Characterization of Depressive Symptom Trajectories in Women between Childbirth and Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure and Questionnaires
2.3. Missing Data
2.4. Parameter Overview
2.5. Methods: LCMM
2.6. Methods: MultLCMM
3. Results
3.1. Population
3.2. LCMM EPDS: Model Selection
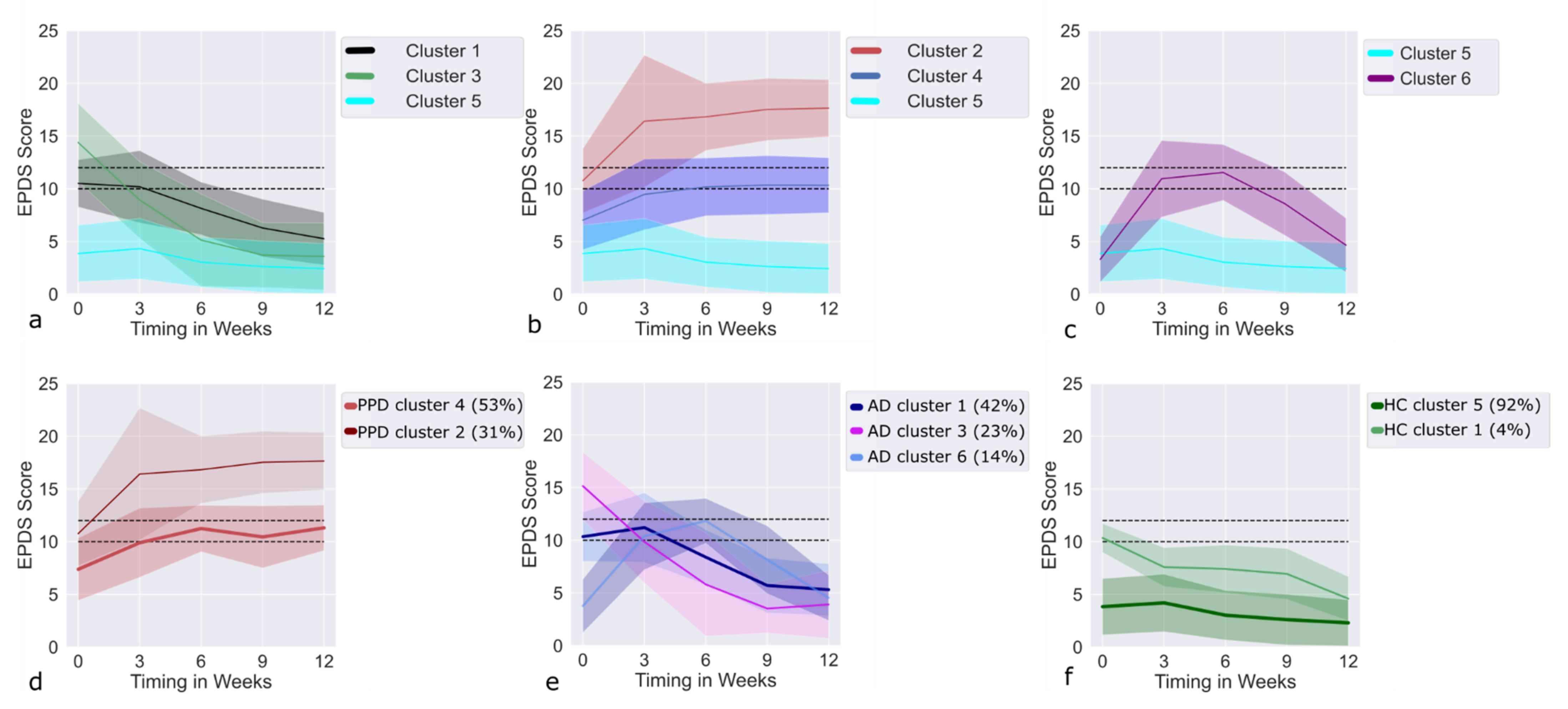
3.3. LCMM EPDS: Clusters
3.4. Recovering Clusters
3.5. Deteriorating Clusters
3.6. Trajectories of AD Cases within the Recovering Clusters
3.7. Trajectories of PPD Cases within the Deteriorating Clusters
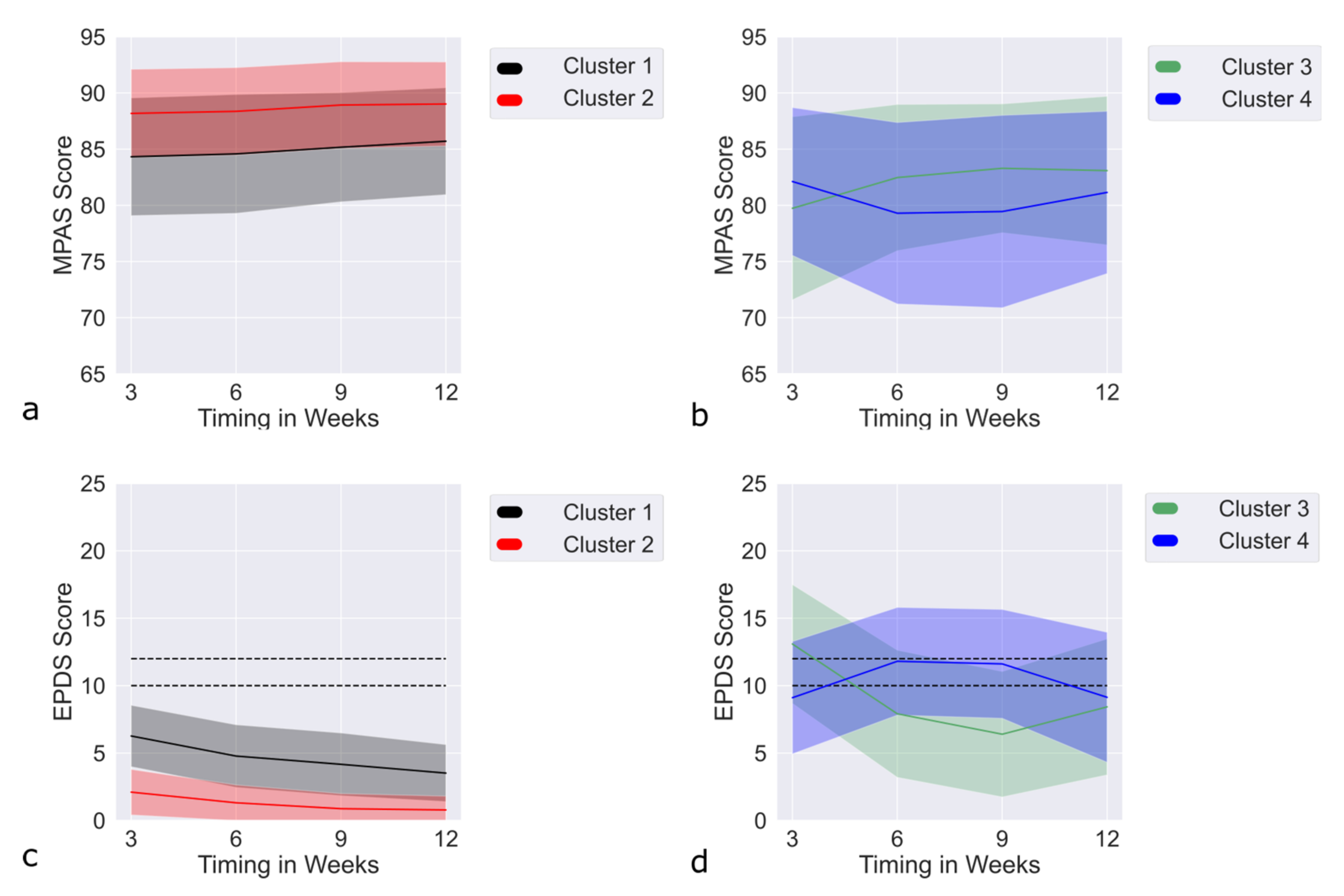
3.8. Results MultLCMM MPAS + EPDS: Model Selection
3.9. MultLCMM MPAS/EPDS: Clusters
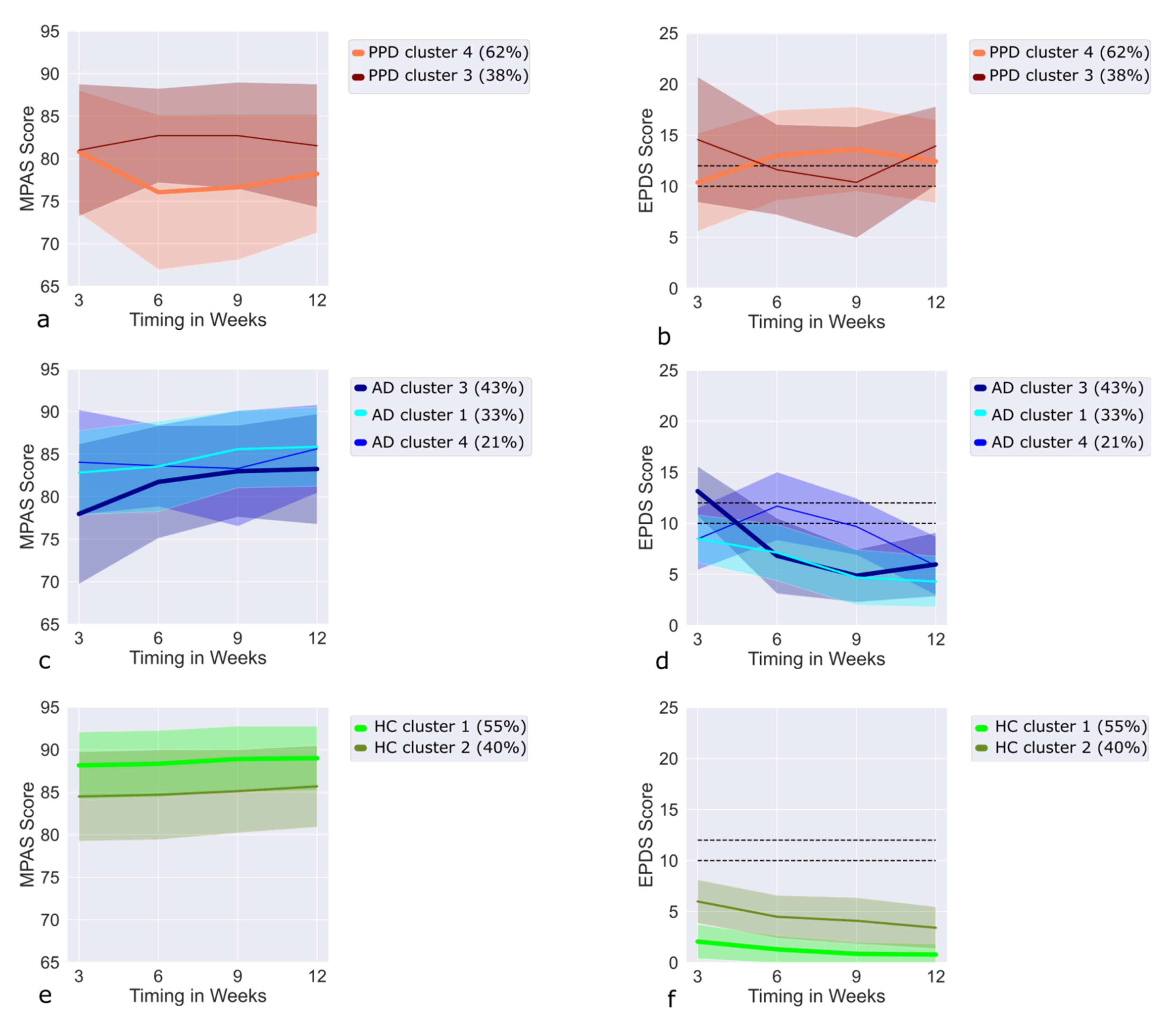
3.10. Trajectories of PPD Cases in the MPAS/EPDS Clusters
3.11. Trajectories of AD Cases in the MPAS/EPDS Clusters
3.12. Trajectories of HC in the MPAS/EPDS Clusters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, J.R.; Nieman, L.K. The Hypothalamic-Pituitary-Adrenal Axis in Pregnancy: Challenges in Disease Detection and Treatment. Endocr. Rev. 2005, 26, 775–799. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.A.; Frokjaer, V. Perinatal Depression: Embracing Variability toward Better Treatment and Outcomes. Neuron 2019, 102, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Hahn, L.; Eickhoff, S.B.; Habel, U.; Stickeler, E.; Schnakenberg, P.; Goecke, T.W.; Stickel, S.; Franz, M.; Dukart, J.; Chechko, N. Early identification of postpartum depression using demographic, clinical, and digital phenotyping. Transl. Psychiatry 2021, 11, 121. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Stickel, S.; Eickhoff, S.B.; Habel, U.; Stickeler, E.; Goecke, T.; Lang, J.; Chechko, N. Endocrine stress response in pregnancy and 12 weeks postpartum—Exploring risk factors for postpartum depression. Psychoneuroendocrinology 2020, 125, 105122. [Google Scholar] [CrossRef]
- Field, T. Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant Behav. Dev. 2010, 33, 1–6. [Google Scholar] [CrossRef]
- Barker, E.D.; Kirkham, N.; Ng, J.; Jensen, S.K.G. Prenatal maternal depression symptoms and nutrition, and child cognitive function. Br. J. Psychiatry 2013, 203, 417–421. [Google Scholar] [CrossRef]
