Neutralization Assessments Reveal High Cardiothoracic Ratio and Old Age as Independent Predictors of Low Neutralizing Antibody Titers in Hemodialysis Patients Receiving a Single Dose of COVID-19 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Sample Collection
2.3. Immunogenicity Response Assessment
2.4. Quantifying nAbs by a Two-Variable Generalized Additive Model
2.5. WHO International Standard Unit (IU) Conversion
2.6. Statistical Analysis
3. Results
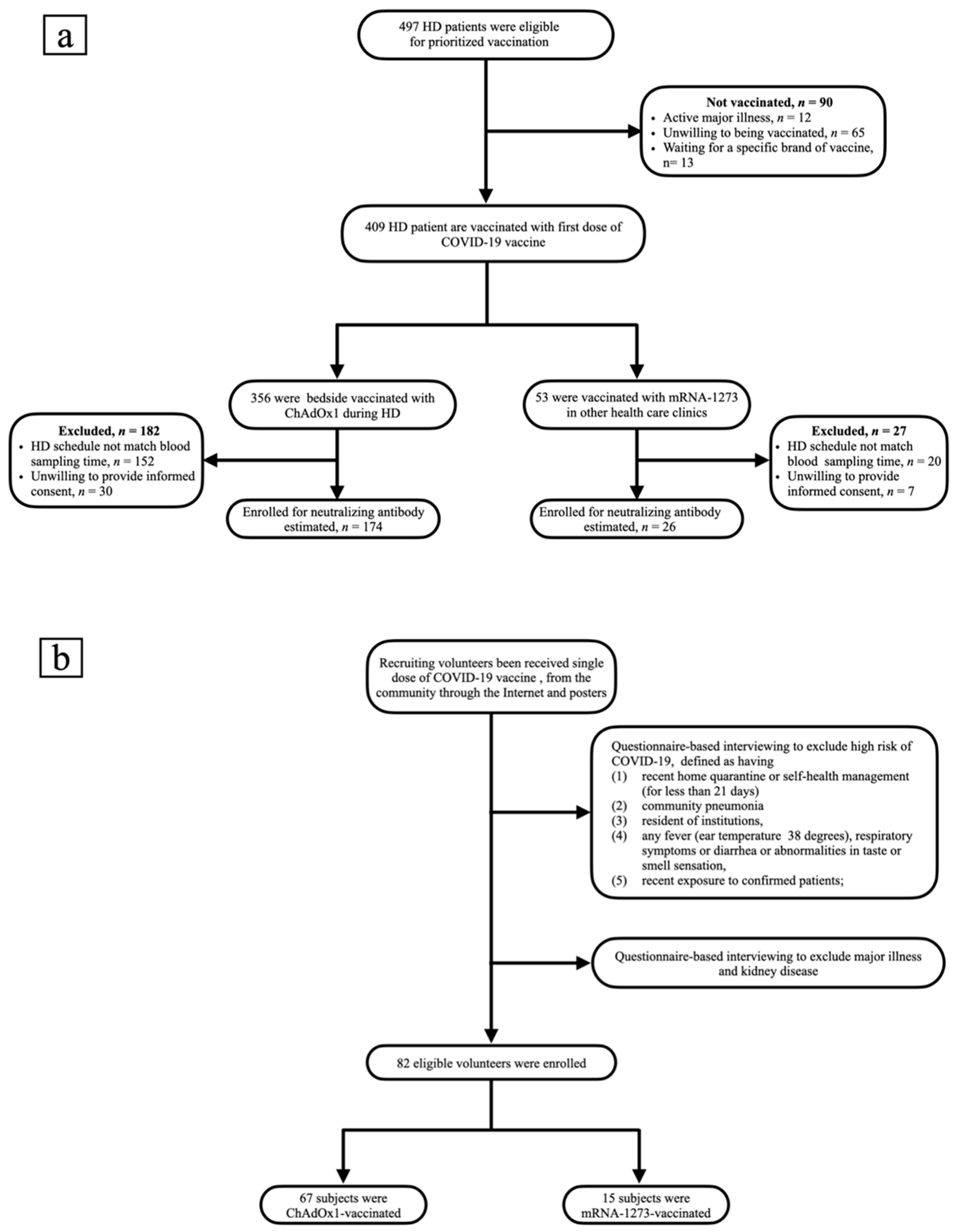
3.1. Study Design and Subject Characteristics
3.2. NT50 and Clinical Characteristics of ChAdOx1-Vaccinated HD Patients
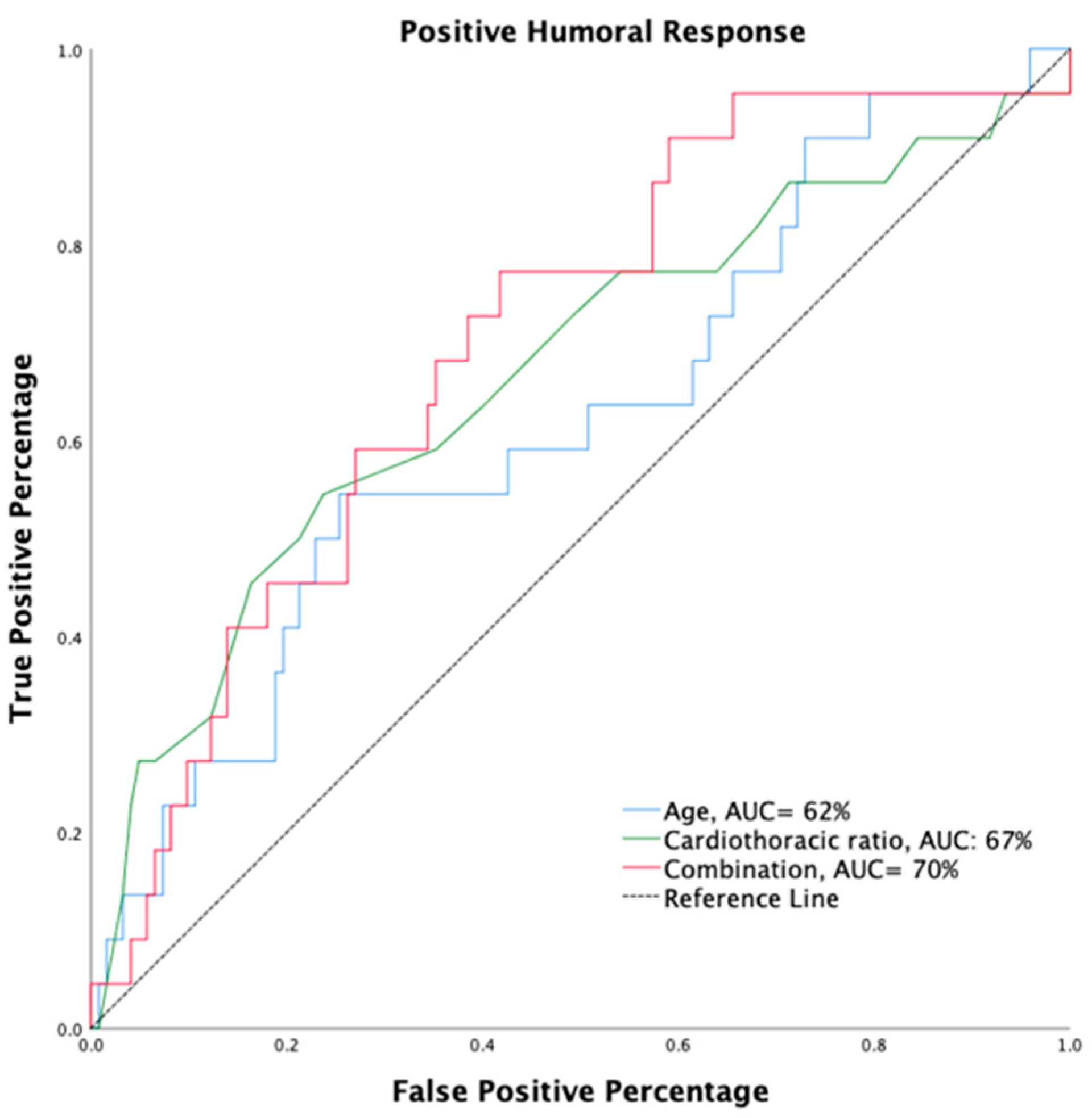
