Machine Learning-Based Approaches for Prediction of Patients’ Functional Outcome and Mortality after Spontaneous Intracerebral Hemorrhage
Abstract
:1. Introduction
2. Materials and Methods
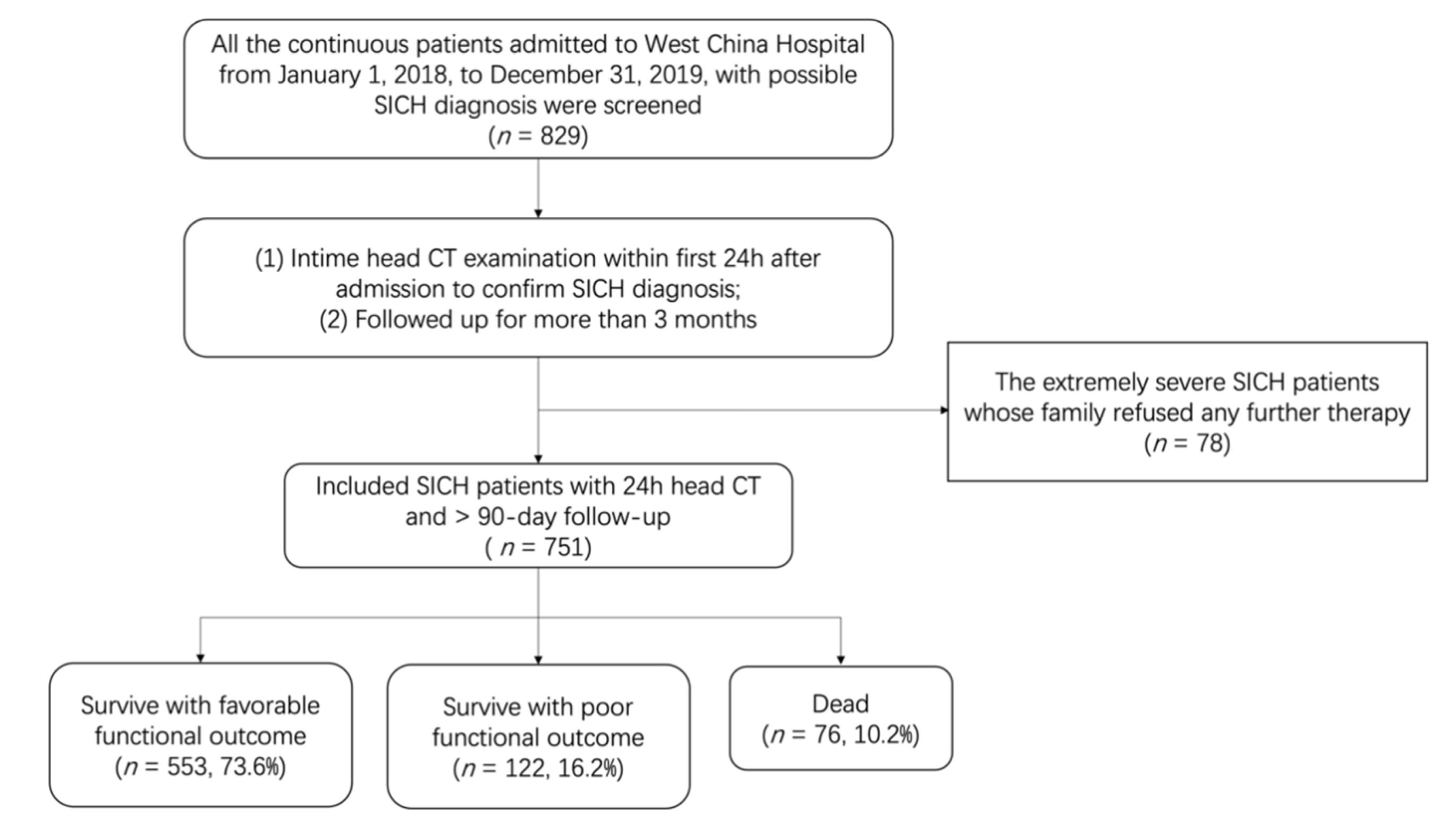
2.1. Study Population
2.2. Data Collection
2.3. In-Hospital Treatments and Outcomes
2.4. Machine Learning ML Algorithms
2.5. Comparison to the Intracerebral Hemorrhage (ICH) Score
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
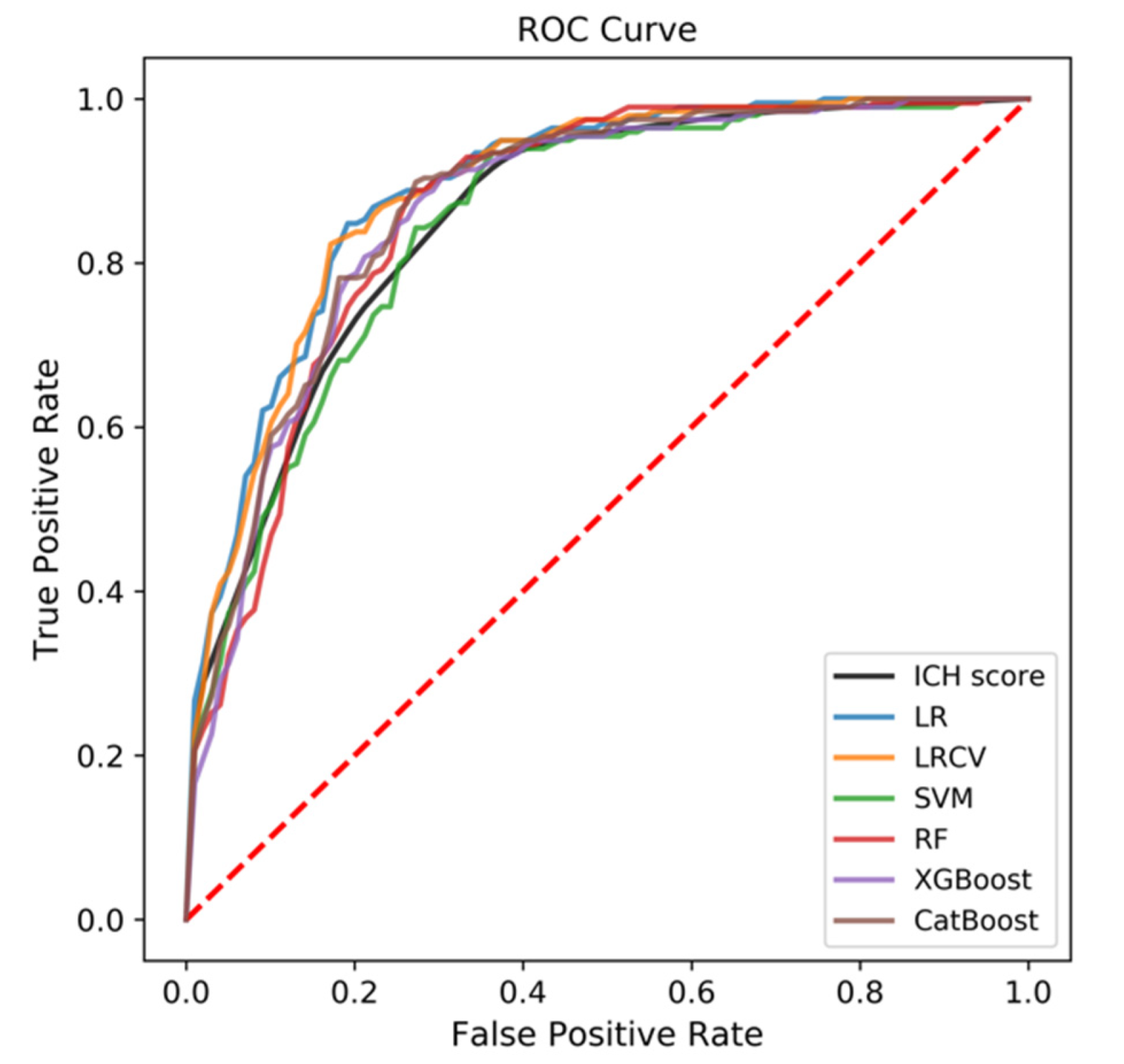
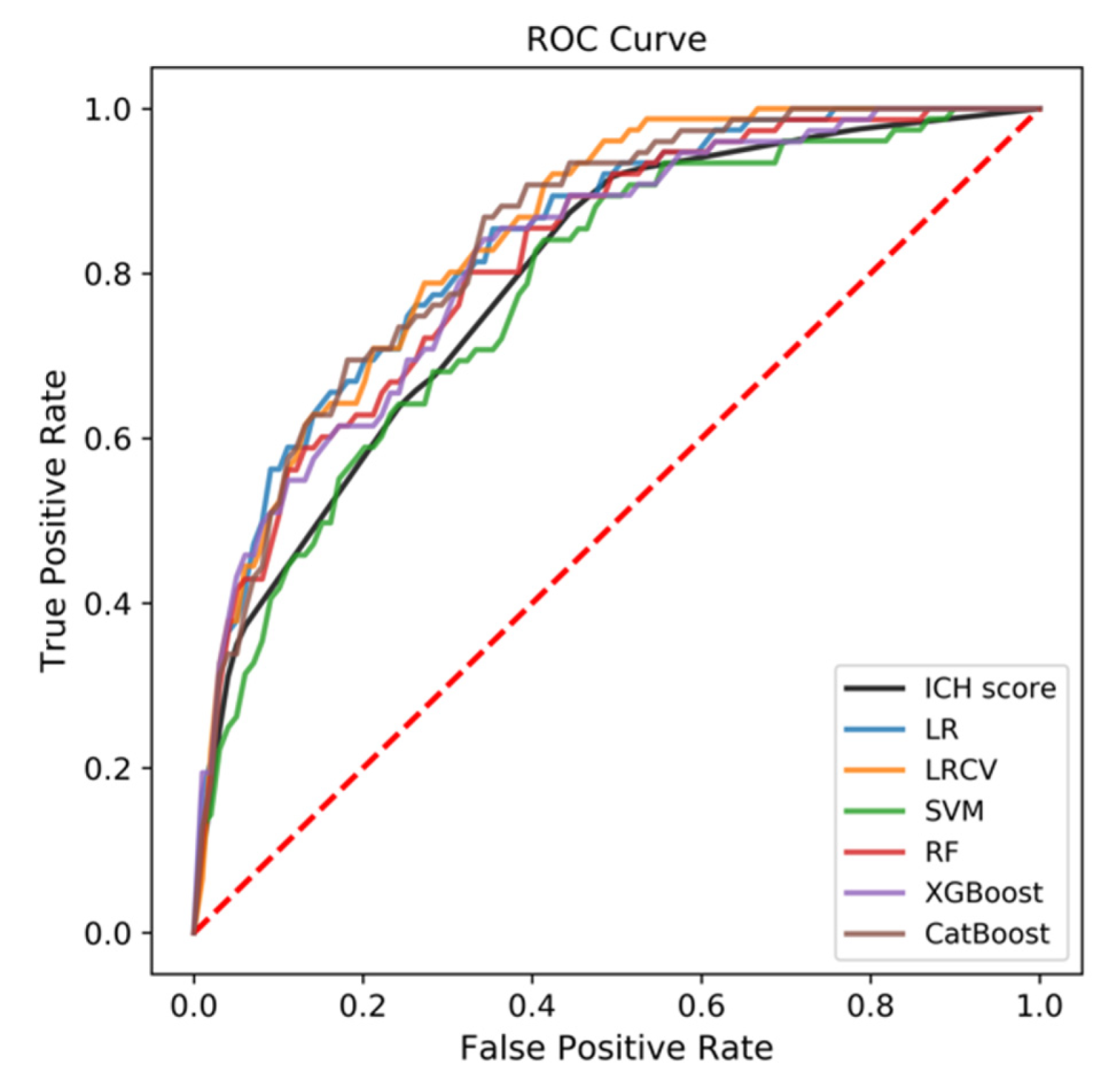
3.2. Predictive Performance of the ML-Based Models
3.3. Comparison to ICH Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Patrice Lindsay, M.; Castellucci, L.A.; Cayley, A.; Crowther, M.; de Wit, K.; English, S.W.; Hoosein, S.; Huynh, T.; Kelly, M.; et al. Canadian stroke best practice recommendations: Management of Spontaneous Intracerebral Hemorrhage, 7th Edition Update 2020. Int. J. Stroke Off. J. Int. Stroke Soc. 2021, 16, 321–341. [Google Scholar] [CrossRef]
- Steiner, T.; Salman, R.A.-S.; Beer, R.; Christensen, H.; Cordonnier, C.; Csiba, L.; Forsting, M.; Harnof, S.; Klijn, C.; Krieger, D.; et al. European Stroke Organisation (ESO) Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Int. J. Stroke 2014, 9, 840–855. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, M.L.; Woo, D.; Haverbusch, M.; Sekar, P.; Khoury, J.; Sauerbeck, L.; Moomaw, C.J.; Schneider, A.; Kissela, B.; Kleindorfer, D.; et al. Racial Variations in Location and Risk of Intracerebral Hemorrhage. Stroke 2005, 36, 934–937. [Google Scholar] [CrossRef] [Green Version]
- Gregório, T.; Pipa, S.; Cavaleiro, P.; Atanásio, G.; Albuquerque, I.; Chaves, P.C.; Azevedo, L. Prognostic models for intracerebral hemorrhage: Systematic review and meta-analysis. BMC Med. Res. Methodol. 2018, 18, 145. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.P.; Garton, A.L.; Sisti, J.A.; Christophe, B.R.; Lord, A.; Lewis, A.; Frey, H.