The Role of Left Ventricular Ejection Fraction and Left Ventricular Outflow Tract Velocity-Time Integral in Assessing Cardiovascular Impairment in Septic Shock
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Study Endpoints
2.3. Transthoracic Echocardiography (TTE) Examination and PiCCO Measurements
- i.
- TTE measurements
- ii.
- PiCCO measurements
2.4. Mathematical Calculation Models for Afterload-Adjusted LVEF and LVOT VTI Values
- i.
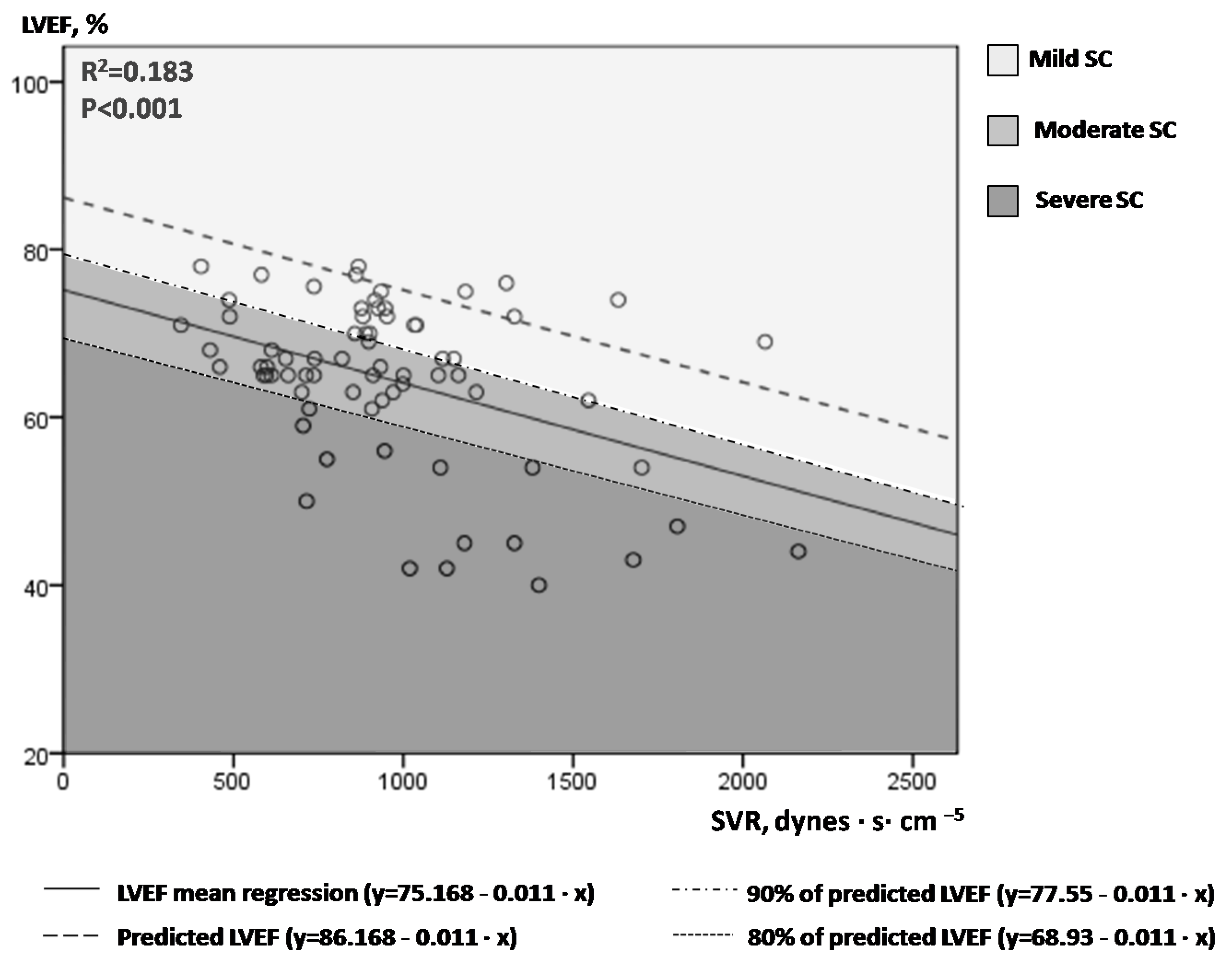
- LVEF plotted against SVR; introduction of the novel index afterload-adjusted LVEF (aLVEF).
- ii.