3.3. Predictors Associated with Positive Humoral Response in HD Patients
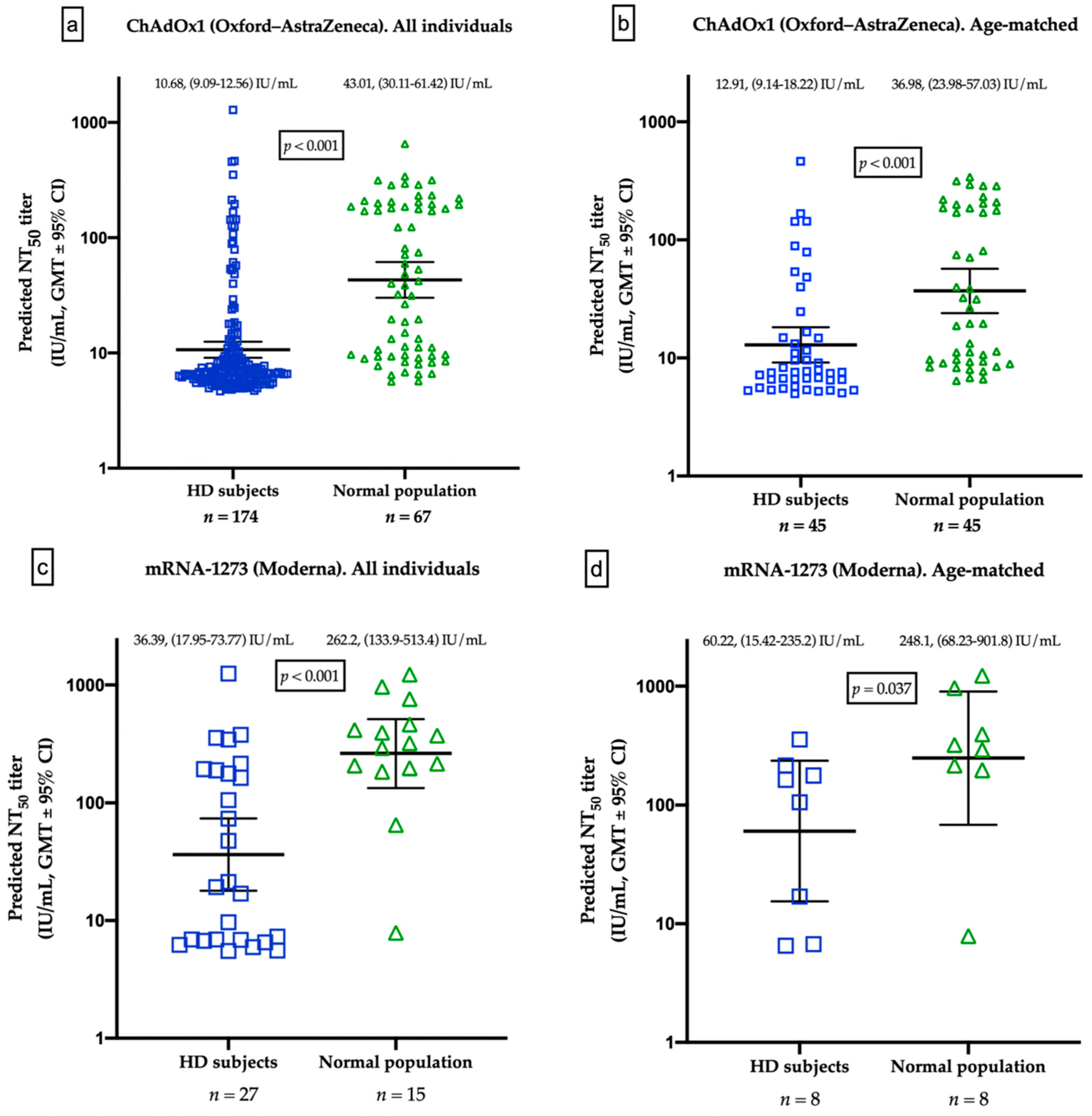
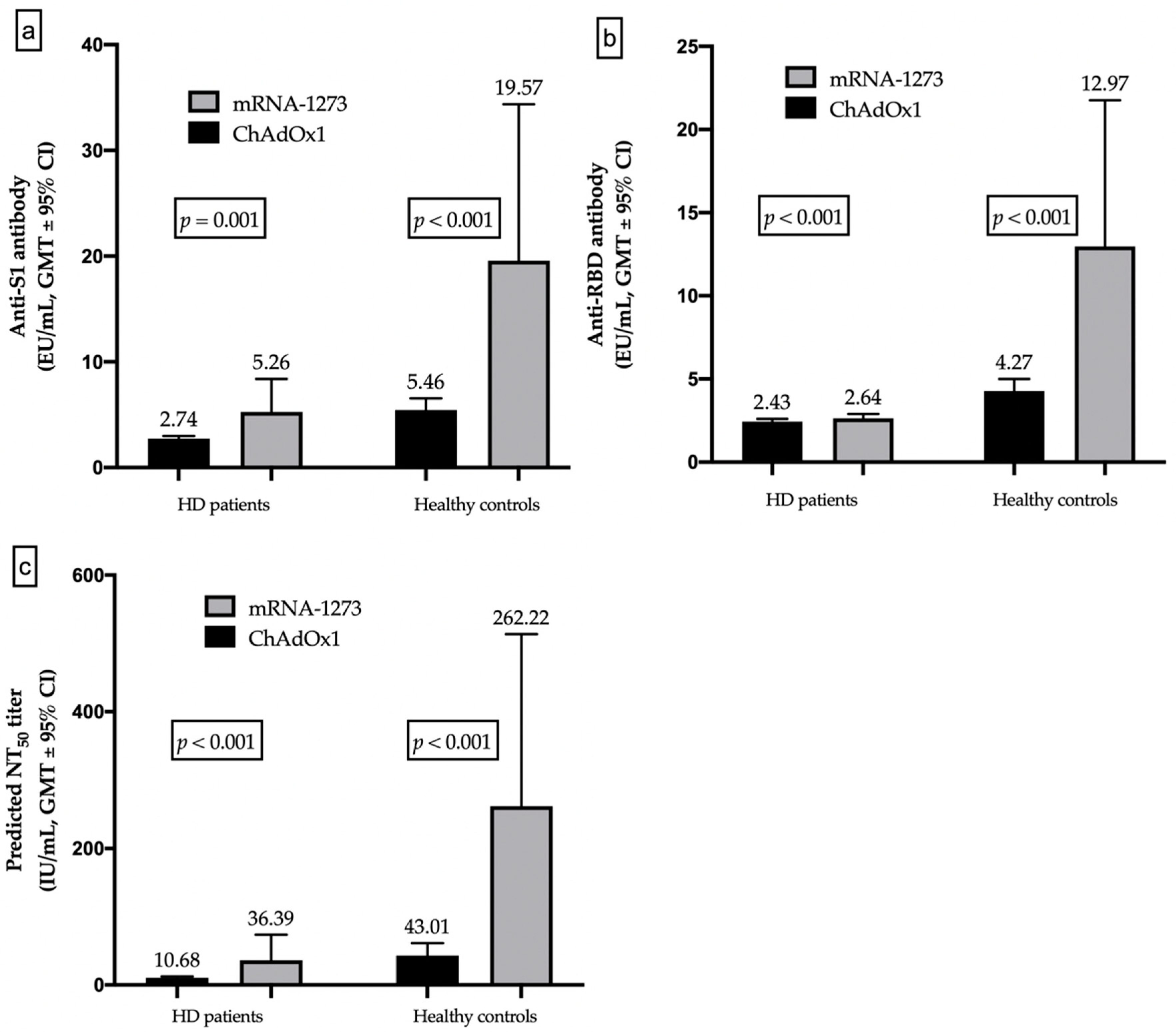
3.4. Different Humoral Responses between HD Patients and Healthy Controls
3.5. Different Humoral Responses between mRNA and Vector-Based Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 5 December 2021).
