A Theory-Informed Systematic Review of Barriers and Enablers to Implementing Multi-Drug Pharmacogenomic Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
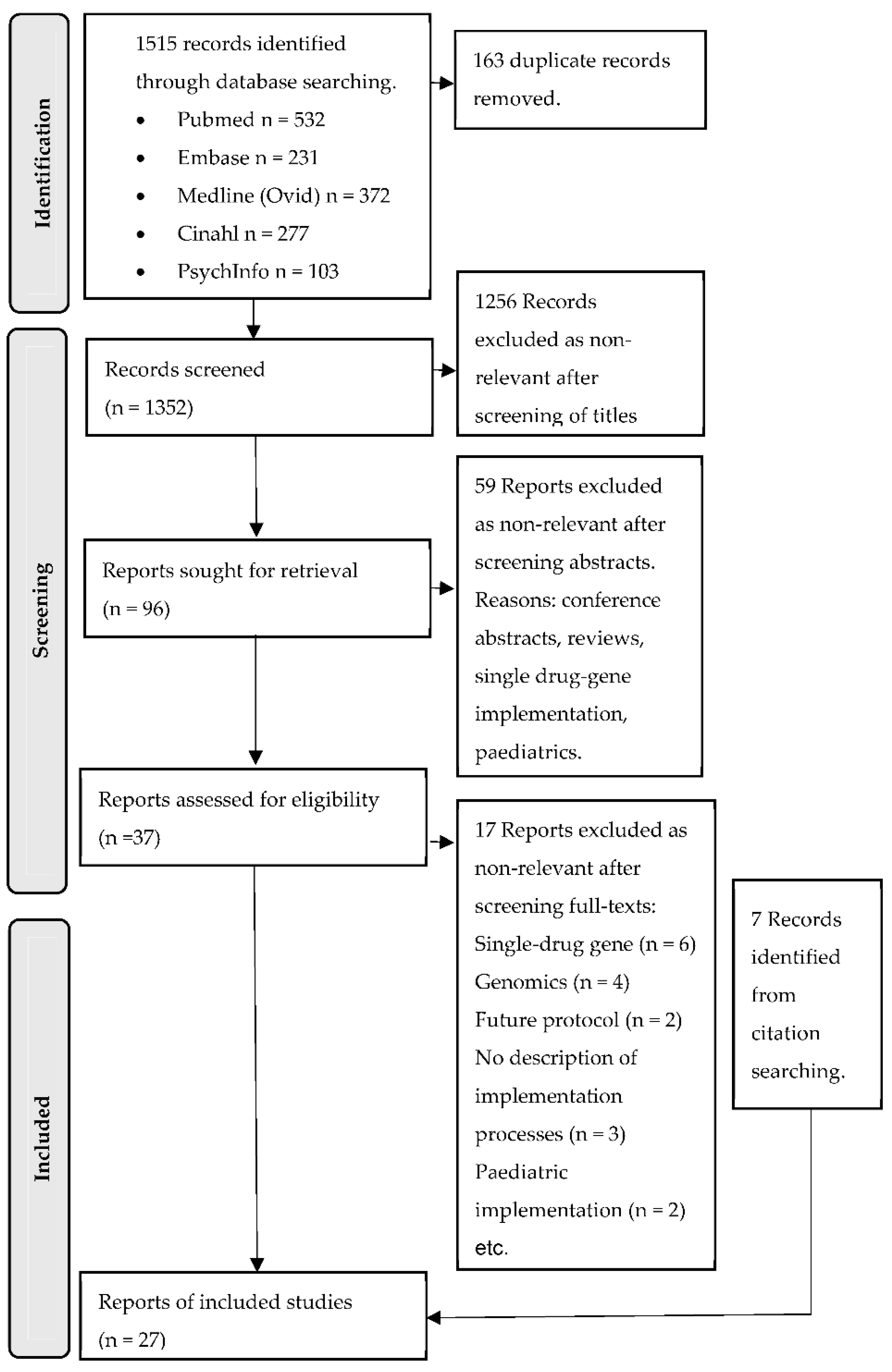
2.3. Screening and Extraction
2.4. Data Synthesis Process
2.5. Quality of Reporting Assessment
3. Results
3.1. Characteristics of Studies
3.2. Target Behaviour Areas
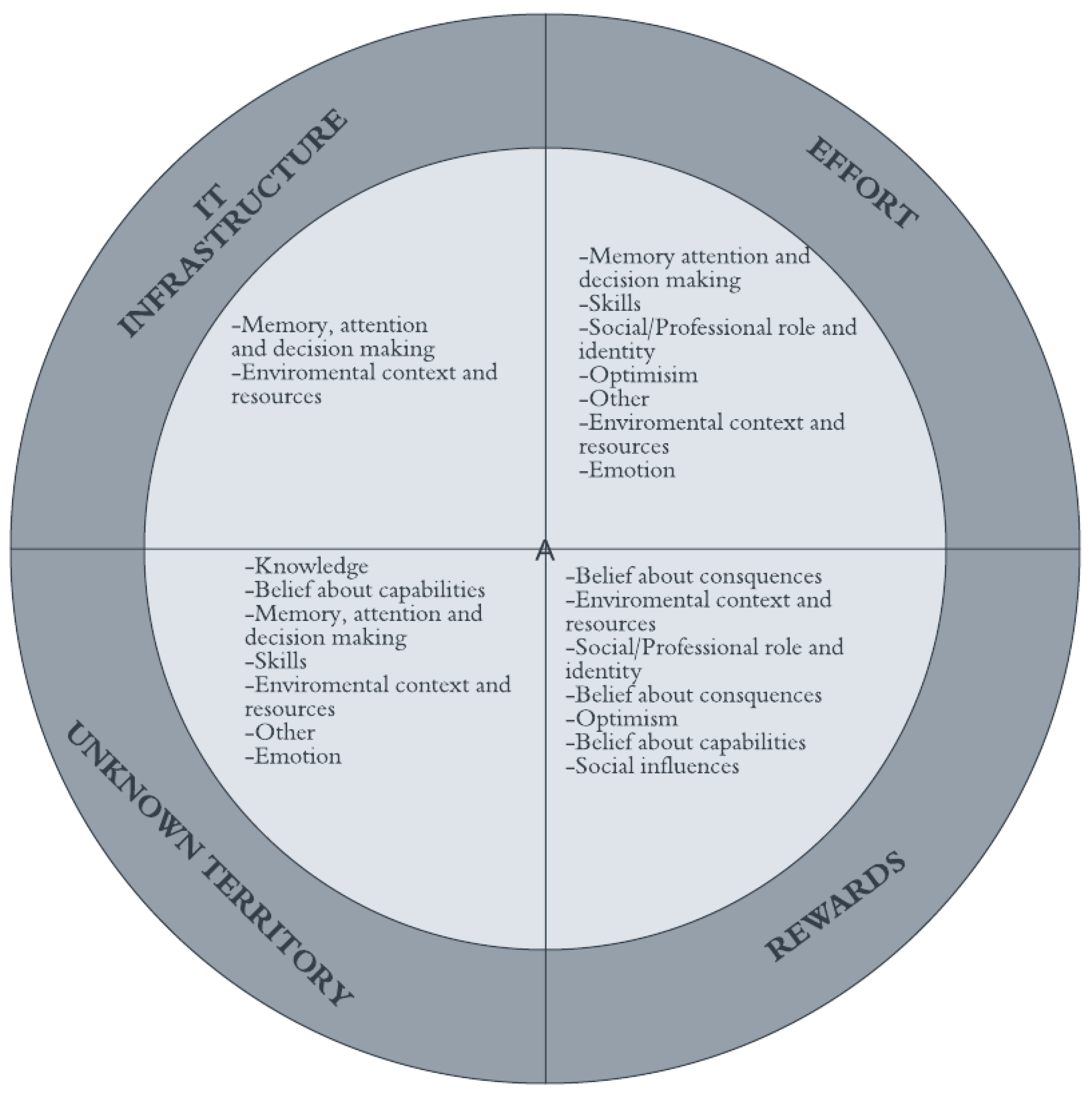
3.3. Themes
3.3.1. IT Infrastructure
3.3.2. Effort
3.3.3. Rewards
3.3.4. Unknown Territory
4. Discussion
4.1. Implications for Future Research
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| PICO Tool a | Search Terms | |||||
|---|---|---|---|---|---|---|
| General Term | PubMed | Ovid EMBASE | Ovid MEDLINE | CINAHL Complete | PsychInfo | |
| Population | Healthcare setting | Primary Health Care [MeSH] OR Secondary Care [MeSH] OR General Practice [MeSH] OR Hospitals [MeSH] OR Pharmacy [MeSH] | Exp Health care delivery [Emtree] OR Exp Primary health care [Emtree] OR Exp Medical care [Emtree] OR Exp health care facility [Emtree] OR EXP Secondary health care [Emtree] OR Exp Pharmacy [Emtree] OR Exp hospital pharmacy [Emtree] | Exp Primary Health Care [MeSH] OR Exp Secondary Care [MeSH]; OR Exp General Practice [MeSH]; OR Exp Hospitals [MeSH] OR Exp Pharmacy [MeSH] | Exp “Health care delivery” OR TX hospital * OR TX pharmacy | MJ “health care service *” OR MA “Secondary Care “[MeSH]; OR MA “General Practice” [MeSH]; OR MA Hospitals [MeSH] OR MA Pharmacy [MeSH] |
| Intervention | PGx testing by doctor or pharmacist or experienced by patient | [Pharmacogenomic testing [MeSH] OR “PGx”.tw; “Pharmacogenetic testing”.tw OR Pharmacogenomic *.tw OR Pharmacogenetic *.tw] AND [Physicians [MeSH] OR Pharmacists [MeSH] OR Patients [MeSH] OR Public.tw OR “service-user *”.tw OR “service user *”.tw OR consumer.tw OR consumers.tw OR customer.tw OR customers] | [Exp Pharmacogenetic testing [Emtree] OR “PGx”.tw OR Pharmacogenomic$.tw OR Pharmacogenetic$.tw] AND [Exp Physician [Emtree] OR Exp Pharmacist [Emtree] OR Exp patient [Emtree] OR Public.tw OR “service-user$”.tw OR “service user$”.tw OR consumer$.tw OR customer$.tw | [Exp Pharmacogenomic testing [MeSH] OR “PGx”.tw; “Pharmacogenetic testing”.tw OR Pharmacogenomic *.tw OR Pharmacogenetic *.tw] AND [Exp Physicians [MeSH] OR Pharmacist *.tw OR Exp Patients [MeSH] OR Public.tw OR “service-user *”.tw OR “service user *”.tw OR consumer *.tw OR customer *] | [AB Pharmacogenetic * OR AB pharmacogenomic * OR AB “PGx”] AND [TX physician * OR TX pharmacist * OR TX nurse * OR MH patients OR TX public OR TX “service user *” OR TX “service-user *”] | [MA “Pharmacogenomic testing” [MeSH] OR AB “PGx” OR AB “Pharmacogenetic testing” OR AB Pharmacogenomic * OR AB Pharmacogenetic *] AND [MA Physicians [MeSH] OR MA Pharmacists [MeSH] OR Patients [MeSH] OR TX Public OR TX “service-user *” OR TX “service user *”] |
| Comparator | n/a | |||||
