Personalized Medicine for Classical Anesthesia Drugs and Cancer Progression
Abstract
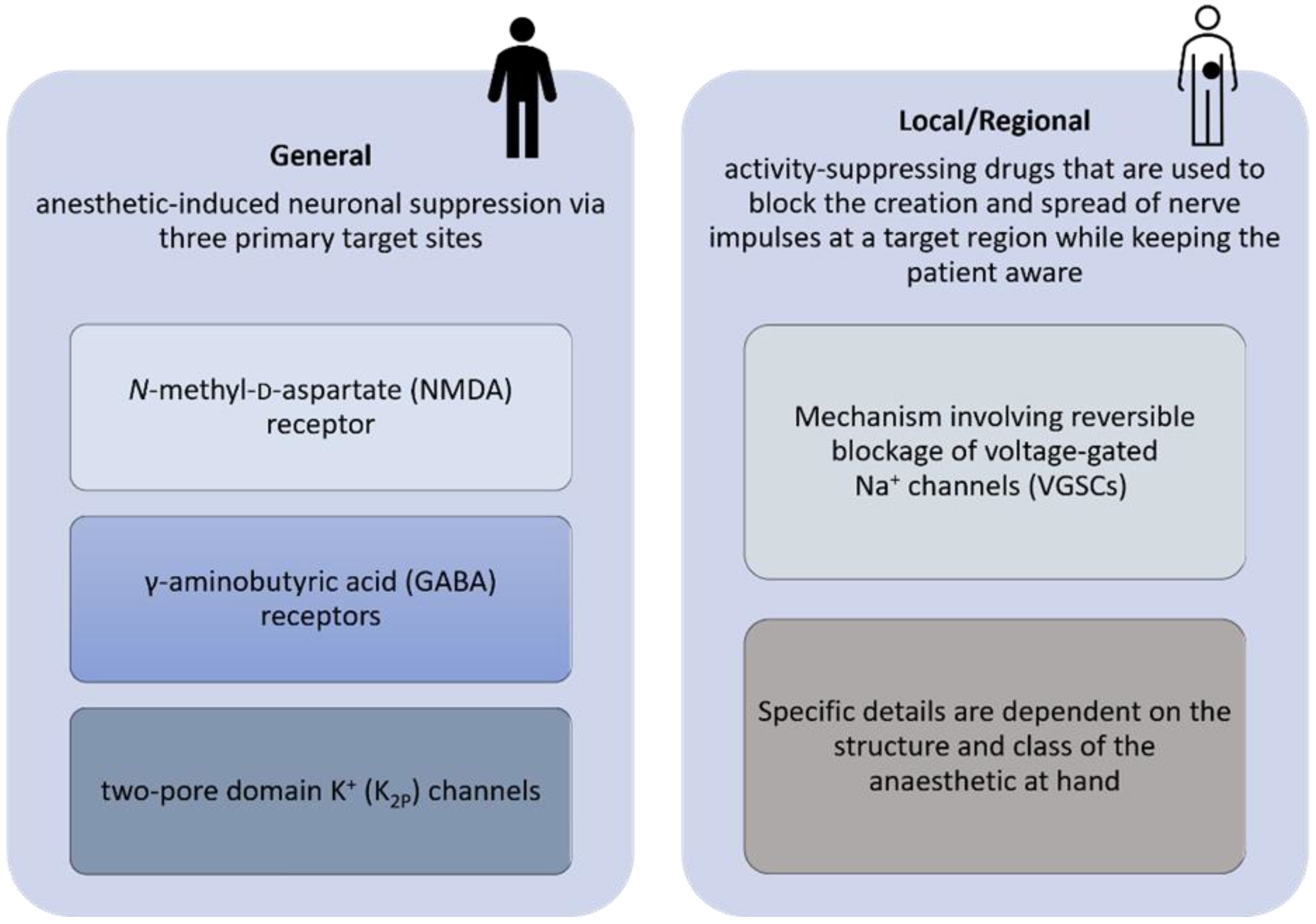
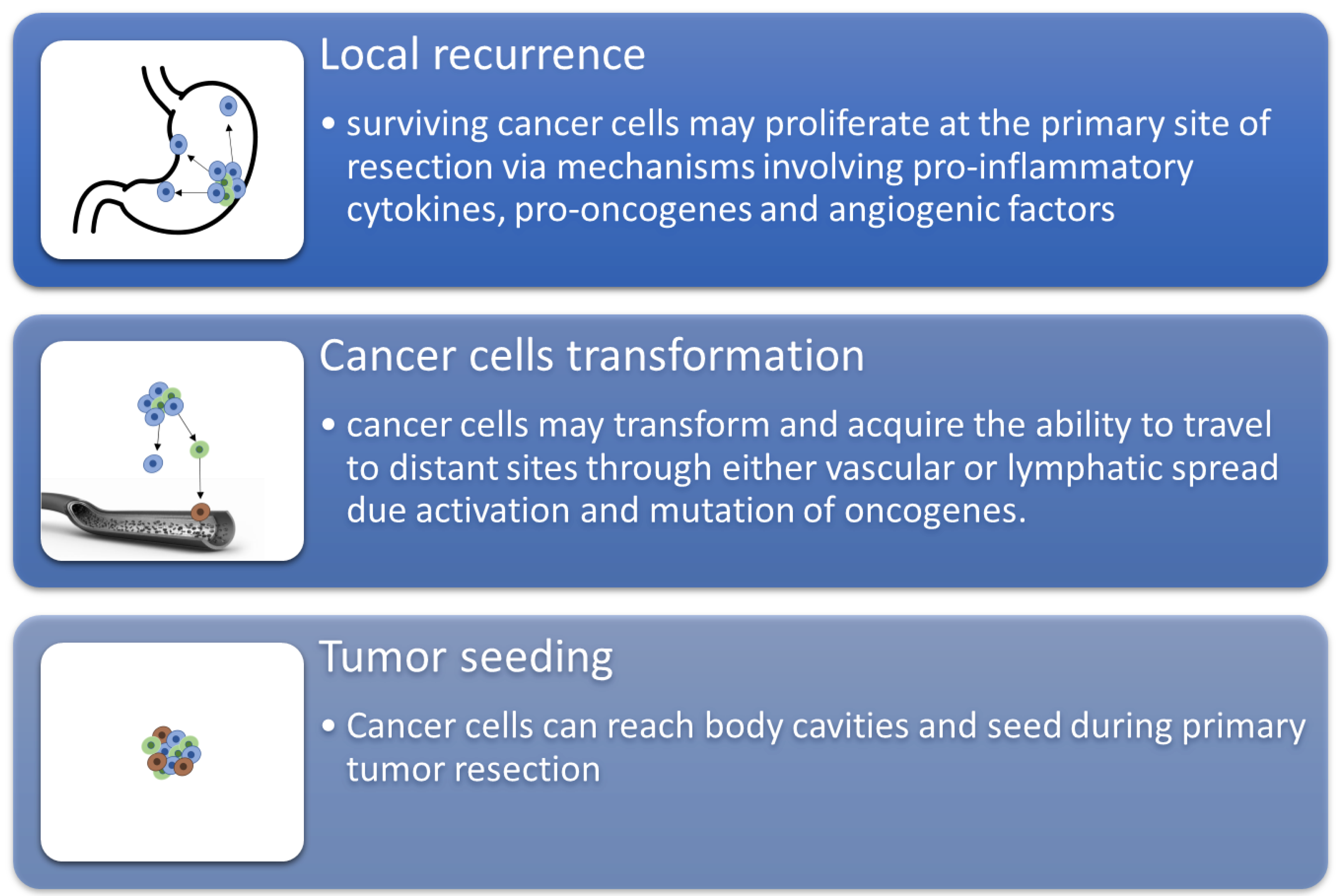
1. Introduction
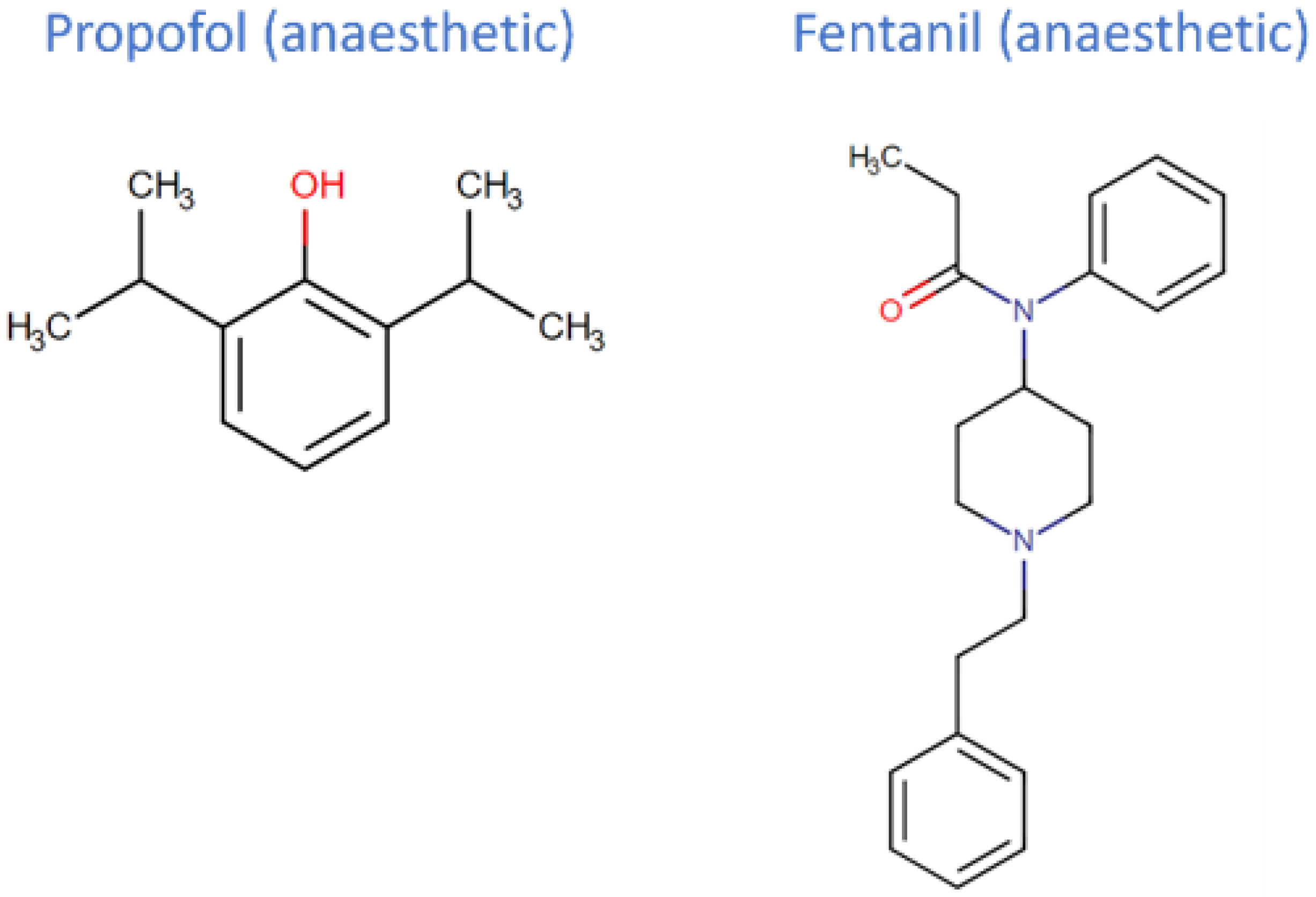
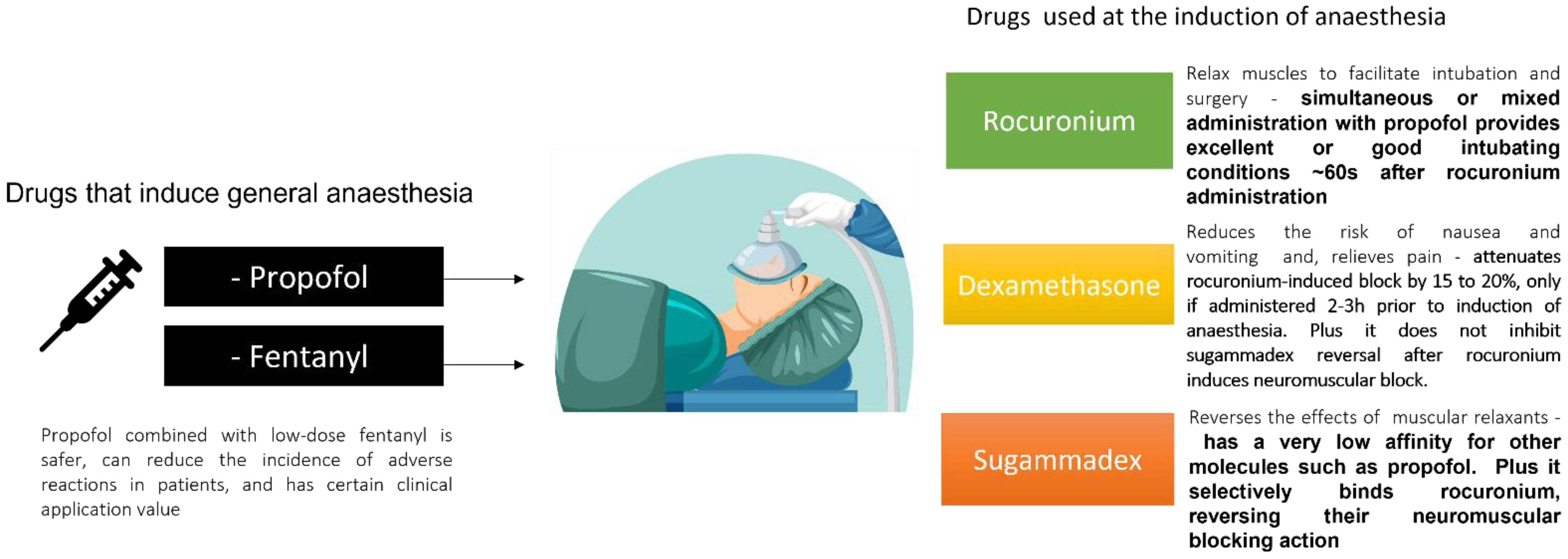
2. Propofol and Fentanyl
3. Complementary Drugs to Propofol and Fentanyl
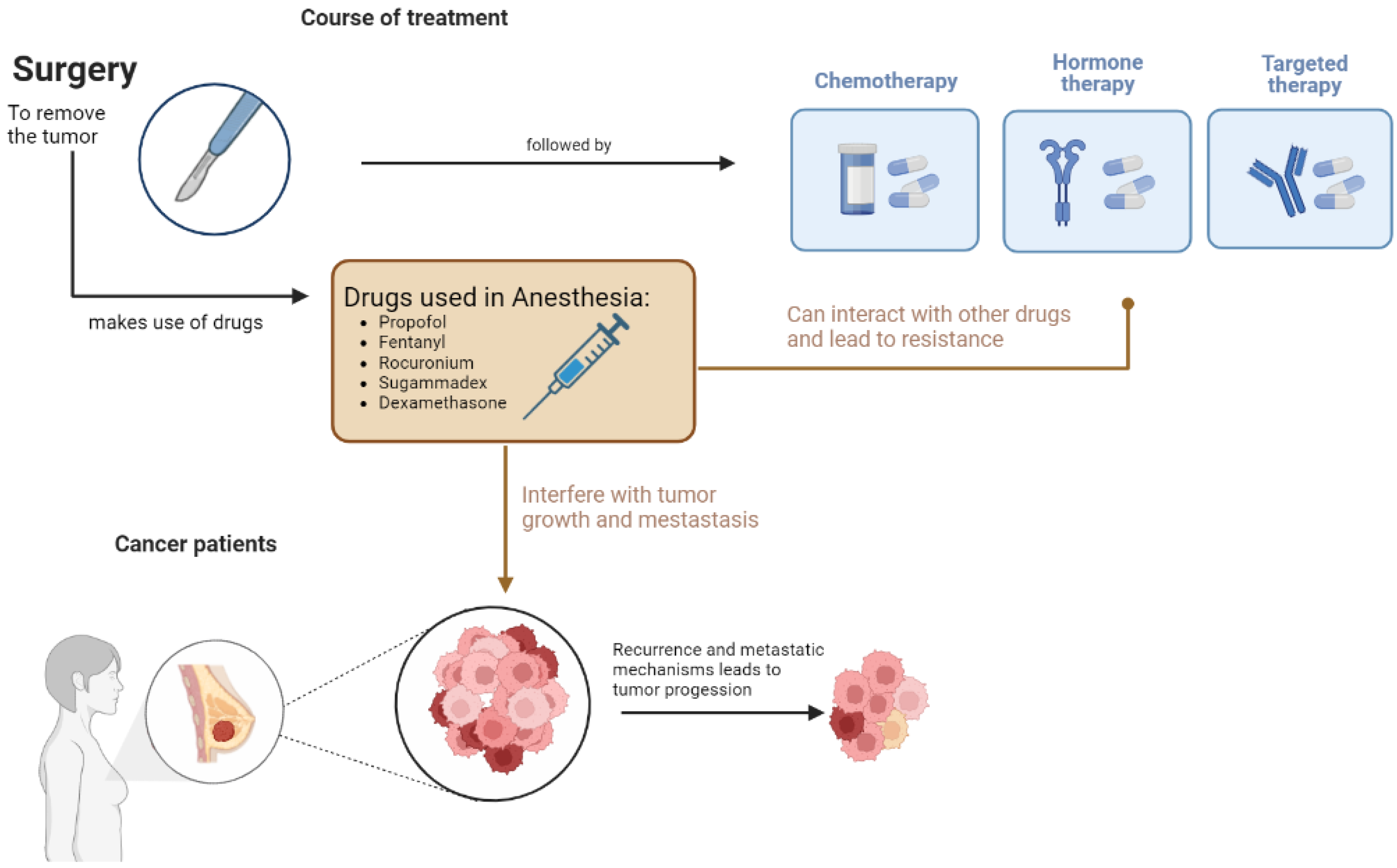
4. Effect on Cancer Treatment
5. Potential Opportunities from In Silico Studies and Pharmacokinetics (Pk)/Pharmacodynamics (PD) Models
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, N.J.S.; Buggy, D.; Ma, D. Can Anesthesia Influence Cancer Outcomes After Surgery? JAMA Surg. 2019, 154, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Rowbotham, D.J.; Lambert, D.G.; Buggy, D.J. Can Anesthetic Techniques or Drugs Affect Cancer Recurrence in Patients Undergoing Cancer Surgery? J. Anesth. 2013, 27, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Huitink, J.M.; Heimerikxs, M.; Nieuwland, M.; Loer, S.A.; Brugman, W.; Velds, A.; Sie, D.; Kerkhoven, R.M. Volatile Anesthetics Modulate Gene Expression in Breast and Brain Tumor Cells. Anesth Analg. 2010, 111, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Montejano, J.; Jevtovic-Todorovic, V. Anesthesia and Cancer, Friend or Foe? A Narrative Review. Front. Oncol. 2021, 11, 5525. [Google Scholar] [CrossRef]
- Iqbal, F.; Thompson, A.J.; Riaz, S.; Pehar, M.; Rice, T.; Syed, N.I. Anesthetics: From Modes of Action to Unconsciousness and Neurotoxicity. J. Neurophysiol. 2019, 122, 760–787. [Google Scholar] [CrossRef]
- Shin, D.J.; Germann, A.L.; Johnson, A.D.; Forman, S.A.; Steinbach, J.H.; Akk, G. Propofol Is an Allosteric Agonist with Multiple Binding Sites on Concatemeric Ternary GABAA Receptors. Mol. Pharmacol. 2018, 93, 178–189. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Li, K.Y.; Dagraca, R.L.; Delphin, E.; Xiong, M.; Ye, J.H. Behavior and Cellular Evidence for Propofol-Induced Hypnosis Involving Brain Glycine Receptors. Anesthesiology 2009, 110, 326–332. [Google Scholar] [CrossRef]
