Acute respiratory disease | 56 | 54.3 | 0.03 | 54 | 54.4 | −0.01 |
| Chronic liver disease | 8.8 | 9.7 | −0.03 | 9.7 | 9.5 | 0.01 |
| Renal impairment | 1.5 | 5.2 | −0.21 | 2.6 | 2.3 | 0.02 |
| Chronic kidney disease | 0.5 | 4.0 | −0.23 | 1.5 | 1.2 | 0.02 |
| Depressive disorder | 7.5 | 9 | −0.05 | 8.3 | 8.5 | −0.01 |
| Gastroesophageal reflux disease | 8.5 | 7.9 | 0.02 | 7.6 | 7.8 | −0.01 |
| Hyperlipidemia | 50.1 | 43.1 | 0.14 | 41.2 | 42.5 | −0.03 |
| Hypertensive disorder | 55.9 | 63.9 | −0.16 | 63.5 | 62.7 | 0.02 |
| Osteoarthritis | 15.1 | 17.2 | −0.06 | 17.4 | 17.2 | 0 |
| Visual system disorder | 36 | 37.2 | −0.02 | 36.9 | 36.5 | 0.01 |
| Retinal disorder | 6.7 | 7.2 | −0.01 | 7.1 | 6.7 | 0.01 |
| Cerebrovascular disease | 5.4 | 6.9 | −0.06 | 6.6 | 6.5 | 0 |
| Heart disease | 22.8 | 29.5 | −0.15 | 28.5 | 27.7 | 0.02 |
| Heart failure | 5.1 | 8.2 | −0.12 | 7.5 | 7.1 | 0.02 |
| Ischemic heart disease | 12.6 | 17.6 | −0.14 | 16.8 | 16.2 | 0.02 |
| Peripheral vascular disease | 14.4 | 15 | −0.02 | 14.8 | 14.6 | 0 |
| Obesity | 0.3 | 0.1 | 0.03 | 0.2 | 0.1 | 0.02 |
| Medication use, % | ||||||
| Agents acting on the renin-angiotensin system | 35.8 | 38.1 | −0.05 | 36.9 | 36.7 | 0 |
| Antibacterials for systemic use | 65 | 65.7 | −0.01 | 66.2 | 64.9 | 0.03 |
| Antidepressants | 12 | 13.3 | −0.04 | 12.9 | 12.8 | 0 |
| Antiepileptics | 7.7 | 9.1 | −0.05 | 8.4 | 8.5 | 0 |
| Anti-inflammatory and antirheumatic products | 55.5 | 57.9 | −0.05 | 58.3 | 57.6 | 0.01 |
| Antithrombotic agents | 60.6 | 61.7 | −0.02 | 61.2 | 60.7 | 0.01 |
| Beta blocking agents | 20.4 | 26.7 | −0.15 | 25.7 | 24.9 | 0.02 |
| Calcium channel blockers | 35.2 | 44.2 | −0.18 | 43.9 | 42.5 | 0.03 |
| Diuretics | 31.6 | 36.8 | −0.11 | 36.3 | 34.8 | 0.03 |
| Drugs for acid-related disorders | 60.9 | 62.5 | −0.03 | 62.6 | 61.4 | 0.02 |
| Drugs for obstructive airway diseases | 37.2 | 39.1 | −0.04 | 38 | 38.7 | −0.01 |
| Drugs used in diabetes | 11.5 | 5.6 | 0.21 | 5.6 | 5.7 | −0.01 |
| Lipid-modifying agents | 36.1 | 30.7 | 0.12 | 28.6 | 30.2 | −0.04 |
| Opioids | 41 | 41.3 | 0 | 41.1 | 40.6 | 0.01 |
| Psycholeptics | 43.8 | 48.6 | −0.1 | 47.8 | 47.6 | 0 |
| Charlson comorbidity index–Romano adaptation | 4.23 | 4.41 | −0.07 | 4.31 | 4.26 | 0.02 |
| No. of Participants | Person-Years | No. of Case | Incidence Rate a | HR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| CRC incidence | |||||||
| Metformin ≥ 6 months | 8201 | 42,458 | 220 | 5.18 | 0.58 | 0.47–0.71 | 0.001 |
| Non-metformin | 8201 | 39,280 | 319 | 8.12 | Ref. | ||
| Metformin ≥ 1 year | 8021 | 42,073 | 223 | 5.30 | 0.72 | 0.58–0.88 | 0.001 |
| Non-metformin | 8021 | 39,444 | 291 | 7.38 | Ref. | ||
| All-cause mortality | |||||||
| Metformin ≥ 6 months | 8264 | 43,254 | 1111 | 25.69 | 0.71 | 0.64–0.78 | 0.001 |