| Outcome | Implementation captured through the perspective of those who have experience of testing | Implementation.tw OR adoption.tw OR perceive.tw OR perceiving.tw OR perception.tw OR perceptions.tw OR value.tw OR values.tw OR perspective.tw OR perspectives.tw OR view.tw OR views.tw OR experience.tw OR experiences.tw OR need.tw OR needs.tw OR attitude.tw OR attitudes.tw OR belief.tw OR beliefs.tw OR opinion.tw OR opinions.tw OR feelings.tw OR understand.tw | Implementation.tw OR adoption.tw OR perceive$.tw OR perception$.tw OR value$.tw OR perspective$.tw OR view$.tw OR experience$.tw OR need$.tw OR attitude$.tw OR belie$.tw OR opinion$.tw OR feel$.tw OR know$.tw OR understand$.tw | Implementation.tw OR adoption.tw OR perceive *.tw OR perception *.tw OR value *.tw OR perspective *.tw OR view *.tw OR experience *.tw OR need *.tw OR attitude *.tw OR belie *.tw OR opinion *.tw OR feel *.tw OR know *.tw OR understand *.tw | TX Implementation OR TX perceive * OR TX perception * OR TX satisf * OR TX value * OR TX perspective * OR TX view * OR TX experience * OR TX opinion * OR TX TX “consumer satisfaction” OR TX belie * OR MH “patient satisfaction” | TX Implementation OR TX perceive * OR TX perception * OR TX satisf * OR TX value * OR TX perspective * OR TX view * OR TX experience OR TX opinion * OR TX belie * OR MJ “Client attitudes” |
References
- Collins, F.S.; McKusick, V.A. Implications of the Human Genome Project for medical science. JAMA 2001, 285, 540–544. [Google Scholar] [CrossRef]
- Wolf, C.R.; Smith, G.; Smith, R.L. Science, medicine, and the future: Pharmacogenetics. BMJ 2000, 320, 987–990. [Google Scholar] [CrossRef]
- Klein, M.E.; Parvez, M.M.; Shin, J.G. Clinical Implementation of Pharmacogenomics for Personalized Precision Medicine: Barriers and Solutions. J. Pharm. Sci. 2017, 106, 2368–2379. [Google Scholar] [CrossRef]
- Abdullah-Koolmees, H.; van Keulen, A.M.; Nijenhuis, M.; Deneer, V.H.M. Pharmacogenetics Guidelines: Overview and Comparison of the DPWG, CPIC, CPNDS, and RNPGx Guidelines. Front. Pharmacol. 2020, 11, 595219. [Google Scholar] [CrossRef]
- Bousman, C.; Maruf, A.A.; Muller, D.J. Towards the integration of pharmacogenetics in psychiatry: A minimum, evidence-based genetic testing panel. Curr. Opin. Psychiatry 2019, 32, 7–15. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Li, X.; Zhou, B.-t. Barriers and Solutions in Clinical Implementation of Pharmacogenomics for Personalized Medicine. In Pharmacogenomics in Precision Medicine; Springer: Singapore, 2020. [Google Scholar]
- Pratt, V.M.; Cavallari, L.H.; Del Tredici, A.L.; Hachad, H.; Ji, Y.; Moyer, A.M.; Scott, S.A.; Whirl-Carrillo, M.; Weck, K.E. Recommendations for Clinical CYP2C9 Genotyping Allele Selection: A Joint Recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 2019, 21, 746–755. [Google Scholar] [CrossRef]
