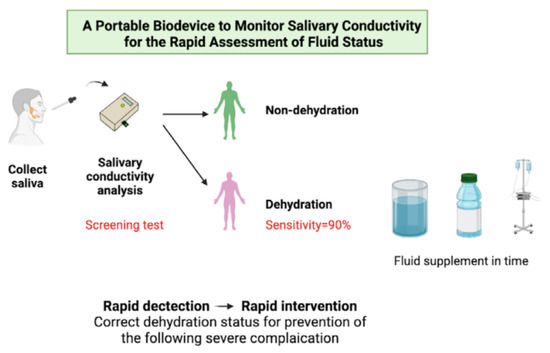
A Portable Biodevice to Monitor Salivary Conductivity for the Rapid Assessment of Fluid Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Subjects
2.3. Experimental Procedures
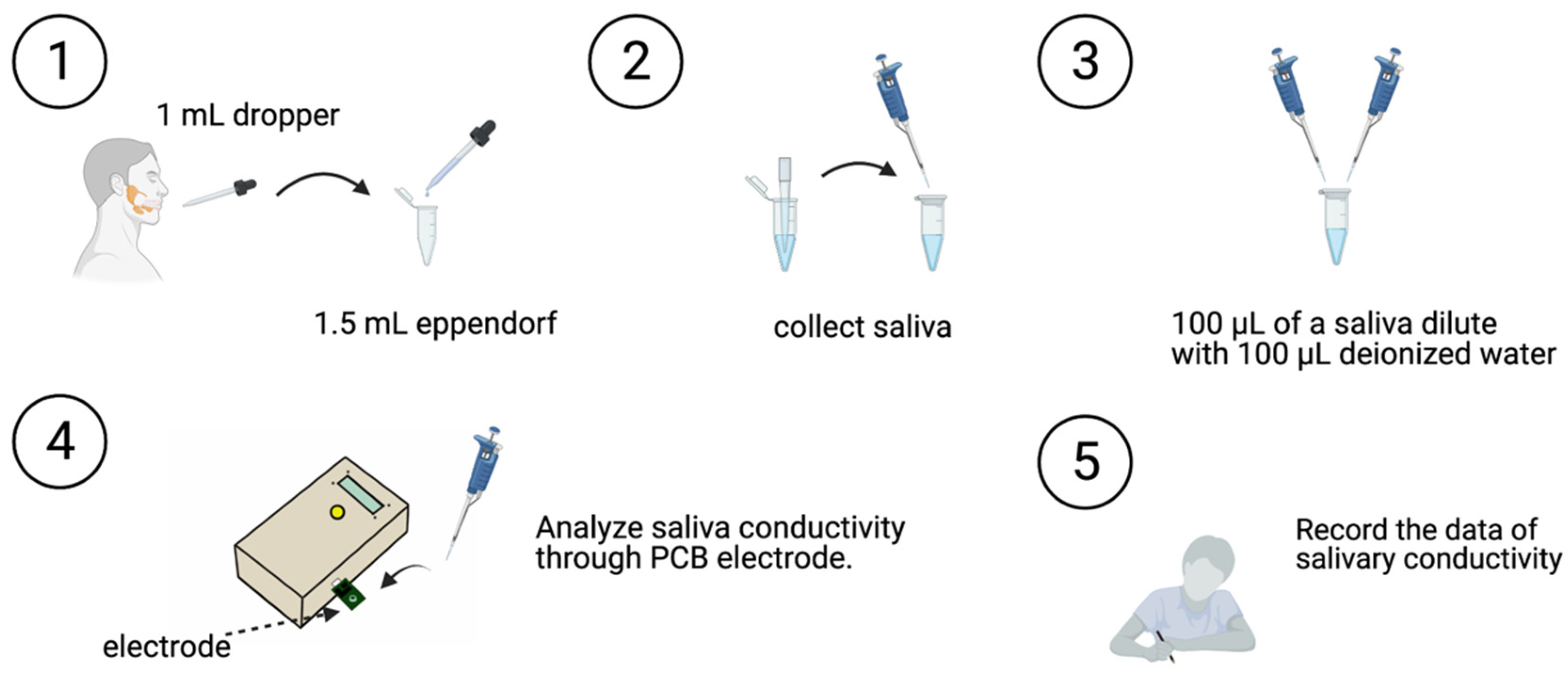
2.4. Saliva Collection and Analysis
- The participant swallowed to empty their mouth.