-P.; Claassen, J.; Connolly, E.S. Prognosticating Functional Outcome After Intracerebral Hemorrhage: The ICHOP Score. World Neurosurg. 2017, 101, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.N.; Weaver, B.; Liotta, E.; Maas, M.B.; Faigle, R.; Mroczek, D.K.; Naidech, A.M. Identifying Modifiable Predictors of Patient Outcomes After Intracerebral Hemorrhage with Machine Learning. Neurocritical Care 2021, 34, 73–84. [Google Scholar] [CrossRef]
- Wang, H.-L.; Hsu, W.-Y.; Lee, M.-H.; Weng, H.-H.; Chang, S.-W.; Yang, J.-T.; Tsai, Y.-H. Automatic Machine-Learning-Based Outcome Prediction in Patients With Primary Intracerebral Hemorrhage. Front. Neurol. 2019, 10, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morotti, A.; Phuah, C.-L.; Anderson, C.D.; Jessel, M.J.; Schwab, K.; Ayres, A.M.; Pezzini, A.; Padovani, A.; Gurol, E.; Viswanathan, A.; et al. Leukocyte Count and Intracerebral Hemorrhage Expansion. Stroke 2016, 47, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeoye, O.; Walsh, K.; Woo, J.G.; Haverbusch, M.; Moomaw, C.J.; Broderick, J.P.; Kissela, B.M.; Kleindorfer, D.; Flaherty, M.L.; Woo, D. Peripheral Monocyte Count Is Associated with Case Fatality after Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, e107–e111. [Google Scholar] [CrossRef] [Green Version]
- Mackey, J.; Blatsioris, A.D.; Saha, C.; Moser, E.A.S.; Carter, R.J.L.; Cohen-Gadol, A.A.; Leipzig, T.J.; Williams, L.S. Higher Monocyte Count is Associated with 30-Day Case Fatality in Intracerebral Hemorrhage. Neurocritical Care 2021, 34, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Sekar, P.; Langefeld, C.D.; Moomaw, C.J.; Elkind, M.S.; Boehme, A.K.; James, M.; Osborne, J.; Sheth, K.N.; Woo, D.; et al. Monocyte Count and 30-Day Case Fatality in Intracerebral Hemorrhage. Stroke 2015, 46, 2302–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yuan, Y.; Liao, X.; Yu, Z.; Li, H.; Zheng, J. Prognostic Significance of Admission Systemic Inflammation Response Index in Patients With Spontaneous Intracerebral Hemorrhage: A Propensity Score Matching Analysis. Front. Neurol. 