| Non-metformin | 8264 | 40,567 | 1449 | 35.72 | Ref. | ||
| Metformin ≥ 1 year | 8077 | 42,791 | 919 | 21.48 | 0.70 | 0.62–0.78 | 0.001 |
| Non-metformin | 8077 | 40,575 | 1192 | 29.38 | Ref. | ||
| CRC-related mortality | |||||||
| Metformin≥ 6 months | 8264 | 43,254 | 22 | 0.51 | 0.55 | 0.26–1.08 | 0.09 |
| Non-metformin | 8264 | 40,567 | 37 | 0.91 | Ref. | ||
| Metformin ≥ 1 year | 8077 | 42,791 | 19 | 0.44 | 0.85 | 0.37–1.89 | 0.69 |
| Non-metformin | 8077 | 40,575 | 32 | 0.79 | Ref. | ||
| Analysis | Lag Period | HR | 95% CI | p-Value |
|---|---|---|---|---|
| CRC incidence | ||||
| PS matching (main analysis) | 6 months | 0.58 | 0.47–0.71 | 0.001 |
| PS matching | 1 year | 0.63 | 0.51–0.79 | 0.001 |
| PS stratification | 6 months | 0.65 | 0.57–0.75 | 0.001 |
| PS stratification | 1 year | 0.68 | 0.59–0.79 | 0.001 |
| All-cause mortality | ||||
| PS matching (main analysis) | 6 months | 0.71 | 0.64–0.78 | 0.001 |
| PS matching | 1 year | 0.72 | 0.66–0.80 | 0.001 |
| PS stratification | 6 months | 0.68 | 0.64–0.73 | 0.001 |
| PS stratification | 1 year | 0.68 | 0.64–0.73 | 0.001 |
| CRC-related mortality | ||||
| PS matching (main analysis) | 6 months | 0.55 | 0.26–1.08 | 0.09 |
| PS matching | 1 year | 0.65 | 0.32–1.29 | 0.23 |
| PS stratification | 6 months | 0.60 | 0.39–0.93 | 0.02 |
| PS stratification | 1 year | 0.67 | 0.43–1.06 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.I.; Kim, T.J.; Park, C.H.; Bang, C.S.; Lee, K.J.; Kim, J.; Kim, H.H.; Shin, W.G. Incidence and Survival Outcomes of Colorectal Cancer in Long-Term Metformin Users with Diabetes: A Population-Based Cohort Study Using a Common Data Model. J. Pers. Med. 2022, 12, 584. https://doi.org/10.3390/jpm12040584
Seo SI, Kim TJ, Park CH, Bang CS, Lee KJ, Kim J, Kim HH, Shin WG. Incidence and Survival Outcomes of Colorectal Cancer in Long-Term Metformin Users with Diabetes: A Population-Based Cohort Study Using a Common Data Model. Journal of Personalized Medicine. 2022; 12(4):584. https://doi.org/10.3390/jpm12040584
Chicago/Turabian StyleSeo, Seung In, Tae Jun Kim, Chan Hyuk Park, Chang Seok Bang, Kyung Joo Lee, Jinseob Kim, Hyon Hee Kim, and Woon Geon Shin. 2022. "Incidence and Survival Outcomes of Colorectal Cancer in Long-Term Metformin Users with Diabetes: A Population-Based Cohort Study Using a Common Data Model" Journal of Personalized Medicine 12, no. 4: 584. https://doi.org/10.3390/jpm12040584
APA StyleSeo, S. I., Kim, T. J., Park, C. H., Bang, C. S., Lee, K. J., Kim, J., Kim, H. H., & Shin, W. G. (2022). Incidence and Survival Outcomes of Colorectal Cancer in Long-Term Metformin Users with Diabetes: A Population-Based Cohort Study Using a Common Data Model. Journal of Personalized Medicine, 12(4), 584. https://doi.org/10.3390/jpm12040584