- McMichael, T.M.; Currie, D.W.; Clark, S.; Pogosjans, S.; Kay, M.; Schwartz, N.G.; Lewis, J.; Baer, A.; Kawakami, V.; Lukoff, M.D.; et al. Epidemiology of COVID-19 in a Long-Term Care Facility in King County, Washington. N. Engl. J. Med. 2020, 382, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Chow, N.; Fleming-Dutra, K.; Gierke, R.; Hall, A.; Hughes, M. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 382. [Google Scholar]
- Rao, K.S.; Suryaprakash, V.; Senthilkumar, R.; Preethy, S.; Katoh, S.; Ikewaki, N.; Abraham, S.J.K. Role of Immune Dysregulation in Increased Mortality among a Specific Subset of COVID-19 Patients and Immune-Enhancement Strategies for Combatting through Nutritional Supplements. Front. Immunol. 2020, 11, 1548. [Google Scholar] [CrossRef]
- Taiwan End Stage Renal Disease Registry. 2020 Annual Report on Kidney Disease in Taiwan. Available online: https://www.tsn.org.tw/UI/L/TWRD/ebook_2020%E5%B9%B4%E5%A0%B1.pdf (accessed on 1 January 2022).
- Bregman, H.D.J.; Ing, T.S. Complications during hemodialysis. In Handbook of Dialysis, 2nd ed.; Dauugirdas, J.T., Ing, T.S., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 1994; p. 149. [Google Scholar]
- Liu, Y.F.; Zhang, Z.; Pan, X.L.; Xing, G.L.; Zhang, Y.; Liu, Z.S.; Tu, S.H. The chronic kidney disease and acute kidney injury involvement in COVID-19 pandemic: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0244779. [Google Scholar] [CrossRef]
- Betjes, M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Corbett, R.W.; Blakey, S.; Nitsch, D.; Loucaidou, M.; McLean, A.; Duncan, N.; Ashby, D.R.; West London Renal and Transplant Centre. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020, 31, 1815–1823. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shao, S.C.; Chen, Y.T.; Hsu, C.K.; Hsu, H.J.; Lee, C.C.; Sun, C.Y.; Chen, Y.C.; Hung, M.J.; Wu, I.W. Incidence and Clinical Impacts of COVID-19 Infection in Patients with Hemodialysis: Systematic Review and Meta-Analysis of 396,062 Hemodialysis Patients. Healthcare 2021, 9, 47. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Rotem, R.S.; Patalon, T.; Gazit, S.; Ben-Tov, A.; Weil, C.; Goldshtein, I.; Twig, G.; Cohen, D.; et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: Analysis of real-world data. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Patel, M.M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; Gaglani, M.; McNeal, T.; et al. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing COVID-19 Hospitalizations in the United States. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Garcia, P.; Anand, S.; Han, J.; Montez-Rath, M.E.; Sun, S.; Shang, T.; Parsonnet, J.; Chertow, G.M.; Schiller, B.; Abra, G. COVID-19 Vaccine Type and Humoral Immune Response in Patients Receiving Dialysis. J. Am. Soc. Nephrol. 2022, 33, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Wu, M.; Harvey, R.; Wall, E.C.; Kelly, G.; Hussain, S.; Howell, M.; Kassiotis, G.; Swanton, C.; Gandhi, S.; et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet 2021, 398, 1038–1041. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef]
- Speer, C.; Goth, D.; Benning, L.; Buylaert, M.; Schaier, M.; Grenz, J.; Nusshag, C.; Kalble, F.; Kreysing, M.; Reichel, P.; et al. Early Humoral Responses of Hemodialysis Patients after COVID-19 Vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021, 16, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers for Disease Control. COVID-19 Statistics of Taiwan. Available online: https://sites.google.com/cdc.gov.tw/2019ncov/taiwan?authuser=0 (accessed on 5 December 2021).
- Chen, C.Y.; Ye, J.J.; Huang, T.S.; Lee, C.C.; Chen, Y.T.; Hsu, C.K.; Hsu, H.J.; Sun, C.Y.; Pan, H.C.; Chen, K.S.; et al. Effective Preventive Strategies to Prevent Secondary Transmission of COVID-19 in Hemodialysis Unit: The First Month of Community Outbreak in Taiwan. Healthcare 2021, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Baigent, C.; Ikizler, T.A.; Cockwell, P.; Jha, V. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: A call to action. Kidney Int. 2021, 99, 791–793. [Google Scholar] [CrossRef] [PubMed]
- American Society of Nephrology. Prioritizing COVID-19 Vaccination in Dialysis. Available online: https://www.kidneynews.org/view/news/policy-advocacy/leading-edge/prioritizing-COVID-19-vaccination-in-dialysis.xml (accessed on 24 November 2021).
- Combe, C.; Kirsch, A.H.; Alfano, G.; Luyckx, V.A.; Shroff, R.; Kanbay, M.; van der Sande, F.; Basile, C.; The EUDIAL Working Group of the ERA-EDTA. At least 156 reasons to prioritize COVID-19 vaccination in patients receiving in-centre haemodialysis. Nephrol. Dial. Transplant. 2021, 36, 571–574. [Google Scholar] [CrossRef]
- Taiwan Society of Nephrology. Taiwan Society of Nephrology. Statement from the Taiwan Society of Nephrology: Regarding the Administration of COVID-19 503 Vaccines for Dialysis Facilities and Renal Patients. Available online: https://www.tsn.org.tw/tsnFile/authority/F8D9162FFC199F7B/%E5%8F%B0%E7%81%A3%E8%85%8E%E8%87%9F%E9%86%AB%E5%AD%B8%E6%9C%83%E8%81%B2%E6%98%8E_v3_clean_%E9%97%9C%E6%96%BC%E9%80%8F%E6%9E%90%E9%99%A2%E6%89%80%E5%8F%8A%E8%85%8E%E8%87%9F%E7%97%85%E4%BA%BACOVID-19%E7%96%AB%E8%8B%97%E6%96%BD%E6%89%93_1100513.pdf (accessed on 24 November 2021).