- Rahman, A.; Surkan, P.J.; Cayetano, C.E.; Rwagatare, P.; Dickson, K.E. Grand Challenges: Integrating Maternal Mental Health into Maternal and Child Health Programmes. PLoS Med. 2013, 10, e1001442. [Google Scholar] [CrossRef]
- Monk, C.; Georgieff, M.K.; Osterholm, E.A. Research Review: Maternal prenatal distress and poor nutrition–mutually influencing risk factors affecting infant neurocognitive development. J. Child Psychol. Psychiatry 2013, 54, 115–130. [Google Scholar] [CrossRef]
- Pearson, R.M.; Bornstein, M.H.; Cordero, M.; Scerif, G.; Mahedy, L.; Evans, J.; Abioye, A.; Stein, A. Maternal perinatal mental health and offspring academic achievement at age 16: The mediating role of childhood executive function. J. Child Psychol. Psychiatry 2016, 57, 491–501. [Google Scholar] [CrossRef]
- Goodman, S.H.; Rouse, M.H.; Connell, A.M.; Broth, M.R.; Hall, C.M.; Heyward, D. Maternal Depression and Child Psychopathology: A Meta-Analytic Review. Clin. Child Fam. Psychol. Rev. 2011, 14, 1–27. [Google Scholar] [CrossRef]
- Murray, L.; Arteche, A.; Fearon, P.; Halligan, S.; Croudace, T.; Cooper, P. The effects of maternal postnatal depression and child sex on academic performance at age 16 years: A developmental approach: PND & child cognitive and academic outcomes. J. Child Psychol. Psychiatry 2010, 51, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Magnusson, C.; Rai, D.; Lundberg, M.; Lê-Scherban, F.; Dalman, C.; Lee, B.K. Associations of Parental Depression with Child School Performance at Age 16 Years in Sweden. JAMA Psychiatry 2016, 73, 239. [Google Scholar] [CrossRef] [PubMed]
- Weissman, M.M.; Wickramaratne, P.; Nomura, Y.; Warner, V.; Pilowsky, D.; Verdeli, H. Offspring of Depressed Parents: 20 Years Later. Am. J. Psychiatry 2006, 163, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; Evans, J.; Kounali, D.; Lewis, G.; Heron, J.; Ramchandani, P.; O’Connor, T.G.; Stein, A. Maternal Depression During Pregnancy and the Postnatal Period: Risks and Possible Mechanisms for Offspring Depression at Age 18 Years. JAMA Psychiatry 2013, 70, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, P.; Wei, D.; Wang, K.; Li, Y.; Qiu, J. Effects of parental emotional warmth on the relationship between regional gray matter volume and depression-related personality traits. Soc. Neurosci. 2017, 12, 337–348. [Google Scholar] [CrossRef]