- Court, M.H.; Duan, S.X.; Hesse, L.M.; Venkatakrishnan, K.; Greenblatt, D.J. Cytochrome P-450 2B6 Is Responsible for Interindividual Variability of Propofol Hydroxylation by Human Liver Microsomes. Anesthesiology 2001, 94, 110–119. [Google Scholar] [CrossRef]
- Pavlovic, D.; Budic, I.; Stoimenov, T.J.; Stokanovic, D.; Marjanovic, V.; Stevic, M.; Slavkovic, M.; Simic, D. The Effect of UGT1A9, CYP2B6 and CYP2C9 Genes Polymorphism on Propofol Pharmacokinetics in Children. Pharmgenomics. Pers. Med. 2020, 13, 13. [Google Scholar] [CrossRef]
- Schneider, E.; Brune, K. Opioid Activity and Distribution of Fentanyl Metabolites. Naunyn. Schmiedebergs. Arch. Pharmacol. 1986, 334, 267–274. [Google Scholar] [CrossRef]
- Ma, D.; Sapsed-Byrne, S.M.; Chakrabarti, M.K.; Whitwam, J.G. Synergistic Interaction between the Effects of Propofol and Midazolam with Fentanyl on Phrenic Nerve Activity in Rabbits. Acta Anaesthesiol. Scand. 1998, 42, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Kazama, T.; Ikeda, K.; Morita, K. Reduction by Fentanyl of the Cp50 Values of Propofol and Hemodynamic Responses to Various Noxious Stimuli. Anesthesiology 1997, 87, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Vullo, P.A.; Real Navacerrada, M.I.; Navarro Suay, R. Hemodynamic Impact of Increasing Time between Fentanyl and Propofol Administration during Anesthesia Induction: A Randomised, Clinical Trial. Braz. J. Anesthesiol. (English Ed.) 2021. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Dolatabadi, A.A.; Kariman, H.; Hatamabadi, H.; Memary, E.; Salimi, S.; Shokrzadeh, S. Low-Dose Fentanyl, Propofol, Midazolam, Ketamine and Lidocaine Combination vs. Regular Dose Propofol and Fentanyl Combination for Deep Sedation Induction; a Randomized Clinical Trial. Emergency 2018, 6, e57. [Google Scholar] [PubMed]
- Hillier, K. Rocuronium. xPharm Compr. Pharmacol. Ref. 2007, 1–5. [Google Scholar] [CrossRef]
- Appiah-Ankam, J.; Hunter, J.M. Pharmacology of Neuromuscular Blocking Drugs. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 2–7. [Google Scholar] [CrossRef]