2021, 12, 718032. [Google Scholar] [CrossRef]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Kuang, H.; Qiu, W.; Boers, A.M.; Brown, S.; Muir, K.; Majoie, C.B.; Dippel, D.W.; White, P.; Epstein, J.; Mitchell, P.J.; et al. Computed Tomography Perfusion–Based Machine Learning Model Better Predicts Follow-Up Infarction in Patients with Acute Ischemic Stroke. Stroke 2021, 52, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.H.; Lim, I.C.Z.; Tseng, F.S.; Teo, Y.N.; Kow, C.S.; Ng, Z.H.C.; Ko, N.C.K.; Sia, C.-H.; Leow, A.S.T.; Yeung, W.; et al. Predicting Clinical Outcomes in Acute Ischemic Stroke Patients Undergoing Endovascular Thrombectomy with Machine Learning: A Systematic Review and Meta-analysis. Clin. Neuroradiol. 2021, 31, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Rubbert, C.; Patil, K.R.; Beseoglu, K.; Mathys, C.; May, R.; Kaschner, M.G.; Sigl, B.; Teichert, N.A.; Boos, J.; Turowski, B.; et al. Prediction of outcome after aneurysmal subarachnoid haemorrhage using data from patient admission. Eur. Radiol. 2018, 28, 4949–4958. [Google Scholar] [CrossRef] [PubMed]
- Asadi, H.; Kok, H.K.; Looby, S.; Brennan, P.; O’Hare, A.; Thornton, J. Outcomes and Complications After Endovascular Treatment of Brain Arteriovenous Malformations: A Prognostication Attempt Using Artificial Intelligence. World Neurosurg. 2016, 96, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Divani, A.A.; Majidi, S.; Luo, X.; Souslian, F.G.; Zhang, J.; Abosch, A.; Tummala, R.P. The ABCs of Accurate Volumetric Measurement of Cerebral Hematoma. Stroke 2011, 42, 1569–1574. [Google Scholar] [CrossRef] [Green Version]
- Tolles, J.; Meurer, W.J. Logistic Regression: Relating Patient Characteristics to Outcomes. JAMA 2016, 316, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Ho, T.K. The random subspace method for constructing decision forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Opitz, D.; Maclin, R. Popular Ensemble Methods: An Empirical Study. J. Artif. Intell. Res. 1999, 11, 169–198. [Google Scholar] [CrossRef]