- Miller, L.J. Postpartum Depression. JAMA 2002, 287, 762–765. [Google Scholar] [CrossRef]
- O’Hara, M.W. Postpartum depression: What we know. J. Clin. Psychol. 2009, 65, 1258–1269. [Google Scholar] [CrossRef]
- O’Hara, M.W.; McCabe, J.E. Postpartum Depression: Current Status and Future Directions. Annu. Rev. Clin. Psychol. 2013, 9, 379–407. [Google Scholar] [CrossRef]
- Campbell, S.B.; Matestic, P.; Von Stauffenberg, C.; Mohan, R.; Kirchner, T. Trajectories of maternal depressive symptoms, maternal sensitivity, and children’s functioning at school entry. Dev. Psychol. 2007, 43, 1202–1215. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Qandil, S.; Jabr, S.; Wagler, S.; Collin, S.M. Postpartum depression in the Occupied Palestinian Territory: A longitudinal study in Bethlehem. BMC Pregnancy Childbirth 2016, 16, 375. [Google Scholar] [CrossRef] [PubMed]
- Denckla, C.A.; Mancini, A.D.; Consedine, N.S.; Milanovic, S.M.; Basu, A.; Seedat, S.; Spies, G.; Henderson, D.C.; Bonanno, G.A.; Koenen, K.C. Distinguishing postpartum and antepartum depressive trajectories in a large population-based cohort: The impact of exposure to adversity and offspring gender. Psychol. Med. 2018, 48, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Munk-Olsen, T.; Laursen, T.M.; Pedersen, C.B.; Mors, O.; Mortensen, P.B. New Parents and Mental Disorders: A Population-Based Register Study. JAMA 2006, 296, 2582–2589. [Google Scholar] [CrossRef]
- Taylor, C.L.; Broadbent, M.; Khondoker, M.; Stewart, R.J.; Howard, L.M. Predictors of severe relapse in pregnant women with psychotic or bipolar disorders. J. Psychiatr. Res. 2018, 104, 100–107. [Google Scholar] [CrossRef]
- Halfin, A. Depression: The benefits of early and appropriate treatment. Am. J. Manag. Care 2007, 13, S92. [Google Scholar]
- Fisher, J.; De Mello, M.C.; Patel, V.; Rahman, A.; Tran, T.; Holton, S.; Holmes, W. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: A systematic review. Bull. World Health Organ. 2012, 90, 139H–149H. [Google Scholar] [CrossRef]