- Barclay, K.; Eggers, K.; Asai, T. Low-Dose Rocuronium Improves Conditions for Tracheal Intubation after Induction of Anaesthesia with Propofol and Alfentanil. Br. J. Anaesth. 1997, 78, 92–94. [Google Scholar] [CrossRef]
- Ahmad, N.; Choy, C.Y.; Aris, E.A.; Balan, S. Preventing the Withdrawal Response Associated with Rocuronium Injection: A Comparison of Fentanyl with Lidocaine. Anesth. Analg. 2005, 100, 987–990. [Google Scholar] [CrossRef]
- Kosare, S.S.; Dave, N.M.; Garasia, M. Fentanyl or Ketamine Pre-Treatment to Prevent Withdrawal Response to Rocuronium. Indian J. Anaesth. 2017, 61, 350. [Google Scholar] [CrossRef]
- Nag, K.; Singh, D.R.; Shetti, A.N.; Kumar, H.; Sivashanmugam, T.; Parthasarathy, S. Sugammadex: A Revolutionary Drug in Neuromuscular Pharmacology. Anesth. Essays Res. 2013, 7, 302. [Google Scholar] [CrossRef]
- Chang, H.C.; Liu, S.Y.; Lee, M.J.; Lee, S.O.; Wong, C.S. Sugammadex Reversal of Muscle Relaxant Blockade Provided Less Post-Anesthesia Care Unit Adverse Effects than Neostigmine/Glycopyrrolate. J. Formos. Med. Assoc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.; Passier, P.; Smeets, J.; Zwiers, A.; De Zwart, M.; Van De Wetering-Krebbers, S.; Van Iersel, M.; Van Marle, S.; Van Den Dobbelsteen, D. Sugammadex Is Cleared Rapidly and Primarily Unchanged via Renal Excretion. Biopharm. Drug Dispos. 2011, 32, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, B.F.; Vermeyen, K.M.; Struys, M.M.R.F.; Rietbergen, H.; Vandermeersch, E.; Saldien, V.; Kalmar, A.F.; Prins, M.E. Reversal of Rocuronium-Induced Neuromuscular Block with the Novel Drug Sugammadex Is Equally Effective under Maintenance Anesthesia with Propofol or Sevoflurane. Anesth. Analg. 2007, 104, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Hristovska, A.M.; Duch, P.; Allingstrup, M.; Afshari, A. Efficacy and Safety of Sugammadex versus Neostigmine in Reversing Neuromuscular Blockade in Adults. Cochrane Database Syst. Rev. 2017, 8, CD012763. [Google Scholar] [CrossRef]