- Caudle, K.E.; Dunnenberger, H.M.; Freimuth, R.R.; Peterson, J.F.; Burlison, J.D.; Whirl-Carrillo, M.; Scott, S.A.; Rehm, H.L.; Williams, M.S.; Klein, T.E.; et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. 2017, 19, 215–223. [Google Scholar] [CrossRef]
- Gaedigk, A.; Ingelman-Sundberg, M.; Miller, N.A.; Leeder, J.S.; Whirl-Carrillo, M.; Klein, T.E.; PharmVar Steering, C. The Pharmacogene Variation (PharmVar) Consortium: Incorporation of the Human Cytochrome P450 (CYP) Allele Nomenclature Database. Clin. Pharmacol. Ther. 2018, 103, 399–401. [Google Scholar] [CrossRef]
- Tsermpini, E.E.; Al-Mahayri, Z.N.; Ali, B.R.; Patrinos, G.P. Clinical implementation of drug metabolizing gene-based therapeutic interventions worldwide. Hum. Genet. 2021, 141, 1137–1157. [Google Scholar] [CrossRef]
- Best, S.; Long, J.C.; Gaff, C.; Braithwaite, J.; Taylor, N. Investigating the Adoption of Clinical Genomics in Australia. An Implementation Science Case Study. Genes 2021, 12, 317. [Google Scholar] [CrossRef]
- Michie, S.; Johnston, M.; Abraham, C.; Lawton, R.; Parker, D.; Walker, A. Making psychological theory useful for implementing evidence based practice: A consensus approach. Qual. Saf. Health Care 2005, 14, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.; Richardson, M.; Johnston, M.; Ladha, R.; Michie, S. From lists of behaviour change techniques (BCTs) to structured hierarchies: Comparison of two methods of developing a hierarchy of BCTs. Br. J. Health Psychol. 2015, 20, 130–150. [Google Scholar] [CrossRef] [PubMed]
- Birken, S.A.; Powell, B.J.; Presseau, J.; Kirk, M.A.; Lorencatto, F.; Gould, N.J.; Shea, C.M.; Weiner, B.J.; Francis, J.J.; Yu, Y.; et al. Combined use of the Consolidated Framework for Implementation Research (CFIR) and the Theoretical Domains Framework (TDF): A systematic review. Implement Sci. 2017, 12, 2. [Google Scholar] [CrossRef]
- Michie, S.; Johnston, M.; Francis, J.; Hardeman, W.; Eccles, M. From Theory to Intervention: Mapping Theoretically Derived Behavioural Determinants to Behaviour Change Techniques. Appl. Psychol. 2008, 57, 660–680. [Google Scholar] [CrossRef]
- White, S.; Jacobs, C.; Phillips, J. Mainstreaming genetics and genomics: A systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020, 22, 1149–1155. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cohen, J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Center for Evidence Based Management. Critical Appraisal Checklist for a Meta-Analysis or Systematic Review. 2014. Available online: https://www.cebma.org (accessed on 30 June 2022).
- Critical Appraisal Skills Programme (CASP). 10 Questions to Help You Make Sense of Qualitative Research; CASP Qualitative Checklist: Oxford, UK, 2017; Available online: https://casp-uk.net/ (accessed on 30 June 2022).
- Center for Evidence Based Management. Critical Appraisal Checklist for Cross-Sectional Study. July 2014. Available online: https://www.cebma.org (accessed on 30 June 2022).