- Al-Mallah, M.H.; Elshawi, R.; Ahmed, A.M.; Qureshi, W.T.; Brawner, C.A.; Blaha, M.J.; Ahmed, H.M.; Ehrman, J.K.; Keteyian, S.J.; Sakr, S. Using Machine Learning to Define the Association between Cardiorespiratory Fitness and All-Cause Mortality (from the Henry Ford Exercise Testing Project). Am. J. Cardiol. 2017, 120, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Hadanny, A.; Shlomo, N.; Iakobishvili, Z.; Unger, R.; Zahger, D.; Alcalai, R.; Atar, S.; Gottlieb, S.; Matetzky, S.; et al. Machine learning for prediction of 30-day mortality after ST elevation myocardial infraction: An Acute Coronary Syndrome Israeli Survey data mining study. Int. J. Cardiol. 2017, 246, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Hong, X.; Liu, P.; Bao, X.; Yao, Y.; Xing, B.; Li, Y.; Huang, Y.; Zhu, H.; et al. Prediction of Recurrence after Transsphenoidal Surgery for Cushing’s Disease: The Use of Machine Learning Algorithms. Neuroendocrinology 2019, 108, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion During Inflammation and Injury. Arter. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Functional Outcome | Mortality | ||||
|---|---|---|---|---|---|---|
| Favorable (n = 553) | Unfavorable (n = 198) | p-Value | Survival (n = 675) | Death (n = 76) | p-Value | |
| Demographics | ||||||
| Age, years | 54.0 (46.0–66.0) | 58.9 (43.7–74.0) | 0.004 ** | 54.0 (46.0–66.0) | 65.5 (52.5–77.0) | <0.001 *** |
| Gender, n (%) | 0.70 | 0.20 | ||||
| Female | 189 (74.70%) | 64 (25.30%) | 232 (92.06%) | 20 (7.94%) | ||
| Male | 364 (73.09%) | 134 (26.91%) | 443 (88.78%) | 56 (11.22%) | ||
| Clinical features | ||||||
| Location, n (%) | <0.001 *** | <0.001 *** | ||||
| Supratentorial | 475 (78.51%) | 130 (21.49%) | 556 (91.90%) | 49 (8.10%) | ||
| Infratentorial | 72 (58.06%) | 52 (41.94%) | 106 (85.48%) | 18 (14.52%) | ||
| Supra and Infra | 6 (27.27%) | 16 (72.73%) | 13 (59.09%) | 9 (40.91%) | ||