- Myles, P.S.; Corcoran, T. Benefits and Risks of Dexamethasone in Noncardiac Surgery. Anesthesiology 2021, 135, 895–903. [Google Scholar] [CrossRef]
- Bartlett, R.; Hartle, A.J. Routine Use of Dexamethasone for Postoperative Nausea and Vomiting: The Case Against. Anaesthesia 2013, 68, 892–896. [Google Scholar] [CrossRef]
- Erdem, A.F.; Yoruk, O.; Alici, H.A.; Cesur, M.; Atalay, C.; Altas, E.; Yuksek, M.S. Subhypnotic propofol infusion plus dexamethasone is more effective than dexamethasone alone for the prevention of vomiting in children after tonsillectomy. Pediatr. Anesth. 2008, 18, 878–883. [Google Scholar] [CrossRef]
- Raff, H.; Norton, A.J.; Flemma, R.J.; Findling, J.W. Inhibition of the Adrenocorticotropin Response to Surgery in Humans: Interaction between Dexamethasone and Fentanyl. J. Clin. Endocrinol. Metab. 1987, 65, 295–298. [Google Scholar] [CrossRef]
- Lin, J.A.; Chen, F.C.; Lee, M.S.; Horng, H.C.; Cherng, C.H.; Yeh, C.C.; Wong, C.S. Intravenous Dexamethasone Pretreatment Reduces Fentanyl-Induced Cough. J. Formos. Med. Assoc. 2007, 106, 649–655. [Google Scholar] [CrossRef]
- Buonanno, P.; Laiola, A.; Palumbo, C.; Spinelli, G.; Servillo, G.; Di Minno, R.M.; Cafiero, T.; Di Iorio, C. Dexamethasone Does Not Inhibit Sugammadex Reversal After Rocuronium-Induced Neuromuscular Block. Anesth. Analg. 2016, 122, 1826–1830. [Google Scholar] [CrossRef]
- Koo, C.H.; Hwang, J.Y.; Min, S.W.; Ryu, J.H. A Meta-Analysis on the Effect of Dexamethasone on the Sugammadex Reversal of Rocuronium-Induced Neuromuscular Block. J. Clin. Med. 2020, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.G.; Won, Y.J.; Kim, H. The Effect of Dexamethasone on Sugammadex Reversal of Rocuronium-Induced Neuromuscular Blockade in Surgical Patients Undergoing General Anesthesia: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e23992. [Google Scholar] [CrossRef] [PubMed]