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102329. [Google Scholar] [CrossRef]
- United States Renal Data System. 2020 USRDS Annual Data Report: Incidence, Prevalence, Patient Characteristics, and Treatment Modalities. Available online: https://adr.usrds.org/2020/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities (accessed on 24 November 2021).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Clarke, C.L.; Martin, P.; Gleeson, S.; Thomson, T.; Edwards, H.; Mortimer, P.; McIntyre, S.; Deborah, J.; Cox, A.; Pickard, G.; et al. Comparison of immunogenicity between BNT162b2 and ChAdOx1 SARS-CoV-2 vaccines in a large haemodialysis population. medRxiv 2021. [Google Scholar] [CrossRef]
- Billany, R.E.; Selvaskandan, H.; Adenwalla, S.F.; Hull, K.L.; March, D.S.; Burton, J.O.; Bishop, N.C.; Carr, E.J.; Beale, R.; Tang, J.W.; et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: A call to arms. Kidney Int. 2021, 99, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Lesny, P.; Anderson, M.; Cloherty, G.; Stec, M.; Haase-Fielitz, A.; Haarhaus, M.; Santos, C.; Lucas, C.; Macario, F.; Haase, M. Immunogenicity of a first dose of mRNA- or vector-based SARS-CoV-2 vaccination in dialysis patients: A multicenter prospective observational pilot study. J. Nephrol. 2021, 34, 975–983. [Google Scholar] [CrossRef]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Kerschmann, R.; Beyer, P.; Dittrich, M.; Block, G.A.; et al. Antibody Response to COVID-19 Vaccination in Patients Receiving Dialysis. J. Am. Soc. Nephrol. 2021, 32, 2435–2438. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.A.; Huang, C.G.; Huang, S.Y.; Liu, K.T.; Huang, P.N.; Yu, K.Y.; Yang, S.L.; Chen, C.P.; Cheng, C.Y.; Lin, Y.T.; et al. Antibody titers measured by commercial assays are correlated with neutralizing antibody titers calibrated by international standards. medRxiv 2021. [Google Scholar] [CrossRef]
- Sharif, M.R.; Chitsazian, Z.; Moosavian, M.; Raygan, F.; Nikoueinejad, H.; Sharif, A.R.; Einollahi, B. Immune disorders in hemodialysis patients. Iran. J. Kidney Dis. 2015, 9, 84–96. [Google Scholar]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Basic Science and Dialysis: Disturbances of acquired immunity in hemodialysis patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Pahl, M.V.; Gollapudi, S.; Sepassi, L.; Gollapudi, P.; Elahimehr, R.; Vaziri, N.D. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol. Dial. Transplant. 2010, 25, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Lu, K.C.; Kuo, K.L. The Efficacy of COVID-19 Vaccines in Chronic Kidney Disease and Kidney Transplantation Patients: A Narrative Review. Vaccines 2021, 9, 885. [Google Scholar] [CrossRef]
- Kim, K.W.; Chung, B.H.; Jeon, E.J.; Kim, B.M.; Choi, B.S.; Park, C.W.; Kim, Y.S.; Cho, S.G.; Cho, M.L.; Yang, C.W. B cell-associated immune profiles in patients with end-stage renal disease (ESRD). Exp. Mol. Med. 2012, 44, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Stephens, C.; Brown, K.; Amirthalingam, G.; Otter, A.; Hallis, B.; Zuo, J.; Moss, P. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun. Ageing 2021, 18, 34. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Tut, G.; Ali, M.; Stephens, C.; Greenwood, D.; Faustini, S.; Hughes, S.; Huissoon, A.; Meade, R.; et al. Immunogenicity of single vaccination with BNT162b2 or ChAdOx1 nCoV-19 at 5–6 weeks post vaccine in participants aged 80 years or older: An exploratory analysis. Lancet Healthy Longev. 2021, 2, e554–e560. [Google Scholar] [CrossRef]
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Torres-Hernández, P.C.; Hernández-Bello, J. Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines 2021, 9, 1376. [Google Scholar] [CrossRef]