- Kroopnick, M. AM Last Page. Acad. Med. 2013, 88, 737. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.H.; Bush, A.; Spitz, J.; Danahey, K.; Saner, D.; Das, S.; Cox, N.J.; Ratain, M.J. The 1200 patients project: Creating a new medical model system for clinical implementation of pharmacogenomics. Clin. Pharmacol. Ther. 2012, 92, 446–449. [Google Scholar] [CrossRef]
- Bielinski, S.J.; Olson, J.E.; Pathak, J.; Weinshilboum, R.M.; Wang, L.; Lyke, K.J.; Ryu, E.; Targonski, P.V.; Van Norstrand, M.D.; Hathcock, M.A.; et al. Preemptive genotyping for personalized medicine: Design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin. Proc. 2014, 89, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Levy, K.D.; Decker, B.S.; Carpenter, J.S.; Flockhart, D.A.; Dexter, P.R.; Desta, Z.; Skaar, T.C. Prerequisites to implementing a pharmacogenomics program in a large health-care system. Clin. Pharmacol. Ther. 2014, 96, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Formea, C.M.; Nicholson, W.T.; Vitek, C.R. An inter-professional approach to personalized medicine education: One institution’s experience. PerMed 2015, 12, 129–138. [Google Scholar] [CrossRef]
- Haga, S.B.; Moaddeb, J.; Mills, R.; Patel, M.; Kraus, W.; Allen LaPointe, N.M. Incorporation of pharmacogenetic testing into medication therapy management. Pharmacogenomics 2015, 16, 1931–1941. [Google Scholar] [CrossRef]
- Unertl, K.M.; Jaffa, H.; Field, J.R.; Price, L.; Peterson, J.F. Clinician Perspectives on Using Pharmacogenomics in Clinical Practice. PerMed 2015, 12, 339–347. [Google Scholar] [CrossRef]
- Dawes, M.; Aloise, M.N.; Ang, J.S.; Cullis, P.; Dawes, D.; Fraser, R.; Liknaitzky, G.; Paterson, A.; Stanley, P.; Suarez-Gonzalez, A.; et al. Introducing pharmacogenetic testing with clinical decision support into primary care: A feasibility study. CMAJ Open 2016, 4, E528–E534. [Google Scholar] [CrossRef]
- Dunnenberger, H.M.; Biszewski, M.; Bell, G.C.; Sereika, A.; May, H.; Johnson, S.G.; Hulick, P.J.; Khandekar, J. Implementation of a multidisciplinary pharmacogenomics clinic in a community health system. Am. J. Health Syst. Pharm. 2016, 73, 1956–1966. [Google Scholar] [CrossRef]
- Eadon, M.T.; Desta, Z.; Levy, K.D.; Decker, B.S.; Pierson, R.C.; Pratt, V.M.; Callaghan, J.T.; Rosenman, M.B.; Carpenter, J.S.; Holmes, A.M.; et al. Implementation of a pharmacogenomics consult service to support the INGENIOUS trial. Clin. Pharmacol. Ther. 2016, 100, 63–66. [Google Scholar] [CrossRef]
- St Sauver, J.L.; Bielinski, S.J.; Olson, J.E.; Bell, E.J.; Mc Gree, M.E.; Jacobson, D.J.; McCormick, J.B.; Caraballo, P.J.; Takahashi, P.Y.; Roger, V.L.; et al. Integrating Pharmacogenomics into Clinical Practice: Promise vs. Reality. Am. J. Med. 2016, 129, 1093–1099.e1. [Google Scholar] [CrossRef] [PubMed]
- Rosenman, M.B.; Decker, B.; Levy, K.D.; Holmes, A.M.; Pratt, V.M.; Eadon, M.T. Lessons Learned When Introducing Pharmacogenomic Panel Testing into Clinical Practice. Value Health 2017, 20, 54–59. [Google Scholar] [CrossRef]
- Bain, K.T.; Schwartz, E.J.; Knowlton, O.V.; Knowlton, C.H.; Turgeon, J. Implementation of a pharmacist-led pharmacogenomics service for the Program of All-Inclusive Care for the Elderly (PHARM-GENOME-PACE). J. Am. Pharm. Assoc. (2003) 2018, 58, 281–289.e281. [Google Scholar] [CrossRef]
- Borden, B.A.; Lee, S.M.; Danahey, K.; Galecki, P.; Patrick-Miller, L.; Siegler, M.; Sorrentino, M.J.; Sacro, Y.; Davis, A.M.; Rubin, D.T.; et al. Patient-provider communications about pharmacogenomic results increase patient recall of medication changes. Pharm. J. 2019, 19, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Dressler, L.G.; Bell, G.C.; Abernathy, P.M.; Ruch, K.; Denslow, S. Implementing pharmacogenetic testing in rural primary care practices: A pilot feasibility study. Pharmacogenomics 2019, 20, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Gift, M.; Alexander, E. Prioritizing pharmacogenomics implementation initiates: A survey of healthcare professionals. PerMed 2022, 19, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.; Salloum, R.G.; Elchynski, A.L.; Smith, D.M.; Rowe, E.; Blake, K.V.; Limdi, N.A.; Aquilante, C.L.; Bates, J.; Beitelshees, A.L.; et al. Multisite evaluation of institutional processes and implementation determinants for pharmacogenetic testing to guide antidepressant therapy. Clin. Transl. Sci. 2022, 15, 371–383. [Google Scholar] [CrossRef]
- Arwood, M.J.; Dietrich, E.A.; Duong, B.Q.; Smith, D.M.; Cook, K.; Elchynski, A.; Rosenberg, E.I.; Huber, K.N.; Nagoshi, Y.L.; Wright, A.; et al. Design and early implementation successes and challenges of a pharmacogenetics consult clinic. J. Clin. Med. 2020, 9, 2274. [Google Scholar] [CrossRef]
- Marrero, R.J.; Cicali, E.J.; Arwood, M.J.; Eddy, E.; DeRemer, D.; Ramnaraign, B.H.; Daily, K.C.; Jones, D.; Cook, K.J.; Cavallari, L.H.; et al. How to Transition from Single-Gene Pharmacogenetic Testing to Preemptive Panel-Based Testing: A Tutorial. Clin. Pharmacol. Ther. 2020, 108, 557–565. [Google Scholar] [CrossRef]
- Swen, J.J.; Van Der Straaten, T.; Wessels, J.A.M.; Bouvy, M.L.; Vlassak, E.E.W.; Assendelft, W.J.J.; Guchelaar, H.J. Feasibility of pharmacy-initiated pharmacogenetic screening for CYP2D6 and CYP2C19. Eur. J. Clin. Pharmacol. 2012, 68, 363–370. [Google Scholar] [CrossRef][Green Version]
- Moaddeb, J.; Mills, R.; Haga, S.B. Community pharmacists’ experience with pharmacogenetic testing. J. Am. Pharm. Assoc. JAPhA 2015, 55, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bright, D.; Saadeh, C.; Devuyst-Miller, S.; Sohn, M.; Choker, A.; Langerveld, A. Pharmacist consult reports to support pharmacogenomics report interpretation. Pharm. Pers. Med. 2020, 13, 719–724. [Google Scholar] [CrossRef]