- Saliva was collected with a 1-mL dropper, and the collected salivary sample was placed in a 1.5 mL Eppendorf tube.
- The saliva sample (100 μL) was then diluted with 100 μL of deionized water.
- Saliva conductivity was then analyzed using the developed portable monitor with a disposable printed-circuit board (PCB) electrode.
- Data on salivary conductivity was then recorded.
2.5. Blood and Urine Collection and Analysis
2.6. Evaluation of the Thirst Intensity Scales
2.7. Statistical Analysis
3. Results
3.1. Demographics of the Participants
3.2. Biochemical, Urinary, and Hematological Data
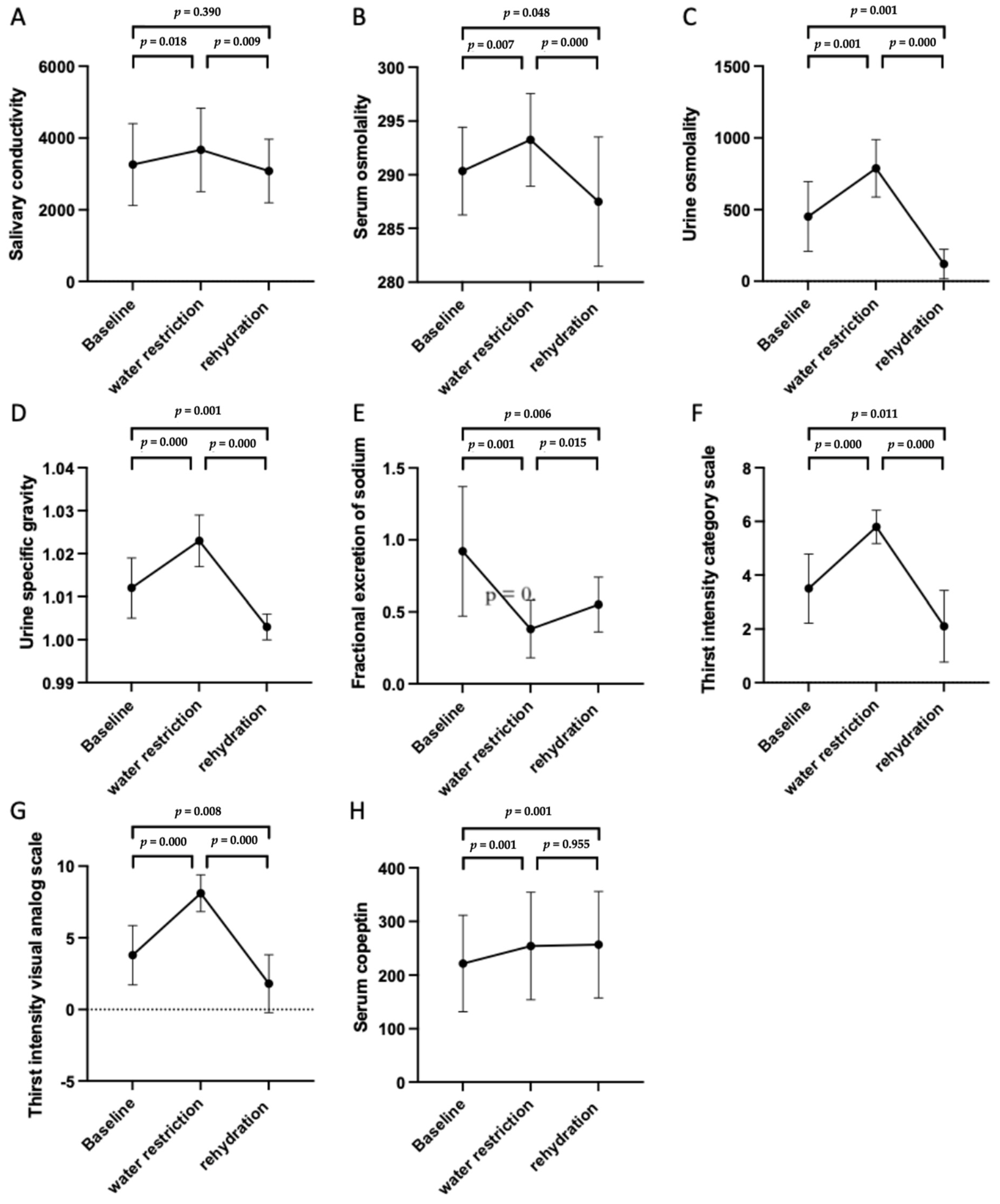
3.3. Trends of the Different Biomarkers and Parameters in Changing Fluid Status
3.4. Use of Salivary Conductivity as a Screening Biomarker in Patients with Body Fluid Deprivement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lier, H.; Bernhard, M.; Hossfeld, B. Hypovolemic and hemorrhagic shock. Anaesthesist 2018, 67, 225–244. [Google Scholar] [CrossRef]
- Manz, F. Hydration and disease. J. Am. Coll. Nutr. 2007, 26, 535s–541s. [Google Scholar] [CrossRef]
- Fortes, L.S.; Nascimento-Júnior, J.R.A.; Mortatti, A.L.; Lima-Júnior, D.; Ferreira, M.E.C. Effect of dehydration on passing decision making in soccer athletes. Res. Q. Exerc. Sport 2018, 89, 332–339. [Google Scholar] [CrossRef]
- Hurley, S.W.; Arseth, H.A.; Johnson, A.K. Orexin neurons couple neural systems mediating fluid balance with motivation-related circuits. Behav. Neurosci. 2018, 132, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, O.C.; Thoma, V. Water supplementation after dehydration improves judgment and decision-making performance. Psychol. Res. 2020, 84, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef]
- Moghadamyeghaneh, Z.; Phelan, M.J.; Carmichael, J.C.; Mills, S.D.; Pigazzi, A.; Nguyen, N.T.; Stamos, M.J. Preoperative dehydration increases risk of postoperative acute renal failure in colon and rectal surgery. J. Gastrointest. Surg. 2014, 18, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Feehally, J.; Khosravi, M. Effects of acute and chronic hypohydration on kidney health and function. Nutr. Rev. 2015, 73 (Suppl. 2), 110–119. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Hoffman, R.; Saharov, G.; Brenner, B.; Nadir, Y. Dehydration as a possible cause of monthly variation in the incidence of venous thromboembolism. Clin. Appl. Thromb. Hemost. 2016, 22, 569–574. [Google Scholar] [CrossRef]
- Fortes, M.B.; Owen, J.A.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J. Am. Med. Dir. Assoc. 2015, 16, 221–228. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Attreed, N.J.; Campbell, W.W.; Channell, A.M.; Chassagne, P.; Culp, K.R.; Fletcher, S.J.; Fortes, M.B.; Fuller, N.; et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst. Rev. 2015, 2015, Cd009647. [Google Scholar] [CrossRef]