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J.; Akil, H.; Gordon, J.; et al. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of Postnatal Depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Bergant, A.M.; Nguyen, T.; Heim, K.; Ulmer, H.; Dapunt, O. Deutschsprachige Fassung und Validierung der »Edinburgh postnatal depression scale. DMW Dtsch. Med. Wochenschr. 2008, 123, 35–40. [Google Scholar] [CrossRef]
- Condon, J.T.; Corkindale, C.J. The assessment of parent-to-infant attachment: Development of a self-report questionnaire instrument. J. Reprod. Infant Psychol. 1998, 16, 57–76. [Google Scholar] [CrossRef]
- Kennerley, H.; Gath, D. Maternity Blues: I. Detection and Measurement by Questionnaire. Br. J. Psychiatry 1989, 155, 356–362. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio; RStudio Team: Boston, MA, USA, 2020. [Google Scholar]
- Harrison, E. Missing Data. Available online: https://cran.r-project.org/web/packages/finalfit/vignettes/missing.html (accessed on 2 February 2022).
- Van De Schoot, R.; Sijbrandij, M.; Winter, S.D.; Depaoli, S.; Vermunt, J.K. The GRoLTS-Checklist: Guidelines for Reporting on Latent Trajectory Studies. Struct. Equ. Model. Multidiscip. J. 2017, 24, 451–467. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Philipps, V.; Liquet, B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. J. Stat. Softw. 2017, 78, 1–56. [Google Scholar] [CrossRef]
- Proust, C.; Jacqmin-Gadda, H. Estimation of linear mixed models with a mixture of distribution for the random effects. Comput. Methods Programs Biomed. 2005, 78, 165–173. [Google Scholar] [CrossRef]
- Carrière, I.; Farré, A.; Proust-Lima, C.; Ryan, J.; Ancelin, M.L.; Ritchie, K. Chronic and remitting trajectories of depressive symptoms in the elderly. Characterisation and risk factors. Epidemiol. Psychiatr. Sci. 2017, 26, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Madigan, S.; Wade, M.; Plamondon, A.; Jenkins, J.M. Trajectories of maternal depressive symptoms in the early childhood period and family-wide clustering of risk. J. Affect. Disord. 2017, 215, 49–55. [Google Scholar] [CrossRef]