- Haga, S.B.; Mills, R.; Moaddeb, J.; Liu, Y.; Voora, D. Independent community pharmacists’ experience in offering pharmacogenetic testing. Pharm. Pers. Med. 2021, 14, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Lanting, P.; Drenth, E.; Boven, L.; van Hoek, A.; Hijlkema, A.; Poot, E.; van der Vries, G.; Schoevers, R.; Horwitz, E.; Gans, R.; et al. Practical barriers and facilitators experienced by patients, pharmacists and physicians to the implementation of pharmacogenomic screening in Dutch outpatient hospital care-an explorative pilot study. J. Pers. Med. 2020, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Liko, I.; Corbin, L.; Tobin, E.; Aquilante, C.L.; Lee, Y.M. Implementation of a pharmacist-provided pharmacogenomics service in an executive health program. Am. J. Health-Syst. Pharm. 2021, 78, 1094–1103. [Google Scholar] [CrossRef]
- Van der Wouden, C.H.; Paasman, E.; Teichert, M.; Crone, M.R.; Guchelaar, H.J.; Swen, J.J. Assessing the implementation of pharmacogenomic panel-testing in primary care in the Netherlands utilizing a theoretical framework. J. Clin. Med. 2020, 9, 814. [Google Scholar] [CrossRef]
- Martin, J.L.; Lee, Y.M.; Corbin, L.W.; Colson, R.; Aquilante, C.L. Patients’ perspectives of a pharmacist-provided clinical pharmacogenomics service. Pharmacogenomics 2022, 23, 463–474. [Google Scholar] [CrossRef]
- Bielinski, S.J.; St Sauver, J.L.; Olson, J.E.; Wieland, M.L.; Vitek, C.R.; Bell, E.J.; Mc Gree, M.E.; Jacobson, D.J.; McCormick, J.B.; Takahashi, P.Y.; et al. Are patients willing to incur out-of-pocket costs for pharmacogenomic testing? Pharm. J. 2017, 17, 1–3. [Google Scholar] [CrossRef]
- Amare, A.T.; Schubert, K.O.; Tekola-Ayele, F.; Hsu, Y.-H.; Sangkuhl, K.; Jenkins, G.; Whaley, R.M.; Barman, P.; Batzler, A.; Altman, R.B.; et al. Association of the polygenic scores for personality traits and response to selective serotonin reuptake inhibitors in patients with major depressive disorder. Front. Psychiatry 2018, 9, 65. [Google Scholar] [CrossRef]
- McDermott, J.H.; Wright, S.; Sharma, V.; Newman, W.G.; Payne, K.; Wilson, P. Characterizing pharmacogenetic programs using the consolidated framework for implementation research: A structured scoping review. Front Med. 2022, 9, 945352. [Google Scholar] [CrossRef]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.; van Schaik, R.H.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From bench to byte—An update of guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Dunnenberger, H.M.; Kevin Hicks, J.; Caudle, K.E.; Whirl Carrillo, M.; Freimuth, R.R.; Williams, M.S.; Klein, T.E.; Peterson, J.F. Developing knowledge resources to support precision medicine: Principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J. Am. Med. Inform. Assoc. JAMIA 2016, 23, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Roosan, D.; Hwang, A.; Law, A.V.; Chok, J.; Roosan, M.R. The inclusion of health data standards in the implementation of pharmacogenomics systems: A scoping review. Pharmacogenomics 2020, 21, 1191–1202. [Google Scholar] [CrossRef]
- Hinderer, M.; Boeker, M.; Wagner, S.A.; Binder, H.; Uckert, F.; Newe, S.; Hulsemann, J.L.; Neumaier, M.; Schade-Brittinger, C.; Acker, T.; et al. The experience of physicians in pharmacogenomic clinical decision support within eight German university hospitals. Pharmacogenomics 2017, 18, 773–785. [Google Scholar] [CrossRef]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Qureshi, S.; Latif, A.; Condon, L.; Akyea, R.K.; Kai, J.; Qureshi, N. Understanding the barriers and enablers of pharmacogenomic testing in primary care: A qualitative systematic review with meta-aggregation synthesis. Pharmacogenomics 2022, 23, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T. How to improve success of technology projects in health and social care. Public Health Res. Pract. 2018, 28, e2831815. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’Court, C.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond Adoption: A New Framework for Theorizing and Evaluating Nonadoption, Abandonment, and Challenges to the Scale-Up, Spread, and Sustainability of Health and Care Technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.; McDermott, J.; Qureshi, N.; Newman, W. Pharmacogenomic testing to support prescribing in primary care: A structured review of implementation models. Pharmacogenomics 2021, 22, 761–776. [Google Scholar] [CrossRef]
- American Society of Health-System Pharmacists. ASHP Statement on the Pharmacist’s Role in Clinical Pharmacogenomics. Am. J. Health-Syst. Pharm. 2021, 79, 704–707. [Google Scholar]
- FDA. Table of Pharmacogenomic Biomarkers in Drug Labeling. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 30 June 2022).
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, K.; Oliver, A.L. A Fresh Look at How Professions Take Shape: Dual-directed Networking Dynamics and Social Boundaries. Organ. Stud. 2016, 28, 661–687. [Google Scholar] [CrossRef]
- Cavallari, L.H.; Lee, C.R.; Beitelshees, A.L.; Cooper-DeHoff, R.M.; Duarte, J.D.; Voora, D.; Kimmel, S.E.; McDonough, C.W.; Gong, Y.; Dave, C.V.; et al. Multi-site Investigation of Outcomes with Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy after Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018, 11, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, M.; Weale, M.E.; Lewis, C.M. Cost-effectiveness of pharmacogenetic-guided treatment: Are we there yet? Pharm. J. 2017, 17, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Youssef, E.; Kirkdale, C.L.; Wright, D.J.; Guchelaar, H.J.; Thornley, T. Estimating the potential impact of implementing pre-emptive pharmacogenetic testing in primary care across the UK. Br. J. Clin. Pharmacol. 2021, 87, 2907–2925. [Google Scholar] [CrossRef] [PubMed]