- Bunn, D.K.; Hooper, L. Signs and Symptoms of Low-Intake Dehydration Do Not Work in Older Care Home Residents-DRIE Diagnostic Accuracy Study. J. Am. Med. Dir. Assoc. 2019, 20, 963–970. [Google Scholar] [CrossRef]
- Popowski, L.A.; Oppliger, R.A.; Patrick Lambert, G.; Johnson, R.F.; Kim Johnson, A.; Gisolf, C.V. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sports Exerc. 2001, 33, 747–753. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Ely, B.R.; Kenefick, R.W.; Sawka, M.N. Biological variation and diagnostic accuracy of dehydration assessment markers. Am. J. Clin. Nutr. 2010, 92, 565–573. [Google Scholar] [CrossRef]
- Morgan, R.M.; Patterson, M.J.; Nimmo, M.A. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat. Acta Physiol. Scand. 2004, 182, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.B.; Diment, B.C.; Di Felice, U.; Gunn, A.E.; Kendall, J.L.; Esmaeelpour, M.; Walsh, N.P. Tear fluid osmolarity as a potential marker of hydration status. Med. Sci. Sports Exerc. 2011, 43, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Ely, B.R.; Cheuvront, S.N.; Kenefick, R.W.; Sawka, M.N. Limitations of salivary osmolality as a marker of hydration status. Med. Sci. Sports Exerc. 2011, 43, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Huang, J.W.; Lee, I.N.; Weng, R.C.; Lin, M.Y.; Yang, J.T.; Lin, C.T. A portable system to monitor saliva conductivity for dehydration diagnosis and kidney healthcare. Sci. Rep. 2019, 9, 14771. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Campbell, R.G. Hunger in humans induced by 2-deoxy-D-glucose: Glucoprivic control of taste preference and food intake. Science 1977, 198, 1065–1068. [Google Scholar] [CrossRef]
- Rolls, B.J.; Wood, R.J.; Rolls, E.T.; Lind, H.; Lind, W.; Ledingham, J.G. Thirst following water deprivation in humans. Am. J. Physiol. 1980, 239, R476–R482. [Google Scholar] [CrossRef]
- Martínez-Camblor, P.; Pardo-Fernández, J.C. The Youden index in the generalized receiver operating characteristic curve context. Int. J. Biostat. 2019, 15. [Google Scholar] [CrossRef]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Yilmaz, S.; Dincer, N.; Ortiz, A.; Sag, A.A.; Covic, A.; Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cherney, D.Z.I.; Johnson, R.J.; et al. Antidiuretic hormone and serum osmolarity physiology and related outcomes: What is old, what is new, and what is unknown? J. Clin. Endocrinol. Metab. 2019, 104, 5406–5420. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.C.; Boilesen, S.N.; Tahan, S.; Melli, L.C.; Morais, M.B. Prevalence of voluntary dehydration according to urine osmolarity in elementary school students in the metropolitan region of São Paulo, Brazil. Clinics (Sao Paulo) 2019, 74, e903. [Google Scholar] [CrossRef]