- Soltész, S.; Fraisl, P.; Noé, K.G.; Hinkelbein, J.; Mellinghoff, H.; Mencke, T. Dexamethasone Decreases the Duration of Rocuronium-Induced Neuromuscular Block: A Randomised Controlled Study. Eur. J. Anaesthesiol. 2014, 31, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R.M.; Pelosi, P. Anti-Inflammatory Properties of Anesthetic Agents. Crit. Care 2017, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, R. Effects of Surgery and Anesthetic Choice on Immunosuppression and Cancer Recurrence. J. Transl. Med. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Y.; Pan, R.; Ding, K.; Chen, R.; Meng, Q. Mechanisms of Cancer Inhibition by Local Anesthetics. Front. Pharmacol. 2021, 12, 770694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, N.; Zhou, S.; Ye, W.; Jing, G.; Zhang, M. Propofol Induces Proliferation and Invasion of Gallbladder Cancer Cells through Activation of Nrf2. J. Exp. Clin. Cancer Res. 2012, 31, 66. [Google Scholar] [CrossRef]
- Chao, M.; Linlin, S.; Juan, W.; Li, D.; Liu, Y.; Cui, X. Propofol Induces Proliferation Partially via Downregulation of P53 Protein and Promotes Migration via Activation of the Nrf2 Pathway in Human Breast Cancer Cell Line MDA-MB-231. Oncol. Rep. 2017, 37, 841–848. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, L.; Li, L.; Zhou, J. Propofol Suppresses Cell Proliferation in Gastric Cancer Cells through NRF2-Mediated Polyol Pathway. Clin. Exp. Pharmacol. Physiol. 2022, 49, 264–274. [Google Scholar] [CrossRef]
- Li, H.; Lu, Y.; Pang, Y.; Li, M.; Cheng, X.; Chen, J. Propofol Enhances the Cisplatin-Induced Apoptosis on Cervical Cancer Cells via EGFR/JAK2/STAT3 Pathway. Biomed. Pharmacother. 2017, 86, 324–333. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.B.; Ye, L.L.; Ma, L.X.; Zou, M.Y.; Cheng, Z.G. Propofol Inhibits Proliferation and Cisplatin Resistance in Ovarian Cancer Cells through Regulating the MicroRNA-374a/Forkhead Box O1 Signaling Axis. Mol. Med. Rep. 2020, 21, 1471. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Tian, M.; Ma, Z.M.; Zhang, L.; Cui, Y.F.; Li, J. long Anesthetic Propofol Epigenetically Regulates Breast Cancer Trastuzumab Resistance through IL-6/MiR-149-5p Axis. Sci. Rep. 2020, 10, 8858. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pan, S.; Jiang, W.; Xue, F.; Zhu, X. Effects of Propofol on the Development of Cancer in Humans. Cell Prolif. 2020, 53, e12867. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Lee, M.S.; Lou, Y.S.; Lai, H.C.; Yu, J.C.; Lu, C.H.; Wong, C.S.; Wu, Z.F. Propofol-Based Total Intravenous Anesthesia Did Not Improve Survival Compared to Desflurane Anesthesia in Breast Cancer Surgery. PLoS ONE 2019, 14, e0224728. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Kim, K.; Jheon, S.; Lee, J.; Do, S.H.; Hwang, J.W.; Song, I.A. Long-Term Oncologic Outcomes for Patients Undergoing Volatile VersusIntravenous Anesthesia for Non-Small Cell Lung Cancer Surgery: A Retrospective Propensity Matching Analysis. Cancer Control. 2018, 25, 1073274818775360. [Google Scholar] [CrossRef]
- Wu, Z.F.; Lee, M.S.; Wong, C.S.; Lu, C.H.; Huang, Y.S.; Lin, K.T.; Lou, Y.S.; Lin, C.; Chang, Y.C.; Lai, H.C. Propofol-Based Total Intravenous Anesthesia Is Associated with Better Survival Than Desflurane Anesthesia in Colon Cancer Surgery. Anesthesiology 2018, 129, 932–941. [Google Scholar] [CrossRef]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904. [Google Scholar] [CrossRef]
- Boland, J.W.; Pockley, A.G. Influence of Opioids on Immune Function in Patients with Cancer Pain: From Bench to Bedside. Br. J. Pharmacol. 2018, 175, 2726. [Google Scholar] [CrossRef]
- Gong, S.; Ying, L.; Fan, Y.; Sun, Z. Fentanyl Inhibits Lung Cancer Viability and Invasion via Upregulation of MiR-331-3p and Repression of HDAC5. Onco. Targets. Ther. 2020, 13, 13131–13141. [Google Scholar] [CrossRef]
- Nomura, Y.; Kawaraguchi, Y.; Sugimoto, H.; Furuya, H.; Kawaguchi, M. Effects of Morphine and Fentanyl on 5-Fluorouracil Sensitivity in Human Colon Cancer HCT116 Cells. J. Anesth. 2014, 28, 298–301. [Google Scholar] [CrossRef]