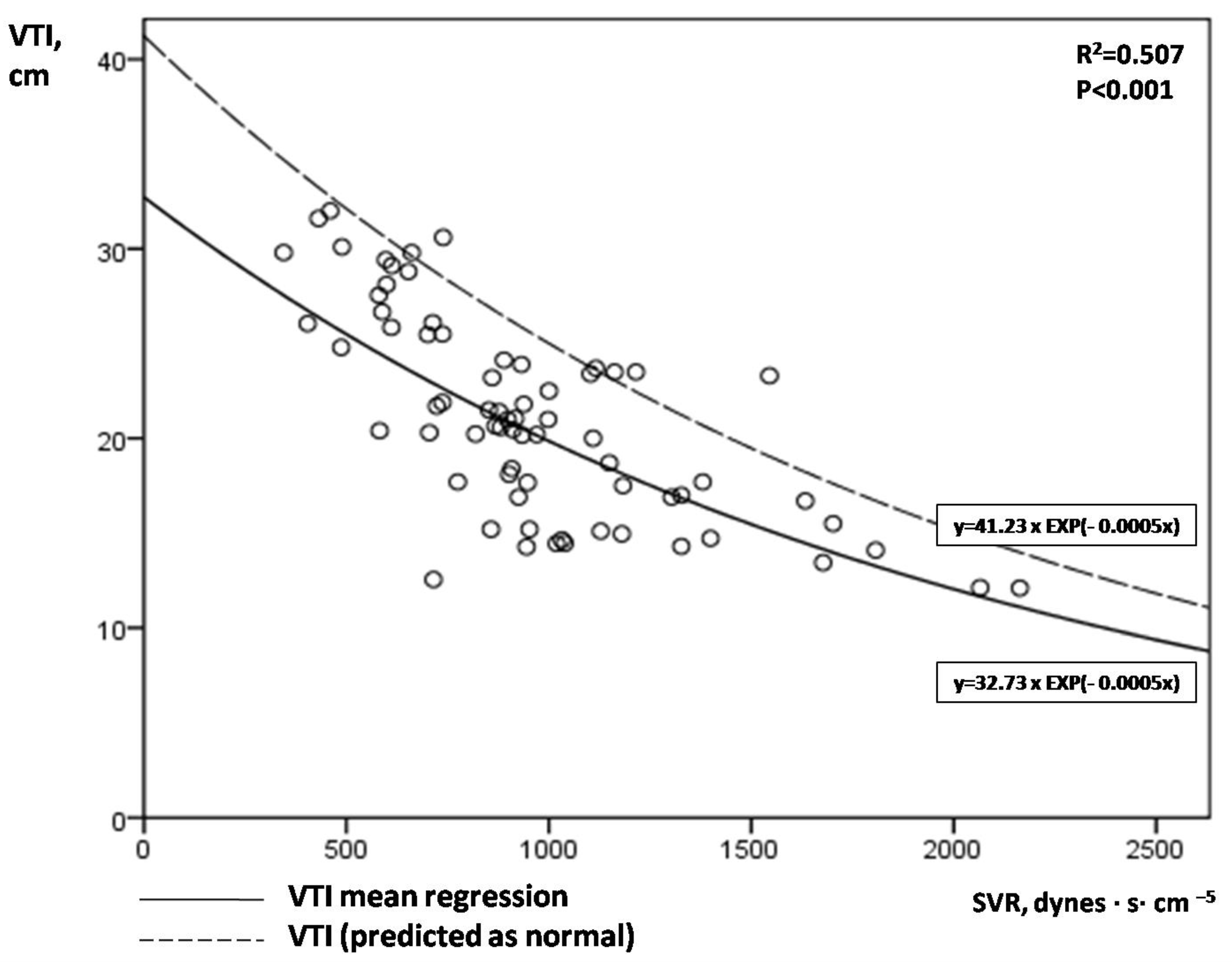
- VTI plotted against SVR; introduction of the novel index afterload-adjusted VTI (aVTI).
2.5. Statistical Analysis
3. Results
- i.
- The role of afterload-adjusted LVEF and VTI indices in SC severity stratification.
- ii.