- Bar-David, Y.; Urkin, J.; Kozminsky, E. The effect of voluntary dehydration on cognitive functions of elementary school children. Acta Paediatr. 2005, 94, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Kavouras, S.A.; Walsh, N.P.; Roberts, W.O. Diagnosing dehydration? Blend evidence with clinical observations. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 434–438. [Google Scholar] [CrossRef]
- Millard-Stafford, M.; Wendland, D.M.; O’Dea, N.K.; Norman, T.L. Thirst and hydration status in everyday life. Nutr. Rev. 2012, 70 (Suppl. 2), S147–S151. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Giersch, G.E.W.; Dunn, L.; Fiol, A.; Muñoz, C.X.; Lee, E.C. Inputs to thirst and drinking during water restriction and rehydration. Nutrients 2020, 12, 2554. [Google Scholar] [CrossRef]
- Davies, H.; Leslie, G.; Jacob, E.; Morgan, D. Estimation of body fluid status by fluid balance and body weight in critically ill adult patients: A systematic review. Worldviews Evid. Based Nurs. 2019, 16, 470–477. [Google Scholar] [CrossRef]
- Hamouti, N.; Del Coso, J.; Avila, A.; Mora-Rodriguez, R. Effects of athletes’ muscle mass on urinary markers of hydration status. Eur. J. Appl. Physiol. 2010, 109, 213–219. [Google Scholar] [CrossRef]
- Hamouti, N.; Del Coso, J.; Mora-Rodriguez, R. Comparison between blood and urinary fluid balance indices during dehydrating exercise and the subsequent hypohydration when fluid is not restored. Eur. J. Appl. Physiol. 2013, 113, 611–620. [Google Scholar] [CrossRef]
- Lemetais, G.; Melander, O.; Vecchio, M.; Bottin, J.H.; Enhörning, S.; Perrier, E.T. Effect of increased water intake on plasma copeptin in healthy adults. Eur. J. Nutr. 2018, 57, 1883–1890. [Google Scholar] [CrossRef]
- Muñoz, C.X.; Johnson, E.C.; Demartini, J.K.; Huggins, R.A.; McKenzie, A.L.; Casa, D.J.; Maresh, C.M.; Armstrong, L.E. Assessment of hydration biomarkers including salivary osmolality during passive and active dehydration. Eur. J. Clin. Nutr. 2013, 67, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.A.; van den Heuvel, A.M.; Kerry, P.; McGhee, S.; Peoples, G.E.; Brown, M.A.; Patterson, M.J. Observations on saliva osmolality during progressive dehydration and partial rehydration. Eur. J. Appl. Physiol. 2012, 112, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Laing, S.J.; Oliver, S.J.; Montague, J.C.; Walters, R.; Bilzon, J.L. Saliva parameters as potential indices of hydration status during acute dehydration. Med. Sci. Sports Exerc. 2004, 36, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Pross, N.; Demazières, A.; Girard, N.; Barnouin, R.; Santoro, F.; Chevillotte, E.; Klein, A.; Le Bellego, L. Influence of progressive fluid restriction on mood and physiological markers of dehydration in women. Br. J. Nutr. 2013, 109, 313–321. [Google Scholar] [CrossRef]