- Morales-Nunez, J.J.; Munoz-Valle, J.F.; Meza-Lopez, C.; Wang, L.F.; Machado Sulbaran, A.C.; Torres-Hernandez, P.C.; Bedolla-Barajas, M.; la O-Gómez, D.; Balcazar-Felix, P.; Hernandez-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines 2021, 9, 742. [Google Scholar] [CrossRef]
- Chen, K.H.; Lin-Tan, D.T.; Huang, W.H.; Hung, C.C.; Chang, C.T.; Huang, J.Y.; Lin, J.L. Cardiothoracic ratio, malnutrition, inflammation, and two-year mortality in non-diabetic patients on maintenance hemodialysis. Kidney Blood Press. Res. 2008, 31, 143–151. [Google Scholar] [CrossRef]
- Yen, T.H.; Lin, J.L.; Lin-Tan, D.T.; Hsu, K.H. Cardiothoracic ratio, inflammation, malnutrition, and mortality in diabetes patients on maintenance hemodialysis. Am. J. Med. Sci. 2009, 337, 421–428. [Google Scholar] [CrossRef]
- Ito, K.; Ookawara, S.; Ueda, Y.; Miyazawa, H.; Yamada, H.; Goto, S.; Ishii, H.; Shindo, M.; Kitano, T.; Hirai, K.; et al. A Higher Cardiothoracic Ratio Is Associated with 2-Year Mortality after Hemodialysis Initiation. Nephron Extra 2015, 5, 100–110. [Google Scholar] [CrossRef]
- Yotsueda, R.; Taniguchi, M.; Tanaka, S.; Eriguchi, M.; Fujisaki, K.; Torisu, K.; Masutani, K.; Hirakata, H.; Kitazono, T.; Tsuruya, K. Cardiothoracic Ratio and All-Cause Mortality and Cardiovascular Disease Events in Hemodialysis Patients: The Q-Cohort Study. Am. J. Kidney Dis. 2017, 70, 84–92. [Google Scholar] [CrossRef]
- Chung, T.L.; Liu, Y.H.; Huang, J.C.; Wu, P.Y.; Tu, H.P.; Chen, S.C.; Chang, J.M. Prognostic Implication of Longitudinal Changes in Cardiothoracic Ratio and Aortic Arch Calcification in Hemodialysis Patients. J. Pers. Med. 2021, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.H.; Liu, Y.H.; Chung, T.L.; Huang, J.C.; Wu, P.Y.; Su, H.M.; Chen, S.C. Aortic Arch Calcification and Cardiomegaly Are Associated with Overall and Cardiovascular Mortality in Hemodialysis Patients. J. Pers. Med. 2021, 11, 657. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; El Burai Felix, S.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, January 22–May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| NT50 < 12.31 IU/mL (n = 135) | 12.31 ≤ NT50 Titer < 35.13 (n = 16) | NT50 Titer ≥ 35.13 (n = 23) | p-Value | |
|---|---|---|---|---|
| Age, year | 65.67 ± 12.76 | 61.69 ± 13.70 | 60.70 ± 14.63 | 0.094 & |
| Male, n (%) | 72 (53.3) | 8 (50) | 14 (60.9) | 0.754 |
| Comorbidities, n (%) | ||||
| Diabetes | 74 (54.8) | 9 (56.3) | 13 (56.5) | 0.984 |
| Dyslipidemia | 49 (36.3) | 8 (50) | 10 (43.5) | 0.494 |
| Liver cirrhosis | 4 (3) | 0 (0) | 0 (0) | 0.551 |
| Cardiovascular disease | 49 (36.3) | 7 (43.8) | 8 (34.8) | 0.824 |
| Baseline medications, n (%) | ||||
| Immunosuppressants | 8 (5.9) | 1 (6.3) | 0 (0) | 0.485 |
| RAAS blockade | 60 (44.4) | 4 (25) | 11(47.8) | 0.294 |
| β-blockers | 55 (40.7) | 6 (37.5) | 12 (52.2) | 0.549 |
| Statins | 45 (33.3) | 7 (43.8) | 10 (43.5) | 0.500 |
| Anti-S1 antibody (EU/mL) | 2.11 (1.96–2.41) | 3.49 (3.14–3.86) | 5.99 (5.18–9.61) | <0.001 $ |
| Anti-RBD antibody (EU/mL) | 2.07 (1.86–2.26) | 3.16 (3.04–3.55) | 3.79 (3.22–5.37) | <0.001 $ |
| Hemoglobin (g/dL) | 10.04 ± 1.14 | 10.23 ± 1.55 | 10.20 ± 1.19 | 0.561 & |
| WBC (1000/μL) | 5.90 (4.70–7.50) | 7.10 (5.80-7.85) | 6.30 (5.30–7.80) | 0.183 $ |
| Platelet (1000/μL) | 187.65 ± 65.41 | 240.56 ± 86.20 | 189.52 ± 76.62 | 0.905 & |
| Albumin (g/dL) | 4.025 ± 0.38 | 3.96 ± 0.32 | 4.09 ± 0.32 | 0.465 & |
| Cholesterol (mg/dL) | 154.85 ± 37.23 | 160.44 ± 32.32 | 147.91 ± 35.72 | 0.556 & |
| Triglyceride (mg/dL) | 114.00 (78.00–173.50) | 152.50 (111.00–304.25) | 128.00 (77.00–164.00) | 0.160 $ |
| AST (U/L) | 16.0 (13.0–20.5) | 18.50 (14.00–25.25) | 17 (14–21) | 0.380 $ |
| ALT (U/L) | 13 (10–19) | 16.50 (10.25–21.50) | 17 (13–21) | 0.043 * $ |
| Alk-P (U/L) | 92 (71–146) | 114.00 (80.00–235.25) | 79 (65–116) | 0.136 $ |