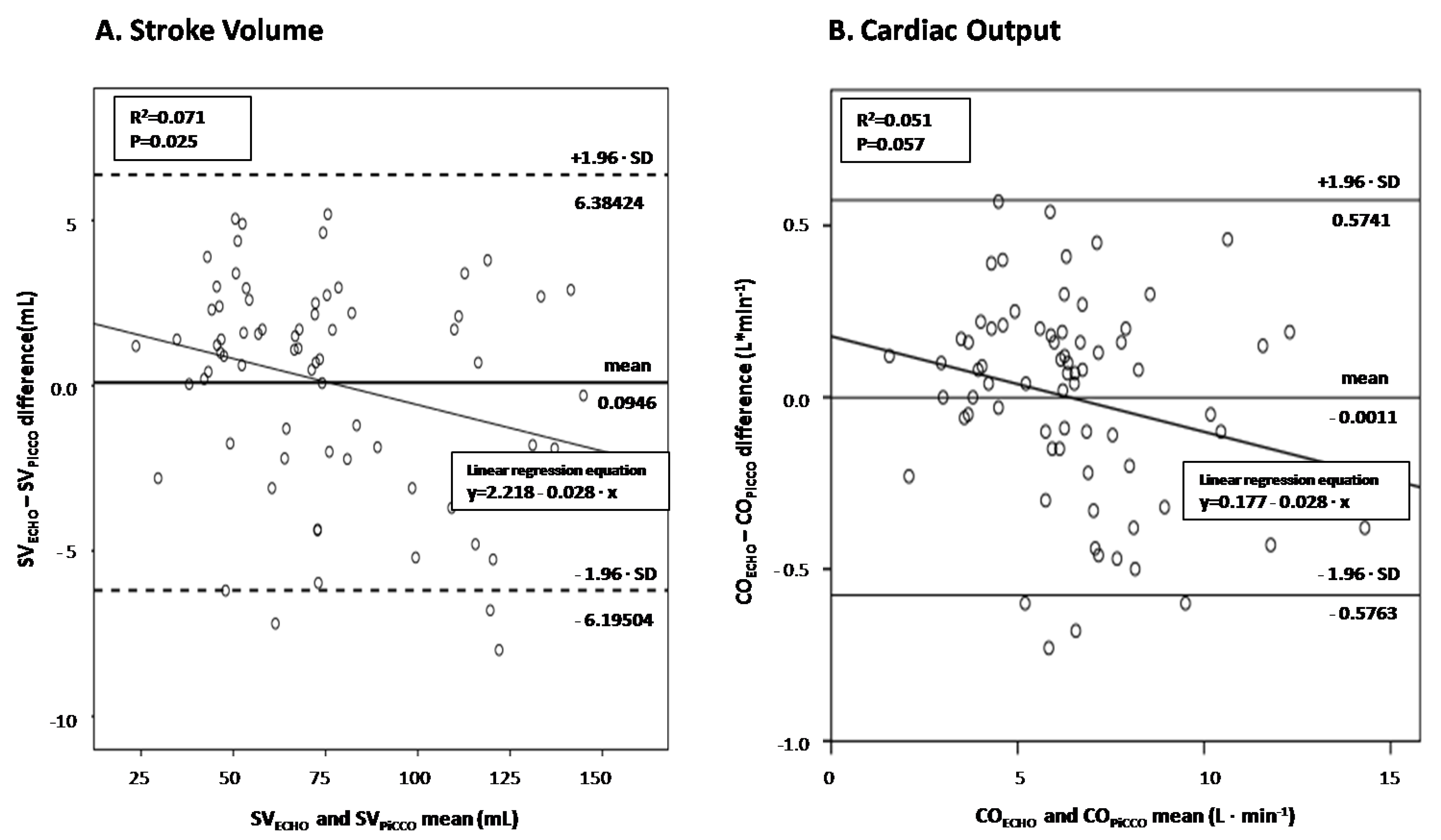
- LVOT VTI in assessing cardiac output in septic shock.
- iii.
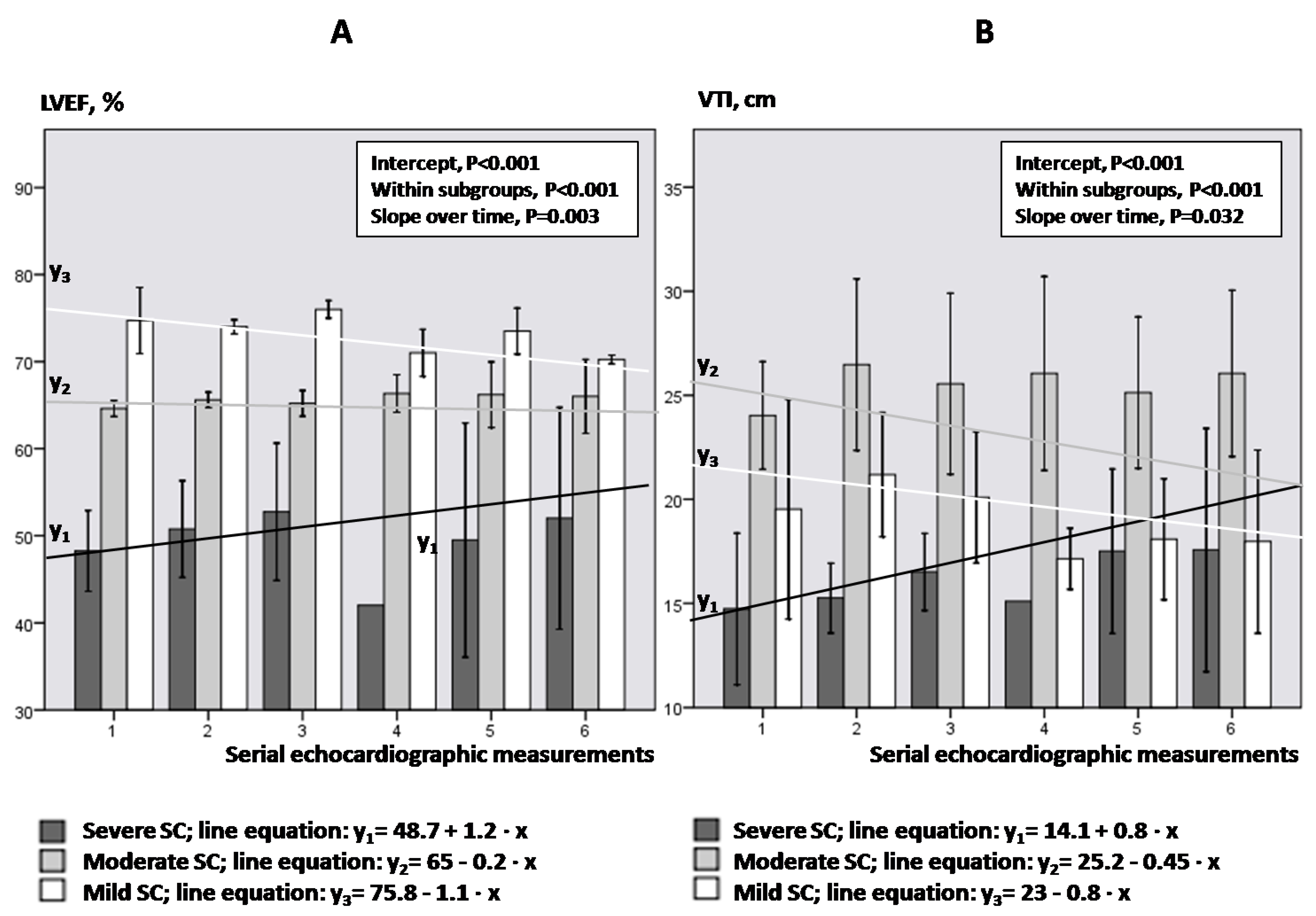
- Kinetics of LVEF and VTI in SC subgroups.
- iv.
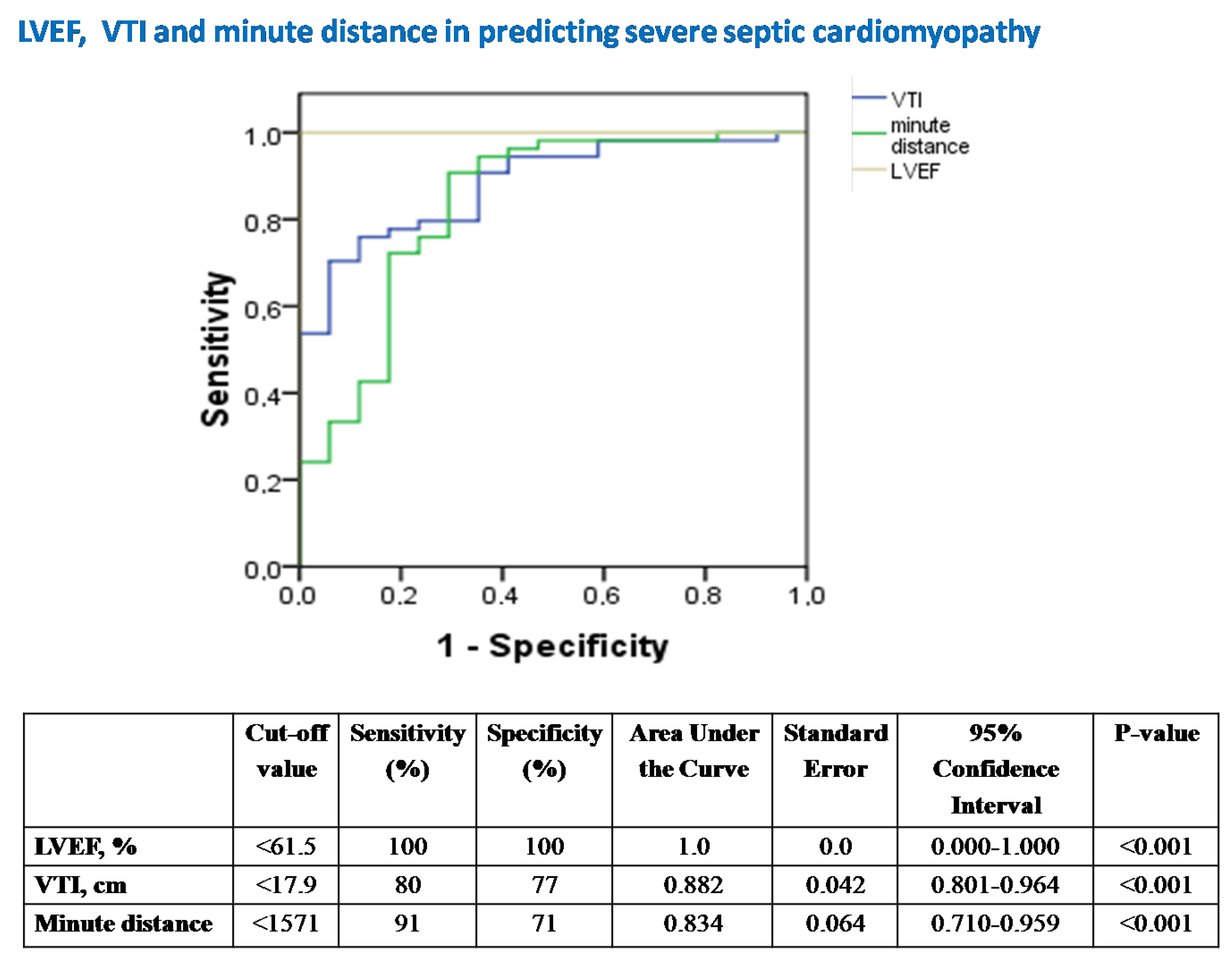
- Conventional echocardiography in assessing severe SC and abnormal SVR.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vieillard-Baron, A.; Millington, S.J.; Sanfilippo, F.; Chew, M.; Diaz-Gomez, J.; McLean, A.; Pinsky, M.R.; Pulido, J.; Mayo, P.; Fletcher, N. A decade of progress in critical care echocardiography: A narrative review. Intensive Care Med. 2019, 45, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Noritomi, D.T.; Vieira, M.L.C.; Mohovic, T.; Bastos, J.F.; Cordioli, R.L.; Akamine, N.; Fischer, C.H. Echocardiography for Hemodynamic Evaluation in the Intensive Care Unit. Shock 2010, 34, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K. Echocardiography as a Noninvasive Swan-Ganz Catheter. Circulation 2005, 111, 3192–3194. [Google Scholar] [CrossRef] [PubMed]
- Burgdorff, A.-M.; Bucher, M.; Schumann, J. Vasoplegia in patients with sepsis and septic shock: Pathways and mechanisms. J. Int. Med. Res. 2018, 46, 1303–1310. [Google Scholar] [CrossRef]
- Hunter, J.D.; Doddi, M. Sepsis and the heart. Br. J. Anaesth. 2010, 104, 3–11. [Google Scholar] [CrossRef]
- Vieillard-Baron, A. Septic cardiomyopathy. Ann. Intensive Care 2011, 1, 6. [Google Scholar] [CrossRef]
- Ciampi, Q.; Villari, B. Role of echocardiography in diagnosis and risk stratification in heart failure with left ventricular systolic dysfunction. Cardiovasc. Ultrasound 2007, 5, 34. [Google Scholar] [CrossRef]
- Tan, C.; Rubenson, D.; Srivastava, A.; Mohan, R.; Smith, M.R.; Billick, K.; Bardarian, S.; Heywood, J.T. Left ventricular outflow tract velocity time integral outperforms ejection fraction and Doppler-derived cardiac output for predicting outcomes in a select advanced heart failure cohort. Cardiovasc. Ultrasound 2017, 15, 18. [Google Scholar] [CrossRef]
- Monnet, X.; Teboul, J.-L. Assessment of volume responsiveness during mechanical ventilation: Recent advances. Crit. Care 2013, 17, 217. [Google Scholar] [CrossRef]