- Smith, D.L.; Shalmiyeva, I.; Deblois, J.; Winke, M. Use of salivary osmolality to assess dehydration. Prehosp. Emerg. Care 2012, 16, 128–135. [Google Scholar] [CrossRef]
- Rogers, B.B.; Shankar, P.; Jerris, R.C.; Kotzbauer, D.; Anderson, E.J.; Watson, J.R.; O’Brien, L.A.; Uwindatwa, F.; McNamara, K.; Bost, J.E. Impact of a rapid respiratory panel test on patient outcomes. Arch. Pathol. Lab. Med. 2015, 139, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Lee, H.S.; Park, M.S.; Kim, I.S.; Lee, S.M. Evaluation of the barricor tube in 28 routine chemical tests and its impact on turnaround time in an outpatient clinic. Ann. Lab. Med. 2021, 41, 277–284. [Google Scholar] [CrossRef]
- Choi, K.; Chang, I.; Lee, J.C.; do Kyun, K.; Noh, S.; Ahn, H.; Cho, J.H.; Kwak, Y.H.; Kim, S.; Kim, H.C. Smartphone-based urine reagent strip test in the emergency department. Telemed. J. E Health 2016, 22, 534–540. [Google Scholar] [CrossRef]
- Hooper, L.; Bunn, D.; Jimoh, F.O.; Fairweather-Tait, S.J. Water-loss dehydration and aging. Mech. Ageing Dev. 2014, 136–137, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Harr, K.E. Sample collection. Vet. Clin. North Am. Exot. Anim. Pract. 2018, 21, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Stadler, G.; Lane, S.P.; McClure, M.J.; Jackson, G.L.; Clavél, F.D.; Iida, M.; Gleason, M.E.J.; Xu, J.H.; Bolger, N. Initial elevation bias in subjective reports. Proc. Natl. Acad. Sci. USA 2018, 115, E15–E23. [Google Scholar] [CrossRef] [PubMed]


| Variables | All patients (N = 20) | Male (N = 10) | Female (N = 10) | p Value |
|---|---|---|---|---|
| Age, years | 35.15 ± 4.09 | 35.50 ± 4.48 | 34.80 ± 3.88 | 0.684 |
| HbA1c, % | 5.43 ± 0.26 | 5.43 ± 0.29 | 5.43 ± 0.25 | 0.280 |
| eGFR, mL/min/1.73 m2 | 96.12 ± 16.67 | 91.70 ± 16.90 | 100.54 ± 16.04 | 0.912 |
| Height, cm | 168.68 ± 9.08 | 175.03 ± 4.06 | 162.33 ± 8.25 | 0.000 *** |
| Weight, kg | 69.37 ± 14.67 | 76.84 ± 10.80 | 61.89 ± 14.62 | 0.011 * |
| BMI, kg/m2 | 24.22 ± 3.67 | 25.07 ± 3.30 | 23.37 ± 3.99 | 0.123 |
| Variables | Baseline (Day 1, 0600 p.m.) | 12-h Water Restriction (Day 1, 06:00 p.m. to Day 2, 06:00 a.m.) | 1-h Water Rehydration (Day 2, 06:00 a.m. to Day 2, 07:00 a.m.) | p Value |
|---|---|---|---|---|
| Biochemical parameters | ||||
| Blood urea nitrogen, mg/dL | 11.63 ± 1.95 | 11.17 ± 2.11 | 10.89 ± 1.82 | 0.279 |
| Creatinine, mg/dL | 0.8335 ± 0.18 | 0.8240 ± 0.16 | 0.7835 ± 0.17 | 0.001 *** |
| Sodium, mEq/L | 141.15 ± 2.08 | 141.95 ± 1.82 | 140.05 ± 1.99 | 0.001 *** |
| eGFR, mL/min/1.73 m2 | 96.12 ± 16.67 | 96.35 ± 13.32 | 103.10 ± 17.11 | 0.001 *** |
| Serum glucose, mg/dL | 93.45 ± 14.04 | 84.10 ± 8.84 | 85.80 ± 10.27 | 0.010 ** |
| HbA1c, % | 5.43 ± 0.26 | 5.42 ± 0.26 | 5.42 ± 0.24 | 0.815 |
| eAG, mg/dL | 109.2 ± 7.46 | 108.70 ± 7.51 | 108.90 ± 6.77 | 0.815 |