- Thorne, A.C.; Orazem, J.P.; Shah, N.K.; Matarazzo, D.; Dwyer, D.; Pierri, M.K.; Hoskins, W.J.; Rubin, S.C.; Bedford, R.F. Isoflurane versus Fentanyl: Hemodynamic Effects in Cancer Patients Treated with Anthracyclines. J. Cardiothorac. Vasc. Anesth. 1993, 7, 307–311. [Google Scholar] [CrossRef]
- Korucu, F.C.; Senyigit, E.; Köstek, O.; Demircan, N.C.; Erdogan, B.; Uzunoglu, S.; Cicin, I. A Retrospective Study on Potential Drug Interactions: A Single Center Experience. J. Oncol. Sci. 2018, 4, 80–84. [Google Scholar] [CrossRef]
- Jiang, A.; Zhao, H.; Cai, J.; Jiang, W.G. Possible Effect of Muscle-Relaxant Anaesthetics on Invasion, Adhesion and Migration of Breast Cancer Cells. Anticancer. Res. 2016, 36, 1259–1265. Available online: https://ar.iiarjournals.org/content/36/3/1259.long (accessed on 12 July 2022). [PubMed]
- Jiang, A.; Zhao, H.; Liu, X.; Yu, M.; Chen, J.; Jiang, W.G. Comparison of Different Muscle-Relaxant Anesthetics on Growth, Migration and Invasion of Gastric Cancer Cells. Anticancer. Res. 2017, 37, 4371–4378. Available online: https://ar.iiarjournals.org/content/37/8/4371.long (accessed on 12 July 2022). [PubMed]
- Gong, W.; Martin, T.A.; Sanders, A.J.; Hargest, R.; Jiang, A.; Sun, P.; Jiang, W.G. Influence of Anaesthetics on the Production of Cancer Cell Motogens, Stromal Cell-Derived Factor-1 and Hepatocyte Growth Factor by Fibroblasts. Oncol. Lett. 2021, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Xie, F.; Tao, Y.; Bai, J. Sugammadex Affects GH/GHR’s Signaling Transduction on Muscle Cells by Regulating the Membrane-Localized GHR Level. Turkish J. Biochem. 2022, 47, 333–339. [Google Scholar] [CrossRef]
- Gunduz Gul, G.; Ozer, A.B.; Demirel, I.; Aksu, A.; Erhan, O.L. The Effect of Sugammadex on Steroid Hormones: A Randomized Clinical Study. J. Clin. Anesth. 2016, 34, 62–67. [Google Scholar] [CrossRef]
- Sekar, V. Center for Drug Evaluation and Research Application Number: 022225Orig1s000 Clinical Pharmacology and Biopharmaceutics Review(s). Available online: https://www.semanticscholar.org/paper/CENTER-FOR-DRUG-EVALUATION-AND-RESEARCH-APPLICATION-Sekar/2d13d0cfdfb159c5a741dbfa5a4f5af36da96a0a (accessed on 12 July 2022).