| Total bilirubin (mg/dL) | 0.40 (0.30–0.40) | 0.35 (0.20–0.40) | 0.40 (0.30–0.40) | 0.749 $ |
| Bun (mg/dL) | 69.97 ± 22.94 | 68.73 ± 19.43 | 67.99 ± 12.97 | 0.686 & |
| Creatinine (mg/dL) | 9.65 ± 2.59 | 9.22 ± 3.14 | 10.06 ± 1.88 | 0.475 & |
| Uric acid (mg/dL) | 6.28 ± 1.95 | 6.21 ± 1.83 | 6.18 ± 1.84 | 0.810 & |
| Na (meq/L) | 138.14 ± 3.08 | 137.5 ± 3.86 | 138.43 ± 2.73 | 0.676 & |
| K (meq/L) | 4.76 ± 0.80 | 4.77 ± 0.78 | 4.57 ± 0.71 | 0.291 & |
| Ca (mg/dL) | 9.35 ± 0.85 | 9.39 ± 0.78 | 9.67 ± 0.62 | 0.081 & |
| P (mg/dL) | 5.26 ± 1.59 | 5.41 ± 1.95 | 5.36 ± 1.45 | 0.772 & |
| C-reactive protein (mg/L) | 3.60 (1.30–9.20) | 6.30 (2.70–9.90) | 4.45 (1.63–9.30) | 0.457 $ |
| Urea reduction rate | 76.00 (70.50–79.50) | 75.00 (68.25–80.00) | 76 (72–79) | 0.695 $ |
| Kt/V (Daugirdes) | 0.65 ± 0.34 | 1.61 ± 0.29 | 1.60 ± 0.35 | 0.562 & |
| nPCR (g/kg/day) | 1.10 ± 0.58 | 1.00 ± 0.28 | 1.04 ± 0.25 | 0.624 & |
| TACurea | 41.69 ± 14.14 | 42.00 ± 12.18 | 39.72 ± 9.97 | 0.519 & |
| Iron (μg/dL) | 65.00 (50.50–89.00) | 55 (46–81) | 78 (54–103) | 0.280 $ |
| Ferritin (ng/mL) | 439.00 (234.00–709.50) | 338.50 (171.50–516.00) | 524 (120–600) | 0.256 $ |
| TSAT (%) | 34.02 ± 13.68 | 29.77 ± 8.66 | 37.82 ± 19.72 | 0.240 & |
| Cardiothoracic ratio | 0.53 ± 0.05 | 0.48 ± 0.06 | 0.48 ± 0.07 | 0.006 * |
| Ca × P product | 49.38 ± 16.95 | 50.66 ± 18.43 | 52.21 ± 16.01 | 0.461 |
| Simple Linear Regression | Multiple Regression Analysis, Model 1 | Multiple Regression Analysis, Model 2 | ||||
|---|---|---|---|---|---|---|
| β ± SE | p | β ± SE | p | β ± SE | p | |
| Age | −0.005 ± 0.003 | 0.075 | −0.003 ± 0.001 | 0.046 * | −0.009 ± 0.003 | 0.011 * |
| Anti-S1 antibody $ | 1.841 ± 0.065 | <0.001 * | 1.490 ± 0.128 | <0.001 * | - | - |
| Anti-RBD antibody $ | 2.250 ± 0.0106 | <0.001 * | 0.589 ± 0.165 | 0.001 * | - | - |
| Hemoglobin | 0.004 ± 0.032 | 0.890 | −0.136 ± 0.056 | 0.017 * | −0.251 ± 0.130 | 0.054 |
| RBC $ | −0.225 ± 0.623 | 0.718 | 2.787 ± 1.313 | 0.036 * | 6.169 ± 3.063 | 0.046 * |
| MCV | 0.004 ± 0.005 | 0.478 | 0.019 ± 0.008 | 0.014 * | 0.042 ± 0.018 | 0.022 * |
| WBC $ | 0.375 ± 0.246 | 0.129 | - | - | - | - |
| Platelet | 0.001 ± 0.001 | 0.254 | - | - | - | - |
| Albumin | 0.061 ± 0.104 | 0.560 | - | - | - | - |
| AST $ | 0.011 ± 0.222 | 0.959 | - | - | - | - |
| ALT $ | 0.308 ± 0.167 | 0.066 | - | - | 0.401 ± 0.191 | 0.038 * |
| Alk-P $ | −0.120 ± 0.137 | 0.380 | - | - | - | - |
| Bilirubin $ | −0.047 ± 0.252 | 0.853 | - | - | - | - |
| Cholesterol | −0.001 ± 0.001 | 0.564 | - | - | - | - |
| Triglyceride $ | 0.034 ± 0.151 | 0.822 | - | - | - | - |
| Creatinine | 0.007 ± 0.015 | 0.628 | 0.016 ± 0.009 | 0.073 | - | - |
| Uric acid | 0.004 ± 0.020 | 0.858 | −0.021 ± 0.010 | 0.038 * | - | - |
| Na | 0.006 ± 0.012 | 0.632 | - | - | - | - |
| K | −0.049 ± 0.048 | 0.312 | - | - | −0.115 ± 0.057 | 0.046 * |
| Ca | 0.083 ± 0.046 | 0.074 | - | - | - | - |
| P | 0.024 ± 0.024 | 0.320 | - | - | - | - |
| C-reactive protein $ | 0.072 ± 0.064 | 0.262 | - | - | - | - |
| URR $ | 0.038 ± 0.240 | 0.875 | −0.655 ± 0.354 | 0.067 | - | - |
| Kt/V | −0.048 ± 0.117 | 0.680 | - | - | - | - |
| nPCR | −0.043 ± 0.074 | 0.559 | - | - | - | - |
| TACurea | −0.001 ± 0.003 | 0.860 | - | - | - | - |
| Ferritin $ | −0.062 ± 0.095 | 0.516 | −0.119 ± 0.048 | 0.016 * | - | - |
| Iron $ | 0.245 ± 0.216 | 0.258 | - | - | - | - |
| TSAT | 0.002 ± 0.003 | 0.465 | 0.003 ± 0.001 | 0.006 * | - | - |
| Intact-PTH | −0.000 ± 0.000 | 0.503 | - | - | - | - |
| Cardiothoracic ratio | −2.026 ± 0.729 | 0.006 * | −0.555 ± 0.305 | 0.071 | −2.259 ± 0.719 | 0.002 * |
| Ca × P product | 0.003 ± 0.002 | 0.158 | −0.003 ± 0.001 | 0.006 * | - | - |
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Male | 0.724 | 0.296–1.775 | 0.481 | 0.809 | 0.214–3.056 | 0.755 |
| Age | 0.975 | 0.944–1.007 | 0.125 | 0.958 | 0.917–1.000 | 0.052 |