| Urinary analysis | ||||
| Urine creatinine, mg/dL | 87.59 ± 81.39 | 206.80 ± 96.26 | 22.96 ± 21.68 | 0.000 *** |
| Urine sodium, mEq/L | 94.9 ± 50.08 | 117.55 ± 46.98 | 28.55 ± 21.05 | 0.001 *** |
| Hematological parameters | ||||
| RBC count, 1000/uL | 4.92 ± 0.66 | 5.01 ± 0.64 | 4.94 ± 0.74 | 0.014 * |
| Hemoglobin, g/dL | 13.90 ± 1.40 | 14.18 ± 1.51 | 13.96 ± 1.66 | 0.018 * |
| Hematocrit, % | 41.19 ± 3.57 | 42.00 ± 4.00 | 41.23 ± 4.26 | 0.002 ** |
| Variables | Baseline (Day 1, 06:00 p.m.) | 12-h Water Restriction (Day 1, 06:00 p.m. to Day 2, 06:00 a.m.) | 1-h Water Rehydration (Day 2, 0600 a.m. to Day 2, 07:00 a.m.) | p Value |
|---|---|---|---|---|
| Salivary conductivity, μs/cm | 3263.141 ± 1140.30 | 3671.445 ± 1161.93 | 3081.006 ± 884.62 | 0.019 * |
| Serum osmolality, mOsm/kgH2O | 290.35 ± 4.08 | 293.25 ± 4.30 | 287.50 ± 6.02 | 0.000 *** |
| Urine Osmolality, mOsm/kgH2O | 451.95 ± 243.96 | 787.80 ± 200.00 | 119.20 ± 102.25 | 0.000 *** |
| Urine SG | 1.012 ± 0.007 | 1.023 ± 0.006 | 1.003 ± 0.003 | 0.000 *** |
| Thirst intensity CS | 3.50 ± 1.28 | 5.80 ± 0.62 | 2.10 ± 1.33 | 0.000 *** |
| Thirst intensity VAS | 3.79 ± 2.07 | 8.11 ± 1.28 | 1.80 ± 2.02 | 0.000 *** |
| Serum copeptin, pg/mL | 221.56 ± 89.87 | 254.31 ± 100.05 | 256.74 ± 99.24 | 0.001 ** |
| FeNa, % | 0.92 ± 0.45 | 0.38 ± 0.20 | 0.55 ± 0.19 | 0.000 *** |
| Weight, kg | 69.37 ± 14.67 | 76.84 ± 10.80 | 61.89 ± 14.62 | 0.011 * |
| ACE test, IU/L | 15.35 ± 4.10 | 15.81 ± 4.01 | 15.48 ± 3.91 | 0.779 |
| Aldosterone, ng/dL | 10.877 ± 8.17 | 11.37 ± 6.77 | 10.32 ± 5.87 | 0.861 |
| Renin, ng/L | 18.196 ± 13.79 | 16.25 ± 8.76 | 14.75 ± 7.03 | 0.287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lu, Y.-P.; Lee, A.-T.; Tung, C.-W.; Tsai, Y.-H.; Tsay, H.-P.; Lin, C.-T.; Yang, J.-T. A Portable Biodevice to Monitor Salivary Conductivity for the Rapid Assessment of Fluid Status. J. Pers. Med. 2021, 11, 577. https://doi.org/10.3390/jpm11060577
Chen C-H, Lu Y-P, Lee A-T, Tung C-W, Tsai Y-H, Tsay H-P, Lin C-T, Yang J-T. A Portable Biodevice to Monitor Salivary Conductivity for the Rapid Assessment of Fluid Status. Journal of Personalized Medicine. 2021; 11(6):577. https://doi.org/10.3390/jpm11060577
Chicago/Turabian StyleChen, Chun-Hao, Yen-Pei Lu, An-Ting Lee, Chun-Wu Tung, Yuan-Hsiung Tsai, Hsin-Pei Tsay, Chih-Ting Lin, and Jen-Tsung Yang. 2021. "A Portable Biodevice to Monitor Salivary Conductivity for the Rapid Assessment of Fluid Status" Journal of Personalized Medicine 11, no. 6: 577. https://doi.org/10.3390/jpm11060577
APA StyleChen, C.-H., Lu, Y.-P., Lee, A.-T., Tung, C.-W., Tsai, Y.-H., Tsay, H.-P., Lin, C.-T., & Yang, J.-T. (2021). A Portable Biodevice to Monitor Salivary Conductivity for the Rapid Assessment of Fluid Status. Journal of Personalized Medicine, 11(6), 577. https://doi.org/10.3390/jpm11060577