| Initial volume, mL | 25.0 (15.0–35.0) | 34.9 (19.3–50.5) | <0.001 *** | 25.0 (15.0–35.0) | 35.0 (20.0–46.2) | <0.001 *** |
| IVH, n (%) | <0.001 *** | 0.001 ** | ||||
| Yes | 253 (63.73%) | 144 (36.27%) | 342 (86.15%) | 55 (13.85%) | ||
| No | 300 (84.75%) | 54 (15.25%) | 333 (94.07%) | 21 (5.93%) | ||
| GCS | 13 (9–15) | 8 (6–8) | <0.001 *** | 13 (8–15) | 7 (4–10) | <0.001 *** |
| Length of time in ER, h | 1.08 (0.57–2.35) | 1.13 (0.65–2.35) | 0.48 | 1.03 (0.57–2.35) | 1.47 (0.85–2.35) | 0.02 * |
| BT, °C | 36.6 (36.5–36.8) | 36.8 (36.5–37.0) | <0.001 *** | 36.6 (36.5–36.9) | 36.8 (36.5–37.0) | 0.02 * |
| HR, bpm | 82 (72–92) | 86 (75 -102) | 0.001 ** | 82 (72–93) | 94 (80–112) | <0.001 *** |
| Systolic BP, mmHg | 165 (144–183) | 164 (130–199) | 0.48 | 165 (144–182) | 168 (128 -208) | 0.18 |
| Diastolic BP, mmHg | 96 (82–107) | 92 (81–109) | 0.10 | 96 (82–108) | 93.5 (78–107) | 0.12 |
| Medical history | ||||||
| Hypertension, n (%) | 0.48 | 0.24 | ||||
| Yes | 429 (72.96%) | 159 (27.04%) | 524 (77.63%) | 64 (82.89%) | ||
| No | 124 (76.07%) | 39 (23.93%) | 151 (22.37%) | 12 (15.79%) | ||
| DM, n (%) | 0.09 | 0.007 ** | ||||
| Yes | 50 (64.94%) | 27 (35.06%) | 62 (80.52%) | 15 (19.48%) | ||
| No | 503 (74.63%) | 171 (25.37%) | 613 (90.95%) | 61 (9.05%) | ||
| Coronary heart disease, n (%) | 0.21 | 0.29 | ||||
| Yes | 34 (65.38%) | 18 (34.62%) | 44 (84.62%) | 8 (15.38%) | ||
| No | 519 (74.25%) | 180 (25.75%) | 631 (90.27%) | 68 (9.73%) | ||
| Kidney diseases, n (%) | 0.16 | 0.15 | ||||
| Yes | 30 (63.83%) | 167 (36.17%) | 38 (82.61%) | 8 (17.39%) | ||
| No | 523 (74.29%) | 181 (25.71%) | 637 (90.35%) | 68 (9.65%) | ||
| Pulmonary diseases, n (%) | 0.07 | 0.15 | ||||
| Yes | 68 (66.02%) | 35 (33.98%) | 88 (85.44%) | 15 (14.56%) | ||
| No | 485 (74.85%) | 163 (25.15%) | 587 (90.59%) | 61 (9.41%) | ||
| Cigarette smoking, n (%) | 0.43 | 0.31 | ||||
| Yes | 175 (75.76%) | 56 (24.24%) | 212 (91.77%) | 19 (8.23%) | ||
| No | 378 (75.76%) | 142 (27.31%) | 463 (89.04%) | 57 (10.96%) | ||
| Alcohol consumption, n (%) | 0.41 | 0.76 | ||||