- Maizel, J.; Airapetian, N.; Lorne, E.; Tribouilloy, C.; Massy, Z.; Slama, M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007, 33, 1133–1138. [Google Scholar] [CrossRef]
- Lamia, B.; Ochagavia, A.; Monnet, X.; Chemla, D.; Richard, C.; Teboul, J.-L. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007, 33, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.M.; Shelhamer, J.H.; Bacharach, S.L.; Green, M.V.; Natanson, C.; Frederick, T.M.; Damske, B.A.; Parrillo, J.E. Profound but Reversible Myocardial Depression in Patients with Septic Shock. Ann. Intern. Med. 1984, 100, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Schweller, M.; Gontijo-Coutinho, C.; Franci, D.; Nocera, P.; Guerra-Grangeia, T.; Matos-Souza, J.; Carvalho-Filho, M. Sepsis survivors present with higher values of cardiac index and velocity time integral in the emergency department. Crit. Care 2015, 19, P137. [Google Scholar] [CrossRef]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Boissier, F.; Razazi, K.; Seemann, A.; Bedet, A.; Thille, A.W.; De Prost, N.; Lim, P.; Brun-Buisson, C.; Dessap, A.M. Left ventricular systolic dysfunction during septic shock: The role of loading conditions. Intensive Care Med. 2017, 43, 633–642. [Google Scholar] [CrossRef]
- Repessé, X.; Charron, C.; Vieillard-Baron, A. Evaluation of left ventricular systolic function revisited in septic shock. Crit. Care 2013, 17, 164. [Google Scholar] [CrossRef]
- Bergenzaun, L.; Gudmundsson, P.; Öhlin, H.; Düring, J.; Ersson, A.; Ihrman, L.; Willenheimer, R.; Chew, M.S. Assessing left ventricular systolic function in shock: Evaluation of echocar-diographic parameters in intensive care. Crit. Care 2011, 15, R200. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Moreno, R.; Vincent, J.-L.; Matos, R.T.; Mendonça, A.; Cantraine, F.; Thijs, L.; Takala, J.; Sprung, C.; Antonelli, M.; Bruining, H.; et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive Care Med. 1999, 25, 686–696. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance defnition of health care-associated infection and criteria for specifc types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Caille, V.; Charron, C.; Belliard, G.; Page, B.; Jardin, F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit. Care Med. 2008, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. J. Am. Soc. Echocardiogr. 