- Kimpton, J.E.; Carey, I.M.; Threapleton, C.J.D.; Robinson, A.; Harris, T.; Cook, D.G.; DeWilde, S.; Baker, E.H. Longitudinal exposure of English primary care patients to pharmacogenomic drugs: An analysis to inform design of pre-emptive pharmacogenomic testing. Br. J. Clin. Pharmacol. 2019, 85, 2734–2746. [Google Scholar] [CrossRef]
- Lunenburg, C.A.T.C.; Thirstrup, J.P.; Bybjerg-Grauholm, J.; Bækvad-Hansen, M.; Hougaard, D.M.; Nordentoft, M.; Werge, T.M.; Børglum, A.D.; Mors, O.; Mortensen, P.B.; et al. Pharmacogenetic genotype and phenotype frequencies in a large Danish population-based case-cohort sample. Transl. Psychiatry 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Chanfreau-Coffinier, C.; Hull, L.E.; Lynch, J.A.; Duvall, S.L.; Damrauer, S.M.; Cunningham, F.E.; Voight, B.F.; Matheny, M.E.; Oslin, D.W.; Icardi, M.; et al. Projected Prevalence of Actionable Pharmacogenetic Variants and Level A Drugs Prescribed Among US Veterans Health Administration Pharmacy Users. JAMA Netw. Open 2019, 2, e195345. [Google Scholar] [CrossRef]
- Blagec, K.; Koopmann, R.; Crommentuijn-van Rhenen, M.; Holsappel, I.; van der Wouden, C.H.; Konta, L.; Hong, X.; Steinberger, D.; Just, E.; Swen, J.J.; et al. Implementing pharmacogenomics decision support across seven European countries: The Ubiquitous Pharmacogenomics (U-PGx) project. J. Am. Med. Inform. Assoc. 2018, 25, 893–898. [Google Scholar] [CrossRef]
- Turner, R.M.; Newman, W.G.; Bramon, E.; McNamee, C.; Wong, W.L.; Misbah, S.A.; Hill, S.L.; Caulfield, M.J.; Pirmohamed, M. Pharmacogenomics in the UK National Health Service: Opportunities and challenges. Pharmacogenomics 2020, 21, 1237–1246. [Google Scholar] [CrossRef]
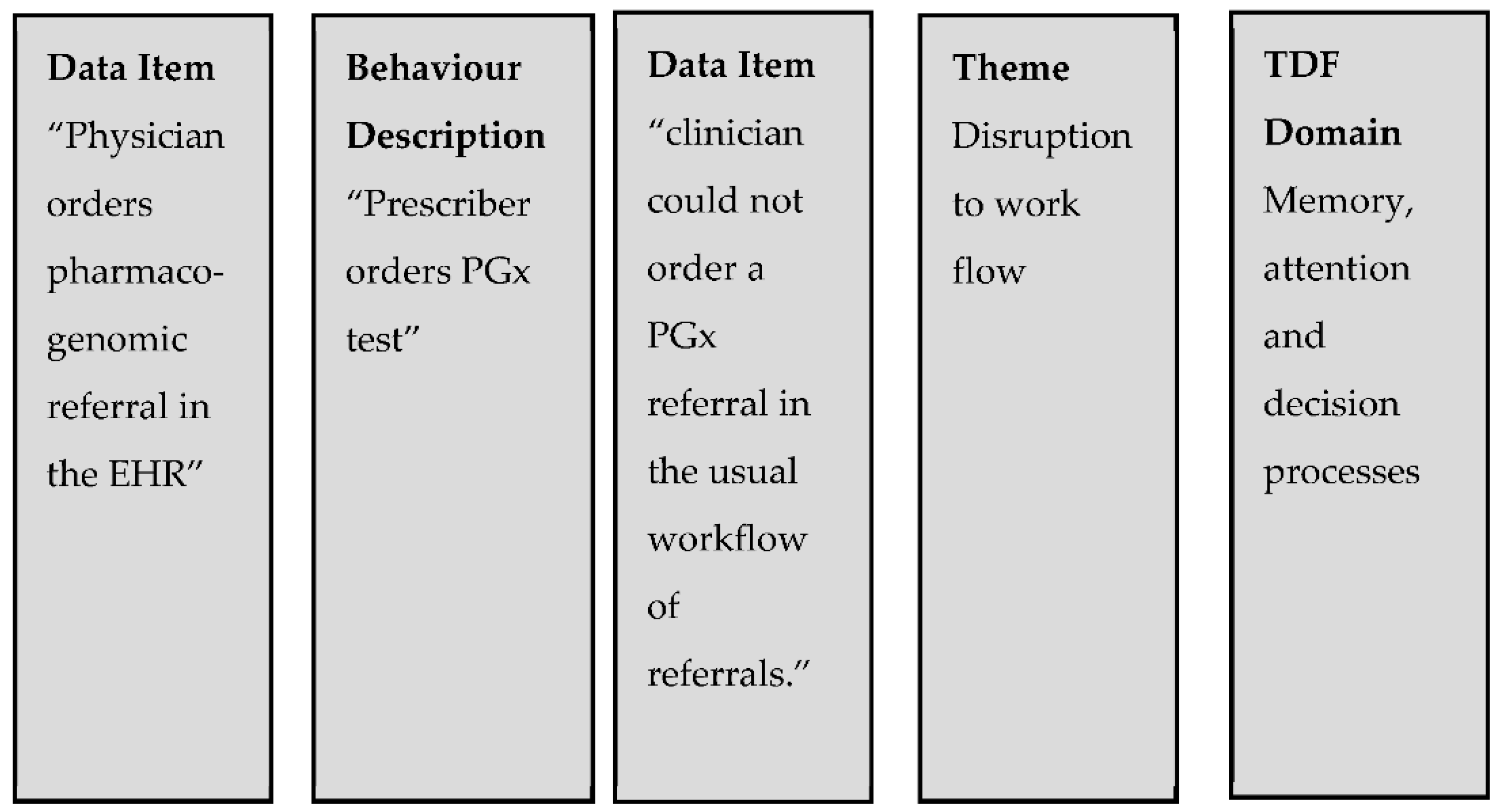
| TDF Domain | TDF Domain Definition [13] | Definition in Context |
|---|---|---|
| Knowledge | An awareness of the existence of something. | Awareness of pharmacogenomics by prescribers, pharmacists, and patients. |
| Skills | An ability or proficiency acquired through practice. | The ability or proficiency prescribers, pharmacists, or patients have acquired to use pharmacogenomics through practice. |
| Social/Professional Role and Identity | A coherent set of behaviours and displayed personal qualities of an individual in a social or work setting. | The perceived professional role and personal identity of prescribers, pharmacists, and patients in relation to using pharmacogenomics. |
| Belief about capabilities | Acceptance of the truth, reality, or validity about an ability, talent, or facility that a person can put to constructive use. | Perception of prescribers, pharmacists, and patients about their own capability to use pharmacogenomics. |
| Optimism | The confidence that things will happen for the best or that desired goals will be attained. | The confidence, or otherwise, of prescribers, pharmacists, or patients around the use of pharmacogenomics in their practice. |
| Belief about consequences | Acceptance of the truth, reality, or validity about outcomes of a behaviour in a given situation. | Belief of prescribers, pharmacists, or patients about the value of using pharmacogenomics in their practice. |
| Reinforcement | Increasing the probability of a response by arranging a dependent relationship, or contingency between the response and a given stimulus. | Incentives, rewards, sanctions, and reinforcement from any level, including patient feedback, clinician perspectives, funding, and external views that facilitate the use of pharmacogenomics in practice. |
| Intentions | A conscious decision to perform a behaviour or a resolve to act in a certain way. | Intentions of prescribers, pharmacists, and patients to consider using pharmacogenomics in their practice. |
| Goals | Mental representations of outcomes or end states that an individual wants to achieve. | Perceptions by prescribers, pharmacists, and patients that pharmacogenomics can be potentially used in their practice. |
| Memory, Attention and Decision Processes | The ability to retain information, focus selectively on aspects of the environment and choose between two or more alternatives. | The ability for prescribers, pharmacists, and patients to remember to consider using pharmacogenomics. |
| Environmental Context and Resources | Any circumstances of a person’s situation or environment that discourages or encourages the development of skills and abilities, independence, social competence, and adaptive behaviour. | Any circumstance of the organisations situation or environment that discourages or encourages the ability of prescribers, pharmacists, or patients to use pharmacogenomics in practice including independence, social competence, and adaptive behaviour. |
| Social Influences | Those interpersonal processes that can cause individuals to change their thoughts, feelings, or behaviours. | Interpersonal interactions within and outside the organisation that can influence the thoughts, feelings, or behaviours of prescribers, pharmacists, or patients in relation to the use of pharmacogenomics. |
| Emotions | A complex reaction pattern, involving experimental, behavioural, and physiological elements, by which the individual attempts to deal with a personally significant matter or event. | Feelings by prescribers, pharmacists, or patients related to the use of pharmacogenomics in their practice. |
| Behavioural Regulation | Anything aimed at managing or changing the objectives of the observed or measured actions. | Anything prescribers, pharmacists, or patients have proactively created to help make decisions about and make changes in using pharmacogenomics. |