- Lo, Y.; Mendell, N.R.; Rubin, D.B. Testing the number of components in a normal mixture. Biometrika 2001, 88, 767–778. [Google Scholar] [CrossRef]
- Nylund, K.L.; Asparouhov, T.; Muthén, B.O. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct. Equ. Model. A Multidiscip. J. 2007, 14, 535–569. [Google Scholar] [CrossRef]
- Pietrzak, R.H.; Feder, A.; Singh, R.; Schechter, C.B.; Bromet, E.J.; Katz, C.L.; Reissman, D.B.; Ozbay, F.; Sharma, V.; Crane, M.; et al. Trajectories of PTSD risk and resilience in World Trade Center responders: An 8-year prospective cohort study. Psychol. Med. 2014, 44, 205–219. [Google Scholar] [CrossRef]
- Fleishman, A.I. A method for simulating non-normal distributions. Psychometrika 1978, 43, 521–532. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.T. Predictors of Postpartum Depression: An Update. Nurs. Res. 2001, 50, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Luoma, I.; Korhonen, M.; Salmelin, R.K.; Helminen, M.; Tamminen, T. Long-term trajectories of maternal depressive symptoms and their antenatal predictors. J. Affect. Disord. 2015, 170, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Righetti-Veltema, M.; Conne-Perréard, E.; Bousquet, A.; Manzano, J. Risk factors and predictive signs of postpartum depression. J. Affect. Disord. 1998, 49, 167–180. [Google Scholar] [CrossRef]
- Sundström-Poromaa, I.; Comasco, E.; Sumners, R.; Luders, E. Progesterone–Friend or foe? Front. Neuroendocr. 2020, 59, 100856. [Google Scholar] [CrossRef] [PubMed]
- Harris, B. Biological and Hormonal Aspects of Postpartum Depressed Mood: Working Towards Strategies for Prophylaxis and Treatment. Br. J. Psychiatry 1994, 164, 288–292. [Google Scholar] [CrossRef][Green Version]
- Patel, M.; Jabeen, S.; Osiezagha, K.; Bailey, R.K.; Ali, S.; Barker, N.C. Postpartum Depression: A Review. J. Health Care Poor Underserved 2012, 23, 534–542. [Google Scholar] [CrossRef]
| Risk Factors | Cluster 1 vs. Baseline | Cluster 3 vs. Baseline | Cluster 6 vs. Baseline | Cluster 2 vs. Baseline | Cluster 4 vs. Baseline |
|---|---|---|---|---|---|
| Family psychiatric history | X | ||||
| Psychiatric diagnosis in previous pregnancy | X | X | X | ||
| Relocation to another ward | X | ||||
| Baby blues | X | X | X | X | |