2009, 22, 442. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P. Rationale for using the velocity–time integral and the minute distance for assessing the stroke volume and cardiac output in point-of-care settings. Ultrasound J. 2020, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Shiran, A.; Adawi, S.; Ganaeem, M.; Asmer, E. Accuracy and reproducibility of left ventricular outflow tract diameter measurement using transthoracic when compared with transesophageal echocardiography in systole and diastole. Eur. J. Echocardiogr. 2008, 10, 319–324. [Google Scholar] [CrossRef]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echo-cardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. J. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Pulsion Medical Systems, PiCCO2 Technical Datasheet, © PULSION EN 03/2013 March 2013 Art. No.: PC856EN_R11.
- Renner, L.E.; Morton, M.J.; Sakuma, G.Y. Indicator amount, temperature, and intrinsic cardiac output affect thermodilution cardiac output accuracy and reproducibility. Crit. Care Med. 1993, 21, 586–597. [Google Scholar] [CrossRef]
- Goldman, J.H.; Schiller, N.B.; Lim, D.C.; Redberg, R.F.; Foster, E. Usefulness of stroke distance by echocardiography as a surrogate marker of cardiac output that is independent of gender and size in a normal population. Am. J. Cardiol. 2001, 87, 499–502. [Google Scholar] [CrossRef]
- Bouhemad, B.; Nicolas-Robin, A.; Arbelot, C.; Arthaud, M.; Féger, F.; Rouby, J.-J. Acute left ventricular dilatation and shock-induced myocardial dysfunction. Crit. Care Med. 2009, 37, 441–447. [Google Scholar] [CrossRef]
- Huang, S.J.; Nalos, M.; McLean, A.S. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit. Care 2013, 17, R96. [Google Scholar] [CrossRef] [PubMed]
- Jardin, F.; Brun-Ney, D.; Auvert, B.; Beauchet, A.; Bourdarias, J.P. Sepsis-related cardiogenic shock. Crit. Care Med. 1990, 18, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Ehrman, R.R.; Sullivan, A.N.; Favot, M.J.; Sherwin, R.L.; Reynolds, C.; Abidov, A.; Levy, P.D. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: A review of the literature. Crit. Care 2018, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, J.; Makris, D.; Mpaka, M.; Palli, E.; Zygoulis, P.; Zakynthinos, E. New insights into the mechanisms involved in B-type natriuretic peptide elevation and its prognostic value in septic patients. Crit. Care 2014, 18, R94. [Google Scholar] [CrossRef] [PubMed]