| Study (Year) Country | Objective | Study Design | Study Setting | Methods Used | Actor |
| Bain et al. (2018) USA | To determine the feasibility of implementing a pharmacist-led pharmacogenomics (PGx) service. | Feasibility Study. | Primary care. (community pharmacy). | Document analysis. | Prescriber. |
| Formea et al. (2015) USA | To describe experiences of implementing pharmacogenomics education in a large, academic healthcare system. | Descriptive case study. | Primary care. | Senior stakeholder observation. | Prescriber. |
| Bielinski et al. (2017) USA | To assess patient experiences and understanding of pharmacogenomics and pharmacogenomics educational materials. | Service evaluation. | Secondary care. | Survey. | Patient. |
| Dawes et al. (2017) Canada | To assess the ability to obtain and genotype saliva samples and determine levels of use of a pharmacogenomic decision support tool. | Prospective cohort study. | Primary care. | Document analysis. | Prescriber, Pharmacist. |
| O′Donnell et al. (2012) USA | To describe an institutional pharmacogenomics-implementation project. | Descriptive case study. | Secondary care. | Senior stakeholder observation. | Prescriber. |
| Haga et al. (2015) USA | To assess the feasibility of a combined pharmacist-delivered medication therapy management (MTM) with pharmacogenetic (PGx) testing. | Feasibility study. | Primary care. | Document analysis, survey. | Prescriber, Pharmacist. |
| Borden et al. (2019) USA | To understand whether pharmacogenomic results are discussed between patient and provider and whether medication recall is impacted by pharmacogenomic testing. | Service evaluation. | Primary care. | Survey. | Prescriber. |
| Study (Year) Country | Objective | Study Design | Study Setting | Methods Used | Actor |
| Levy et al. (2014) USA | To describe the key requirements to ensure a successful and enduring PGx implementation within a large healthcare system. | Descriptive case study. | Secondary care. | Senior stakeholder observation. | Prescriber, Pharmacist. |
| Dunnenberger et al. (2016) USA | To describe the development and implementation of a multidisciplinary pharmacogenomics clinic within a community-based medical genetics program. | Descriptive case study. | Secondary care. | Senior stakeholder observation. | Prescriber, Pharmacist. |
| Swen et al. (2012) Netherlands | To investigate the feasibility of pharmacy-initiated pharmacogenetic screening in primary care. | Feasibility study. | Primary care. | Document analysis, survey. | Pharmacist. |
| Bielinski et al. (2014) USA | To report the design and implementation of a pre-emptive pharmacogenomics (PGx) testing programme. | Descriptive case study. | Primary care, Secondary care. | Survey. | Patient. |
| Eadon et al. (2016) USA | To describe the formation of a pharmacogenomics consultation service at a safety-net hospital, which predominantly serves low-income, uninsured, and vulnerable populations. | Descriptive case study. | Secondary care. | Document analysis, Senior stakeholder observation. | Prescriber. |
| Unertl et al. (2015) USA | To describe the knowledge and attitudes of clinicians participating in a large pharmacogenomics implementation program. | Process evaluation. | Primary care, Secondary care. | Interviews. | Prescriber. |
| St Sauver et al. (2016) USA | To summarise and describe early clinician experience with pharmacogenomics in the clinical setting. | Service evaluation. | Secondary care. | Survey. | Prescriber. |
| Rosenman et al. (2017) USA | To describe challenges and potential solutions based on a pharmacogenomic testing programme. | Descriptive case study. | Secondary care. | Senior stakeholder observation. | Prescriber, Patient. |
| Study (Year) Country | Objective | Study Design | Study Setting | Methods Used | Actor |
| Moeddeb et al. (2015) USA | To characterise the experiences and feasibility of offering pharmacogenetic (PGx) testing in a community pharmacy. | Feasibility study. | Primary care (community pharmacy). | Document analysis. | Pharmacist, Patient. |
| Dressler et al. (2019) USA | To assess the feasibility and perspectives of pharmacogenetic testing in rural, primary care physician practices. | Feasibility study. | Primary care. | Survey. | Prescriber, Patient. |
| Arwood et al. (2020) USA | To describe the development, workflow, and early implementation challenges associated with a pharmacist pharmacogenetic testing clinic. | Service evaluation. | Secondary care. | Document analysis, Senior stakeholder observation. | Prescriber, Pharmacist. |
| Bright et al. (2020) USA | To evaluate the implementation processes relating to a pharmacist pharmacogenetic testing consult service. | Service evaluation. | Secondary care. | Document analysis, Senior stakeholder observation. | Pharmacist. |
| Haga et al. (2021) USA | To assess pharmacist experiences with delivering pharmacogenetic testing in independent community pharmacies. | Process evaluation. | Primary care. | Survey, Document analysis, semi-structured interviews. | Pharmacist. |
| Lanting et al. (2020) Netherlands | To identify barriers and facilitators to the implementation of an outpatient pharmacogenetic screening service. | Process evaluation. | Secondary care. | Survey, interviews, focus group. | Pharmacist, Patient. |
| Liko et al. (2021) USA | To describe the implementation of a pharmacist-provided pharmacogenomic testing service at an academic medical centre. | Descriptive case study. | Secondary care. | Senior stakeholder observation. | Pharmacist. |
| Marrero et al.(2020) USA | To describe the transition from implementing single-gene testing to a pre-emptive panel-based pharmacogenetic testing service. | Descriptive case study. | Secondary care. | Senior stakeholder observation. | Prescriber, Pharmacist. |