| Birth-related psychological and physical traumas | X | X | |||
| Previous depression | X | X | X | X | |
| PMS Severity | X | X | X | ||
| EPDS T0 | X | X | X | X | |
| EPDS T1 | X | X | X | X | X |
| EPDS T2 | X | X | X | X | X |
| EPDS T3 | X | X | X | X | |
| EPDS T4 | X | X | X | X | |
| MPAS T1 | X | X | X | X | |
| MPAS T2 | X | X | X | ||
| MPAS T3 | X | X | X | ||
| MPAS T4 | X | X | |||
| SLE | X | X | |||
| Support at home | X | X | |||
| Family status | X | ||||
| Income | X |
| Risk Factors | Cluster 1 MPAS/EPDS vs. Baseline | Upright U-shaped vs. Baseline | Inverted U-shaped vs. Baseline |
|---|---|---|---|
| Baby blues | X | X | X |
| Previous depression | X | X | |
| PMS Severity | X | X | X |
| EPDS T0 | X | X | X |
| EPDS T1 | X | X | X |
| EPDS T2 | X | X | X |
| EPDS T3 | X | X | X |
| EPDS T4 | X | X | X |
| MPAS T1 | X | X | X |
| MPAS T2 | X | X | X |
| MPAS T3 | X | X | X |
| MPAS T4 | X | X | X |
| SLE | X | ||
| Support at home | X | X | |
| Birth-related psychological and physical traumas | X | X | |
| Psychiatric diagnosis in previous pregnancy | X | ||
| Family psychiatric history | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chechko, N.; Stickel, S.; Losse, E.; Shymanskaya, A.; Habel, U. Characterization of Depressive Symptom Trajectories in Women between Childbirth and Diagnosis. J. Pers. Med. 2022, 12, 538. https://doi.org/10.3390/jpm12040538
Chechko N, Stickel S, Losse E, Shymanskaya A, Habel U. Characterization of Depressive Symptom Trajectories in Women between Childbirth and Diagnosis. Journal of Personalized Medicine. 2022; 12(4):538. https://doi.org/10.3390/jpm12040538
Chicago/Turabian StyleChechko, Natalia, Susanne Stickel, Elena Losse, Aliaksandra Shymanskaya, and Ute Habel. 2022. "Characterization of Depressive Symptom Trajectories in Women between Childbirth and Diagnosis" Journal of Personalized Medicine 12, no. 4: 538. https://doi.org/10.3390/jpm12040538
APA StyleChechko, N., Stickel, S., Losse, E., Shymanskaya, A., & Habel, U. (2022). Characterization of Depressive Symptom Trajectories in Women between Childbirth and Diagnosis. Journal of Personalized Medicine, 12(4), 538. https://doi.org/10.3390/jpm12040538