| Diabetes | 0.939 | 0.388–2.274 | 0.889 | 0.849 | 0.285–2.528 | 0.849 |
| Liver cirrhosis | 2.54 × 108 | 0 | 0.999 | 1.09 × 107 | 0 | 1.000 |
| Cardiovascular disease | 1.105 | 0.441–2.772 | 0.831 | 0.915 | 0.269–3.116 | |
| Immunosuppressants | 0 | 0 | 0.999 | 2.37 × 10−9 | 0 | 0.999 |
| RAAS blockade | 1.246 | 0.517–3.003 | 0.624 | 1.132 | 0.348–3.677 | 0.837 |
| β-blockers | 1.610 | 0.667–3.882 | 0.289 | 0.612 | 0.199–1.885 | 0.392 |
| Hemoglobin | 1.102 | 0.760–1.599 | 0.608 | 0.773 | 0.161–3.721 | 0.748 |
| RBC | 0.993 | 0.440–2.239 | 0.987 | 1.934 | 0.015–252.653 | 0.791 |
| MCV | 1.020 | 0.955–1.090 | 0.560 | 1.078 | 0.853–1.364 | 0.528 |
| Albumin | 1.700 | 0.482–5.999 | 0.410 | 0.852 | 0.119–6.097 | 0.873 |
| ALT | 1.009 | 0.981–1.039 | 0.526 | 1.007 | 0.969–1.046 | 0.720 |
| K | 0.727 | 0.407–1.301 | 0.283 | 0.625 | 0.273–1.432 | 0.267 |
| C-reactive protein | 0.945 | 0.499–1.789 | 0.861 | 1.008 | 0.972–1.045 | 0.670 |
| Kt/V | 0.700 | 0.190–2.570 | 0.591 | 0.977 | 0.131–7.294 | 0.982 |
| nPCR | 0.776 | 0.226–2.663 | 0.687 | 0.556 | 0.053–5.784 | 0.623 |
| TSAT | 1.019 | 0.991–1.047 | 0.189 | 1.015 | 0.982–1.049 | 0.390 |
| Cardiaothoracic ratio | 0.005 | 0.000–0.307 | 0.012 | <0.0001 | 0.000–0.556 | 0.037 * |
| Ca × P product | 1.009 | 0.984–1.035 | 0.477 | 1.019 | 0.981–1.059 | 0.325 |
| ChAdOx1n (Oxford–AstraZeneca) | mRNA-1273 (Moderna) | |||||
|---|---|---|---|---|---|---|
| HD Group (n = 174) | Control Group (n = 67) | p-Value | HD Group (n = 26) | Control Group (n = 15) | p-Value | |
| Age (years) | 64.97 ± 13.20 | 44.86 ± 9.85 | <0.001 * | 68.51 ± 10.35 | 50.07 ± 19.02 | 0.001 * |
| Male, n (%) | 94 (54) | 22 (32.8) | 0.003 * | 7 (26.9) | 13 (86.7) | <0.001 * |
| Predicted NT50 titer (median, IQR) (IU/mL) | 6.85 (5.89–10.68) | 40.01 (9.58–186.71) | <0.001 * | 20.27 (6.83–189.66) | 319.44 (196.54–461.48) | 0.001 * |
| GMTs (95% CI) (IU/mL) | 10.68 (9.10–12.54) | 43.01 (30.11–61.42) | 36.39 (17.95–73.77) | 262.2 (133.9–513.4) | ||
| Humoral response, n (%) | 23 (13.2) | 35 (52.2) | <0.001 * | 12 (46.2) | 14 (93.3) | 0.004 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Liu, K.-T.; Shih, S.-R.; Ye, J.-J.; Chen, Y.-T.; Pan, H.-C.; Hsu, H.-J.; Sun, C.-Y.; Lee, C.-C.; Wu, C.-Y.; et al. Neutralization Assessments Reveal High Cardiothoracic Ratio and Old Age as Independent Predictors of Low Neutralizing Antibody Titers in Hemodialysis Patients Receiving a Single Dose of COVID-19 Vaccine. J. Pers. Med. 2022, 12, 68. https://doi.org/10.3390/jpm12010068
Chen C-Y, Liu K-T, Shih S-R, Ye J-J, Chen Y-T, Pan H-C, Hsu H-J, Sun C-Y, Lee C-C, Wu C-Y, et al. Neutralization Assessments Reveal High Cardiothoracic Ratio and Old Age as Independent Predictors of Low Neutralizing Antibody Titers in Hemodialysis Patients Receiving a Single Dose of COVID-19 Vaccine. Journal of Personalized Medicine. 2022; 12(1):68. https://doi.org/10.3390/jpm12010068
Chicago/Turabian StyleChen, Chun-Yu, Kuan-Ting Liu, Shin-Ru Shih, Jung-Jr Ye, Yih-Ting Chen, Heng-Chih Pan, Heng-Jung Hsu, Chiao-Yin Sun, Chin-Chan Lee, Chun-Ying Wu, and et al. 2022. "Neutralization Assessments Reveal High Cardiothoracic Ratio and Old Age as Independent Predictors of Low Neutralizing Antibody Titers in Hemodialysis Patients Receiving a Single Dose of COVID-19 Vaccine" Journal of Personalized Medicine 12, no. 1: 68. https://doi.org/10.3390/jpm12010068
APA StyleChen, C.-Y., Liu, K.-T., Shih, S.-R., Ye, J.-J., Chen, Y.-T., Pan, H.-C., Hsu, H.-J., Sun, C.-Y., Lee, C.-C., Wu, C.-Y., Lai, C.-C., & Wu, I.-W. (2022). Neutralization Assessments Reveal High Cardiothoracic Ratio and Old Age as Independent Predictors of Low Neutralizing Antibody Titers in Hemodialysis Patients Receiving a Single Dose of COVID-19 Vaccine. Journal of Personalized Medicine, 12(1), 68. https://doi.org/10.3390/jpm12010068