| Study (Year) Country | Objective | Study Design | Study Setting | Methods Used | Actor |
| Tuteja et al. (2021) USA | To evaluate the approaches taken by early adopters to implement a clinical pharmacogenetic testing service. | Service evaluation. | Primary care, Secondary care. | Survey. | Prescriber, Pharmacist. |
| Van der Wouden et al. (2020) Netherlands | To identify pharmacists’ perceived barriers and enablers facilitating the implementation of pharmacist-initiated pharmacogenetic testing in primary care. | Service evaluation. | Primary care. | Interview, Survey. | Pharmacist. |
| Ho et al. (2021) USA | To characterise clinician perceptions, practices, preferences and barriers to integrating pharmacogenomics in a single pharmacogenomic clinic. | Service evaluation. | Secondary care. | Survey. | Prescriber. |
| Martin et al. (2022) USA | To assess the perspectives and experiences of patients participating in a pharmacist-led PGx service. | Service evaluation. | Tertiary care. | Semi-structured interviews. | Patient, Pharmacist. |
| Implementation Step | Description of Behaviour | Theme | TDF Domain | Perspective | Reported Barrier | Reported Enabler |
| Ordering test | Prescriber orders PGx test | IT Infrastructure | Memory, attention, and decision making | Prescriber |
|
|
|
| |||||
| Effort | Memory, attention, and decision making | Prescriber |
|
| ||
|
| |||||
| Skills | Prescriber |
|
| |||
| Social/Professional role and identity | Prescriber |
|
| |||
| Optimism | Prescriber |
|
| |||
| Other | Prescriber |
|
| |||
|
| |||||
| Pharmacist orders PGx test | Rewards | Belief about consequences | Pharmacist |
|
| |
| Unknown territory | Knowledge | Pharmacist |
|
| ||
| Implementation Step | Description of Behaviour | Theme | TDF Domain | Perspective | Reported Barrier | Reported Enabler |
| Ordering test | Prescriber orders PGx test | Rewards | Belief about consequences | Prescriber |
|
|
| Environmental context and resources | Prescriber |
|
| |||
| Prescriber |
|
| ||||
| Unknown territory | Belief about capabilities | Prescriber |
|
| ||
| Memory, attention, and decision making | Prescriber |
|
| |||
| Skills | Prescriber |
|
| |||
| Environmental context and resources | Prescriber |
|
| |||
| Knowledge | Prescriber |
|
| |||
| Prescriber |
|
| ||||
| Other | Prescriber |
|
| |||
| Pharmacist orders PGx test | Effort | Environmental context and resources | Pharmacist |
|
| |
| Rewards | Social/Professional role and identity | Pharmacist |
|
| ||
| Implementation Step | Description of Behaviour | Theme | TDF Domain | Perspective | Reported Barrier | Reported Enabler |
| Facilitating test | HCP collects pts DNA sample | Effort | Skills | Pharmacist |
|
|
| Other | Patient |
|
| |||
| Patient gives consent to PGx test | Effort | Environmental context and resources | Patient |
|
| |
| Emotion | Patient |
|
| |||
| Rewards | Belief about consequences | Patient |
|
| ||
| Optimism | Patient |
|
| |||
| Unknown territory | Emotion | Patient |
|
| ||
|
| |||||
|
| |||||
| Pharmacist shares report with prescriber | IT Infrastructure | Environmental context and resources | Prescriber, Pharmacist |
|
| |
| Implementation Step | Description of Behaviour | Theme | TDF Domain | Perspective | Reported Barrier | Reported Enabler |
| Facilitating the test | HCP counsel′s patient on PGx result | Effort | Environmental context and resources | Prescriber, Pharmacist |
|
|
| Rewards | Environmental context and resources | Patient |
|
| ||
| Unknown territory | Skills | Prescriber |
|
| ||
| Interpretating the test | Pharmacist interprets PGx results | Effort | Social/Professional role and identity | Prescriber, Pharmacist |
|
|
| Prescriber interprets PGx result | Effort | Memory, attention, and decision making | Prescriber |
|
| |
| Emotion | Prescriber |
|
| |||
| Social/Professional role and identity | Prescriber |
|
| |||
| IT Infrastructure | Memory, attention, and decision making | Prescriber |
|
| ||
| Implementation Step | Description of Behaviour | Theme | TDF Domain | Perspective | Reported Barrier | Reported Enabler |
| Application of the test | Prescriber applies PGx result | Effort | Memory, attention and decision making | Prescriber |
|
|
| IT Infrastructure | Environmental context and resources | Prescriber |
|
| ||
| Rewards | Belief about capabilities | Prescriber |
|
| ||
| Belief about consequences | Prescriber |
|
| |||
| Social influences | Prescriber |
|
| |||
| Unknown territory | Environmental context and resources | Prescriber |
|
| ||
| Knowledge | Prescriber |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, E.; Bhattacharya, D.; Sharma, R.; Wright, D.J. A Theory-Informed Systematic Review of Barriers and Enablers to Implementing Multi-Drug Pharmacogenomic Testing. J. Pers. Med. 2022, 12, 1821. https://doi.org/10.3390/jpm12111821
Youssef E, Bhattacharya D, Sharma R, Wright DJ. A Theory-Informed Systematic Review of Barriers and Enablers to Implementing Multi-Drug Pharmacogenomic Testing. Journal of Personalized Medicine. 2022; 12(11):1821. https://doi.org/10.3390/jpm12111821
Chicago/Turabian StyleYoussef, Essra, Debi Bhattacharya, Ravi Sharma, and David J. Wright. 2022. "A Theory-Informed Systematic Review of Barriers and Enablers to Implementing Multi-Drug Pharmacogenomic Testing" Journal of Personalized Medicine 12, no. 11: 1821. https://doi.org/10.3390/jpm12111821
APA StyleYoussef, E., Bhattacharya, D., Sharma, R., & Wright, D. J. (2022). A Theory-Informed Systematic Review of Barriers and Enablers to Implementing Multi-Drug Pharmacogenomic Testing. Journal of Personalized Medicine, 12(11), 1821. https://doi.org/10.3390/jpm12111821