| Yes | 170 (75.89%) | 54 (24.11%) | 203 (90.62%) | 21 (9.38%) | ||
| No | 383 (72.68%) | 144 (27.32%) | 472 (89.56%) | 54 (10.44%) | ||
| Family history of stroke, n (%) | 0.19 | 0.74 | ||||
| Yes | 11 (57.89%) | 8 (42.11%) | 18 (94.74%) | 1 (5.26%) | ||
| No | 542 (74.04%) | 190 (25.96%) | 657 (89.75%) | 75 (10.25%) | ||
| Coagulative disorders, n (%) | 0.05 | 0.86 | ||||
| Yes | 6 (46.15%) | 7 (53.85%) | 11 (84.62%) | 2 (15.38%) | ||
| No | 547 (74.12%) | 191 (25.88%) | 664 (89.97%) | 74 (10.03%) | ||
| Anticoagulation therapy, n (%) | 0.19 | 0.66 | ||||
| Yes | 11 (57.89%) | 8 (42.11%) | 16 (84.21%) | 3 (15.79%) | ||
| No | 542 (74.04%) | 190 (25.96%) | 659 (90.03%) | 73 (9.97%) | ||
| Antiplatelet therapy, n (%) | 0.61 | 0.07 | ||||
| Yes | 2 (50.00%) | 2 (50.00%) | 2 (50.00%) | 2 (50.00%) | ||
| No | 551 (73.76%) | 196 (26.24%) | 673 (90.09%) | 74 (9.91%) | ||
| Laboratory studies | ||||||
| BG, mmol/L | 7.16 (6.07–8.85) | 9.25 (7.35–11.64) | <0.001 *** | 7.38 (6.24–9.41) | 9.37 (7.35–12.45) | <0.001 *** |
| Creatinine, µmol/L | 69 (56–84) | 72 (60–96) | 0.004 ** | 69 (56–85) | 79 (64–116) | <0.001 *** |
| Uric acid, µmol/L | 324 (250–407) | 338 (257–419) | 0.26 | 321 (250–407) | 348 (288–439) | 0.03 * |
| TG, mmol/L | 1.14 (0.80–1.72) | 1.21 (0.87–1.73) | 0.09 | 1.13 (0.81–1.69) | 1.38 (0.88–1.99) | 0.03 * |
| Cholesterol, mmol/L | 4.42 (3.78–5.06) | 4.34 (3.68–5.06) | 0.36 | 4.40 (3.76–5.06) | 4.36 (3.66–5.12) | 0.50 |
| HDLC, mmol/L | 1.29 (1.03–1.61) | 1.33 (1.03–1.66) | 0.19 | 1.30 (1.04–1.63) | 1.31 (1.02–1.61) | 0.33 |
| LDLC, mmol/L | 2.60 (2.08–3.21) | 2.51 (1.91–3.24) | 0.11 | 2.60 (2.05–3.21) | 2.40 (1.83–3.3) | 0.07 |
| Sodium, mmol/L | 138.4 (136.1–140.3) | 138.3 (134.0–142.6) | 0.29 | 138.4 (136.1–140.4) | 137.9 (133.6–142.3) | 0.45 |
| Chlorine, mmol/L | 101.4 (98.8–104.3) | 100.5 (95.5–105.4) | 0.002 ** | 101.3 (98.6–104.3) | 99.6 (94.8–104.4) | 0.001 ** |
| eGFR, mL/min | 91.0 (87.7–103.5) | 91.0 (77.2–100.9) | <0.001 *** | 91.0 (87.0–103.6) | 86.0 (63.6–91.0) | <0.001 *** |