- Israel, G.D. Determining Sample Size. University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS, Florida, FL, USA, 1992.
- Via, G.; Price, S.; Storti, E. Echocardiography in the sepsis syndromes. Crit. Ultrasound J. 2011, 3, 71–85. [Google Scholar] [CrossRef]

| Patient Number | Sex | Age | APACHE II | Underlying Disease | Admitting Etiology | SOFA Score | Noradren. Dose, μg/kg/min | Isolated Pathogen | Outcome of Shock Episode | |
|---|---|---|---|---|---|---|---|---|---|---|
| Blood Culture | Other-Site Culture | |||||||||
| 1 | M | 57 | 18 | AH | ICH | 7.7 ± 0.3 | 0.21 ± 0.07 | - | PsA (BS) | Survived |
| 2 | M | 55 | 22 | AH | ICH | 10.3 ± 0.3 | 0.32 ± 0.05 | AB | - | Survived |
| 3 (3 meas.) | M | 72 | 24 | AH | Bladder cancer | 11 ± 0 | 1.06 ± 0.27 | CA | KP (BS) | Died (day 2) |
| 4 | M | 82 | 19 | AH, AAA | Fecal peritonitis | 10.7 ± 0.3 | 0.09 ± 0.02 | AB | AB (BS) | Survived |
| 5 | F | 75 | 17 | COPD, DM | ARDS | 8 ± 0 | 0.55 ± 0.2 | - | AB (BS) | Survived |
| 6 | M | 65 | 15 | AH | ICH | 8.7 ± 0.3 | 0.14 ± 0.03 | - | PsA (BS) | Survived |
| 7 (5 meas.) | F | 56 | 21 | - | Meningioma post-surgery | 10.3 ± 0.3 | 0.35 ± 0.04 | PM | - | Died (day 3) |
| 8 | F | 81 | 15 | CAD, Ischemic stroke | DTAApost-surgery | 8 ± 0 | 0.84 ± 0.12 | AB | - | Survived |
| 9 | M | 72 | 22 | AH, DM | aSAH | 8.3 ± 0.3 | 0.2 ± 0.04 | AB | - | Survived |
| 10 | F | 62 | 17 | DD | Fecal peritonitis | 11.3 ± 0.9 | 2.36 ± 0.3 | Espp. | - | Survived |
| 11 | F | 65 | 18 | HL | ARDS | 12 ± 0.6 | 1.04 ± 0.13 | - | AB (BS) | Survived |
| 12 (2 meas.) | M | 82 | 25 | AH, CAD | Pancreatitis | 15 | 1.01 | PsA | AB (PLF, PF) | Died (day 2) |
| 13 (1 meas.) | F | 78 | 16 | Colon Cancer | Liver Abscess | 14 | 1.76 | KPC | - | Died (day 1) |
| 14 | F | 67 | 19 | Asthma | ARDS | 8.7 ± 0.3 | 0.38 ± 0.05 | - | KP | Survived |
| Patient Number | LVEF, % | VTI, cm | CO, L/min | SVR,Dynes·s·cm−5 | EVLW, mL | ScvO2, % | CVP, mmHg |