- Kleijn, H.J.; Zollinger, D.P.; van den Heuvel, M.W.; Kerbusch, T. Population Pharmacokinetic–Pharmacodynamic Analysis for Sugammadex-Mediated Reversal of Rocuronium-Induced Neuromuscular Blockade. Br. J. Clin. Pharmacol. 2011, 72, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Hutchinson, M.K.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-Induced Immunosuppression: Mechanisms and Implications for Immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.; Zhang, H.; Xiong, Q.; Cheng, F.; Wang, H.; Luo, J.; Zhang, Y.; Shi, P.; Xu, J.; et al. Dexamethasone Enhances the Lung Metastasis of Breast Cancer via a PI3K-SGK1-CTGF Pathway. Oncogene 2021, 40, 5367–5378. [Google Scholar] [CrossRef]
- Pang, J.M.; Huang, Y.C.; Sun, S.P.; Pan, Y.R.; Shen, C.Y.; Kao, M.C.; Wang, R.H.; Wang, L.H.; Lin, K.T. Effects of Synthetic Glucocorticoids on Breast Cancer Progression. Steroids 2020, 164, 108738. [Google Scholar] [CrossRef] [PubMed]
- Hüppe, T.; Maurer, F.; Sessler, D.I.; Volk, T.; Kreuer, S. Retrospective Comparison of Eleveld, Marsh, and Schnider Propofol Pharmacokinetic Models in 50 Patients. Br. J. Anaesth. 2020, 124, e22–e24. [Google Scholar] [CrossRef] [PubMed]
- Marsh, B.; White, M.; Morton, N.; Kenny, G.N. Pharmacokinetic model driven infusion of propofol in children. Br. J. Anaesth. 1991, 67, 41–48. [Google Scholar] [CrossRef]
- Schnider, T.W.; Minto, C.F.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Shafer, S.L.; Youngs, E.J. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998, 88, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Eleveld, D.J.; Colin, P.; Absalom, A.R.; Struys, M.M.R.F. Pharmacokinetic-pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br. J. Anaesth. 2018, 120, 942–959, Erratum in: Br. J. Anaesth. 2018, 121, 519. [Google Scholar] [CrossRef]
- Przybyłowski, K.; Tyczka, J.; Szczesny, D.; Bienert, A.; Wiczling, P.; Kut, K.; Plenzler, E.; Kaliszan, R.; Grześkowiak, E. Pharmacokinetics and Pharmacodynamics of Propofol in Cancer Patients Undergoing Major Lung Surgery. J. Pharmacokinet. Pharmacodyn. 2015, 42, 111–122. [Google Scholar] [CrossRef]
- Oosten, A.W.; Abrantes, J.A.; Jönsson, S.; De Bruijn, P.; Kuip, E.J.M.; Falcão, A.; Van Der Rijt, C.C.D.; Mathijssen, R.H.J. Treatment with Subcutaneous and Transdermal Fentanyl: Results from a Population Pharmacokinetic Study in Cancer Patients. Eur. J. Clin. Pharmacol. 2016, 72, 459. [Google Scholar] [CrossRef]
- Kaasa, S.; Moksnes, K.; Nolte, T.; Lefebvre-Kuntz, D.; Popper, L.; Kress, H.G. Pharmacokinetics of Intranasal Fentanyl Spray in Patients with Cancer and Breakthrough Pain. J. Opioid Manag. 2010, 6, 17–26. [Google Scholar] [CrossRef]
- Faulkner, C.; de Leeuw, N.H. In Silico Studies of the Interactions between Propofol and Fentanyl Using Gaussian Accelerated Molecular Dynamics. J. Biomol. Struct. Dyn. 2022, 40, 312–324. [Google Scholar] [CrossRef]
- Jackson, R.K.; Liebich, M.; Berry, P.; Errington, J.; Liu, J.; Parker, C.; Moppett, J.; Samarasinghe, S.; Hough, R.; Rowntree, C.; et al. Impact of Dose and Duration of Therapy on Dexamethasone Pharmacokinetics in Childhood Acute Lymphoblastic Leukaemia—A Report from the UKALL 2011 Trial. Eur. J. Cancer 2019, 120, 75–85. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, Q.; Fu, Y.; Tian, X.; An, Q.; Yang, L.; Su, H.; Lu, W.; Hao, C.; Zhou, T. Pharmacokinetic/Pharmacodynamic Modeling of the Anti-Cancer Effect of Dexamethasone in Pancreatic Cancer Xenografts and Anticipation of Human Efficacious Doses. J. Pharm. Sci. 2020, 109, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.Y.; Tsou, M.Y.; Ting, C.K. Response Surface Models in the Field of Anesthesia: A Crash Course. Acta Anaesthesiol. Taiwanica 2015, 53, 139–145. [Google Scholar] [CrossRef] [PubMed]

| STANDARD MODELS | |
|---|---|
| Pk Models for Anesthesia Administration | Application |
| Marsh et al. [64] | Propofol target-controlled infusion (TCI) syringe pumps |
| Schnider et al. [65] | |
| Eleveld et al. [66] | Predicting propofol concentrations and the bispectral index |
| Necessary studies to model Pk/PD of anaesthetics drugs for cancer patients | |
| Objective of the study: | |
| 1. Assess ADMET properties of all anesthetic drugs used in cancer patients (e.g., Gaussian accelerated molecular dynamics) to further understand the interactions between propofol and fentanyl; | |
| 2. Assess the interaction of anesthetics drugs with the chemotherapeutics; | |
| 3. Model tumor growth and assess the involvement of drug anesthesia in its progression and metastasis (e.g., multiparametric imaging data). | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, B.; Mourão, J.; Vale, N. Personalized Medicine for Classical Anesthesia Drugs and Cancer Progression. J. Pers. Med. 2022, 12, 1846. https://doi.org/10.3390/jpm12111846
Costa B, Mourão J, Vale N. Personalized Medicine for Classical Anesthesia Drugs and Cancer Progression. Journal of Personalized Medicine. 2022; 12(11):1846. https://doi.org/10.3390/jpm12111846
Chicago/Turabian StyleCosta, Bárbara, Joana Mourão, and Nuno Vale. 2022. "Personalized Medicine for Classical Anesthesia Drugs and Cancer Progression" Journal of Personalized Medicine 12, no. 11: 1846. https://doi.org/10.3390/jpm12111846
APA StyleCosta, B., Mourão, J., & Vale, N. (2022). Personalized Medicine for Classical Anesthesia Drugs and Cancer Progression. Journal of Personalized Medicine, 12(11), 1846. https://doi.org/10.3390/jpm12111846