| Platelet, 109 cells/L | 170 (129–217) | 184 (136–222) | 0.12 | 175 (131–218) | 175 (98–252) | 0.35 |
| WBC, 109 cells/L | 10.11 (7.58–12.99) | 11.91 (9.22–15.43) | <0.001 *** | 10.54 (7.76–13.22) | 11.62 (8.24–16.45) | 0.006 ** |
| ANC, 109 cells/L | 8.43 (5.67–11.25) | 10.33 (7.09–13.26) | <0.001 *** | 8.81 (5.91–11.51) | 9.64 (6.17–13.57) | 0.03 * |
| ALC, 109 cells/L | 1.09 (0.76–1.48) | 1.17 (0.72–1.82) | 0.08 | 1.09 (0.75–1.51) | 1.19 (0.73–1.99) | 0.06 |
| AMC, 109 cells/L | 0.39 (0.26–0.53) | 0.42 (0.28–0.62) | 0.006 ** | 0.4 (0.26–0.54) | 0.47 (0.30–0.62) | 0.02 * |
| Hematocrit | 0.41 (0.38–0.44) | 0.41 (0.37–0.44) | 0.29 | 0.41 (0.38–0.44) | 0.42 (0.37–0.44) | 0.23 |
| Fibrinogen, g/L | 2.77 (2.26–3.41) | 2.74 (2.16–3.57) | 0.44 | 2.75 (2.24–3.42) | 2.77 (2.28–3.61) | 0.27 |
| D-dimer, mg/L FEU | 0.64 (0.31–1.94) | 1.43 (0.63–2.84) | <0.001 *** | 0.72 (0.32–2.16) | 2.37 (0.80–5.24) | <0.001 *** |
| Treatment, n (%) | 0.92 | 0.74 | ||||
| Surgery | 172 (73.19%) | 63 (26.81%) | 213 (90.64%) | 22 (9.36%) | ||
| Conservative | 381 (73.84%) | 135 (26.16%) | 462 (89.53%) | 54 (10.47%) | ||
| Algorithm | Functional Outcome | Mortality | ||
|---|---|---|---|---|
| AUC, Mean | AUC, 95%CI | AUC, Mean | AUC, 95% CI | |
| ICH score | 0.856 | 0.827–0.884 | 0.790 | 0.712–0.867 |
| LR | 0.890 | 0.858–0.922 | 0.837 | 0.780–0.894 |
| LRCV | 0.887 | 0.855–0.920 | 0.844 | 0.807–0.881 |
| SVM | 0.849 | 0.804–0.894 | 0.777 | 0.720–0.833 |
| RF | 0.862 | 0.813–0.912 | 0.818 | 0.718–0.917 |
| XGBoost | 0.863 | 0.815–0.911 | 0.820 | 0.741–0.899 |
| CatBoost | 0.871 | 0.829–0.913 | 0.841 | 0.774–0.907 |
| Algorithm | Variables for Functional Outcome a | Variables for Mortality a |
|---|---|---|
| LR | Coagulation disorders, Location of the hematoma, GCS, IVH, AMC, BG, BT, D-dimer, Age, ANC, Chlorine | Location of the hematoma, AMC, GCS, DM, WBC, D-Dimer, ANC, BG, Age, Chlorine, IVH, HR, Time in ER, BT |