|---|---|---|---|---|---|---|---|
| 1 | 67.7 ± 2.2 | 29.4 ± 1.7 | 10.7 ± 0.6 | 554 ± 79.9 | 742 ± 63.1 | 84.3 ± 1.3 | 8.7 ± 1.5 |
| 2 | 64 ± 0.6 | 24 ± 1.5 | 7.2 ± 0.1 | 812 ± 93.6 | 635 ± 82.4 | 70.8 ± 1 | 6 ± 1.1 |
| 3 (3 meas.) | 53 ± 1.5 | 15.2 ± 1.5 | 4.5 ± 0.8 | 1064 ± 319.5 | 556 ± 38.4 | 74.9 ± 3.4 | 10 ± 2.5 |
| 4 | 73.9 ± 1.4 | 18.9 ± 2.2 | 6.4 ± 0.4 | 901 ± 86.7 | 537.3 ± 58.2 | 76.4 ± 1.3 | 5 ± 2 |
| 5 | 64 ± 1 | 23 ± 0.3 | 5.4 ± 0.6 | 1216 ± 167.1 | 475.3 ± 74.5 | 73.3 ± 1.2 | 9.7 ± 0.9 |
| 6 | 70.7 ± 4.1 | 21.3 ± 0.9 | 6.9 ± 0.4 | 903.7 ± 33.7 | 724 ± 10.1 | 76.6 ± 0.6 | 12 ± 1.1 |
| 7 (5 meas.) | 73 ± 2 | 15.2 ± 1.6 | 4.1 ± 0.3 | 1667 ± 220.6 | 574.3 ± 67.4 | 55.6 ± 4.8 | 8.3 ± 2.6 |
| 8 | 42.3 ± 1.5 | 14.5 ± 0.1 | 3.8 ± 0.2 | 1248.3 ± 116.5 | 519.7 ± 39.9 | 73.8 ± 39.9 | 7.3 ± 0.3 |
| 9 | 65.3 ± 0.3 | 26.7 ± 0.5 | 7.9 ± 0.1 | 593 ± 9.3 | 520.6 ± 27.2 | 81.6 ± 1.3 | 11 ± 1 |
| 10 | 55 ± 6.7 | 17.8 ± 3.3 | 2.8 ± 0.7 | 1553 ± 314.3 | 990.3 ± 15.7 | 79.8 ± 1.7 | 9 ± 1.5 |
| 11 | 74 ± 3.5 | 20.4 ± 0.1 | 7.4 ± 0.6 | 756.3 ± 88.3 | 751.7 ± 51 | 81.7 ± 2.3 | 5 ± 1.1 |
| 12 (2 meas.) | 76 | 25.4 | 13 | 445.5 | 846.5 | 76.7 | 6.5 |
| 13 (1 meas.) | 73 | 16.9 | 6.9 | 925 | 462 | 65 | 7 |
| 14 | 58 ± 2 | 19.6 ± 0.6 | 6 ± 0.3 | 907.3 ± 116.6 | 868 ± 8.1 | 71.3 ± 1.3 | 3.7 ± 1.5 |
| Severe SC aLVEF < 80%N = 17 | Moderate SC80 ≤ aLVEF < 90%N = 30 | Mild SCaLVEF ≥ 90%N = 24 | p-Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| HR, beats/minute | 88. ± 3.6 | 78 ± 1.6 | 98 ± 2.9 | <0.001 a,b,c |
| MAP, mmHg | 66.9 ± 3.1 | 78.1 ± 1.9 | 80.6 ± 2 | <0.001 a,b |
| SOFA score | 11.2 ± 0.6 | 9.1 ± 0.4 | 9.3 ± 0.4 | 0.007 a,b |
| Noradrenaline dose, μg/kg/min | 0.9 ± 1.3 | 0.55 ± 0.13 | 0.57 ± 0.15 | 0.29 |
| CTnI, ng/mL | 1.22 ± 0.53 | 0.2 ± 0.07 | 0.41 ± 0.19 | 0.011 a,b |
| BNP, pg/mL | 1085 ± 284 | 901 ± 417 | 2006 ± 846 | 0.352 |
| PiCCO-derived Hemodynamics | ||||
| Stroke volume, mL | 48.3 ± 3.2 | 97.7 ± 5.3 | 65.8 ± 4.2 | <0.001 a,c |
| Cardiac Output, L/min | 4.3 ± 0.4 | 7.5 ± 0.4 | 6.5 ± 0.5 | <0.001 a,b |
| SVR, dynes·s·cm−5 | 1215 ± 104 | 799 ± 49 | 990 ± 71 | 0.001 a |
| EVLW, mL | 718 ± 50 | 660 ± 29 | 669 ± 31 | 0.516 |
| ELWI, mL/kg | 10.1 ± 0.9 | 8 ± 0.5 | 8.2 ± 0.4 | 0.035 a |