| LRCV | Coagulation disorders, Location of the hematoma, AMC, GCS, IVH, BG, ANC, WBC, D-dimer, Age, BT | AMC, Location of the hematoma, DM, GCS, WBC, ANC, IVH, D-Dimer, Age, Chlorine, BG, TG, HR, Hematoma volume, BT |
| SVM b | - | - |
| RF | GCS, BG, Hematoma volume, Location of the hematoma, D-Dimer, IVH | GCS, D-dimer, Age, BG, HR, eGFR, Time in ER, Hematoma volume, Chlorine, ANC, WBC, Location of the hematoma, Creatine, Uric acid, TG, BT, IVH, DM |
| XGBoost | GCS, BG, D-dimer, Location of the hematoma, eGFR, Hematoma volume, Age, WBC, Creatine, Chlorine | GCS, D-dimer, Age, WBC, Location of the hematoma, Hematoma volume, eGFR, HR, Chlorine, Time in ER, Creatine, ANC, TG |
| CatBoost | GCS, BG, D-dimer | GCS, Age, D-dimer, HR, Time in ER, Chlorine, eGFR, Location of the hematoma, Hematoma volume |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Zhang, R.; Liu, R.; Liu, Y.; Li, H.; Ma, L.; He, M.; You, C.; Tian, R. Machine Learning-Based Approaches for Prediction of Patients’ Functional Outcome and Mortality after Spontaneous Intracerebral Hemorrhage. J. Pers. Med. 2022, 12, 112. https://doi.org/10.3390/jpm12010112
Guo R, Zhang R, Liu R, Liu Y, Li H, Ma L, He M, You C, Tian R. Machine Learning-Based Approaches for Prediction of Patients’ Functional Outcome and Mortality after Spontaneous Intracerebral Hemorrhage. Journal of Personalized Medicine. 2022; 12(1):112. https://doi.org/10.3390/jpm12010112
Chicago/Turabian StyleGuo, Rui, Renjie Zhang, Ran Liu, Yi Liu, Hao Li, Lu Ma, Min He, Chao You, and Rui Tian. 2022. "Machine Learning-Based Approaches for Prediction of Patients’ Functional Outcome and Mortality after Spontaneous Intracerebral Hemorrhage" Journal of Personalized Medicine 12, no. 1: 112. https://doi.org/10.3390/jpm12010112
APA StyleGuo, R., Zhang, R., Liu, R., Liu, Y., Li, H., Ma, L., He, M., You, C., & Tian, R. (2022). Machine Learning-Based Approaches for Prediction of Patients’ Functional Outcome and Mortality after Spontaneous Intracerebral Hemorrhage. Journal of Personalized Medicine, 12(1), 112. https://doi.org/10.3390/jpm12010112