| ScvO2, % | 76.5 ± 1.9 | 78.9 ± 1.4 | 75.4 ± 2.8 | 0.452 |
| CVP, mmHg | 7 ± 0.8 | 8.9 ± 0.5 | 8.2 ± 0.7 | 0.154 |
| Echocardiographic characteristics | ||||
| VTI, cm | 16 ± 0.7 | 25.5 ± 0.7 | 19 ± 0.7 | <0.001 a,b,c |
| Stroke volume, mL | 47.9 ± 2.9 | 97 ± 5.1 | 67.3 ± 4 | <0.001 a,b,c |
| Cardiac Output, L/min | 4.33 ± 0.3 | 7.5 ± 0.4 | 6.6 ± 0.5 | <0.001 a,b |
| Minute Distance, cm/min | 1416 ± 89 | 1978 ± 50 | 1852 ± 83 | <0.001 a,b |
| aVTI, % | 71.2 ± 3 | 92 ± 1.8 | 74.8 ± 2 | <0.001 a,c |
| LVEDV, mL | 95.5 ± 4.8 | 147.2 ± 7.3 | 91.5 ± 5 | <0.001 a,c |
| LVEF, % | 50.1 ± 1.7 | 65.7 ± 0.4 | 73.2 ± 0.6 | <0.001 a,b,c |
| E/Em | 7.7 ± 0.3 | 7.1 ± 0.1 | 7.2 ± 0.1 | 0.129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spathoulas, K.; Tsolaki, V.; Zakynthinos, G.E.; Karelas, D.; Makris, D.; Zakynthinos, E.; Papanikolaou, J. The Role of Left Ventricular Ejection Fraction and Left Ventricular Outflow Tract Velocity-Time Integral in Assessing Cardiovascular Impairment in Septic Shock. J. Pers. Med. 2022, 12, 1786. https://doi.org/10.3390/jpm12111786
Spathoulas K, Tsolaki V, Zakynthinos GE, Karelas D, Makris D, Zakynthinos E, Papanikolaou J. The Role of Left Ventricular Ejection Fraction and Left Ventricular Outflow Tract Velocity-Time Integral in Assessing Cardiovascular Impairment in Septic Shock. Journal of Personalized Medicine. 2022; 12(11):1786. https://doi.org/10.3390/jpm12111786
Chicago/Turabian StyleSpathoulas, Konstantinos, Vasiliki Tsolaki, George E. Zakynthinos, Dimitrios Karelas, Demosthenes Makris, Epaminondas Zakynthinos, and John Papanikolaou. 2022. "The Role of Left Ventricular Ejection Fraction and Left Ventricular Outflow Tract Velocity-Time Integral in Assessing Cardiovascular Impairment in Septic Shock" Journal of Personalized Medicine 12, no. 11: 1786. https://doi.org/10.3390/jpm12111786
APA StyleSpathoulas, K., Tsolaki, V., Zakynthinos, G. E., Karelas, D., Makris, D., Zakynthinos, E., & Papanikolaou, J. (2022). The Role of Left Ventricular Ejection Fraction and Left Ventricular Outflow Tract Velocity-Time Integral in Assessing Cardiovascular Impairment in Septic Shock. Journal of Personalized Medicine, 12(11), 1786. https://doi.org/10.3390/jpm12111786









