What Can We Change in Diet and Behaviour in Order to Decrease Carotid Intima-Media Thickness in Patients with Obesity?
Abstract
1. Introduction
2. Intima-Media Thickness in General Practice
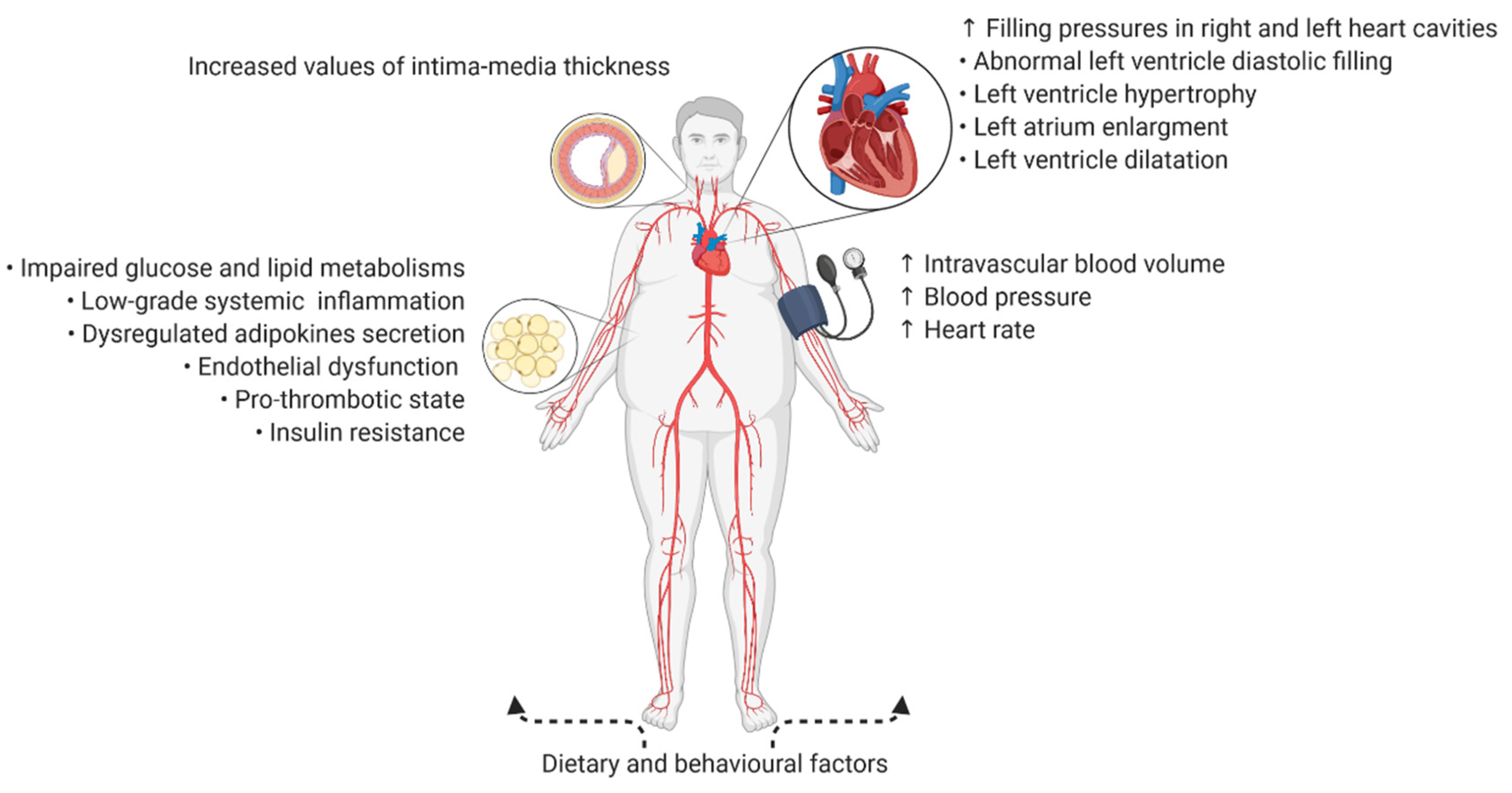
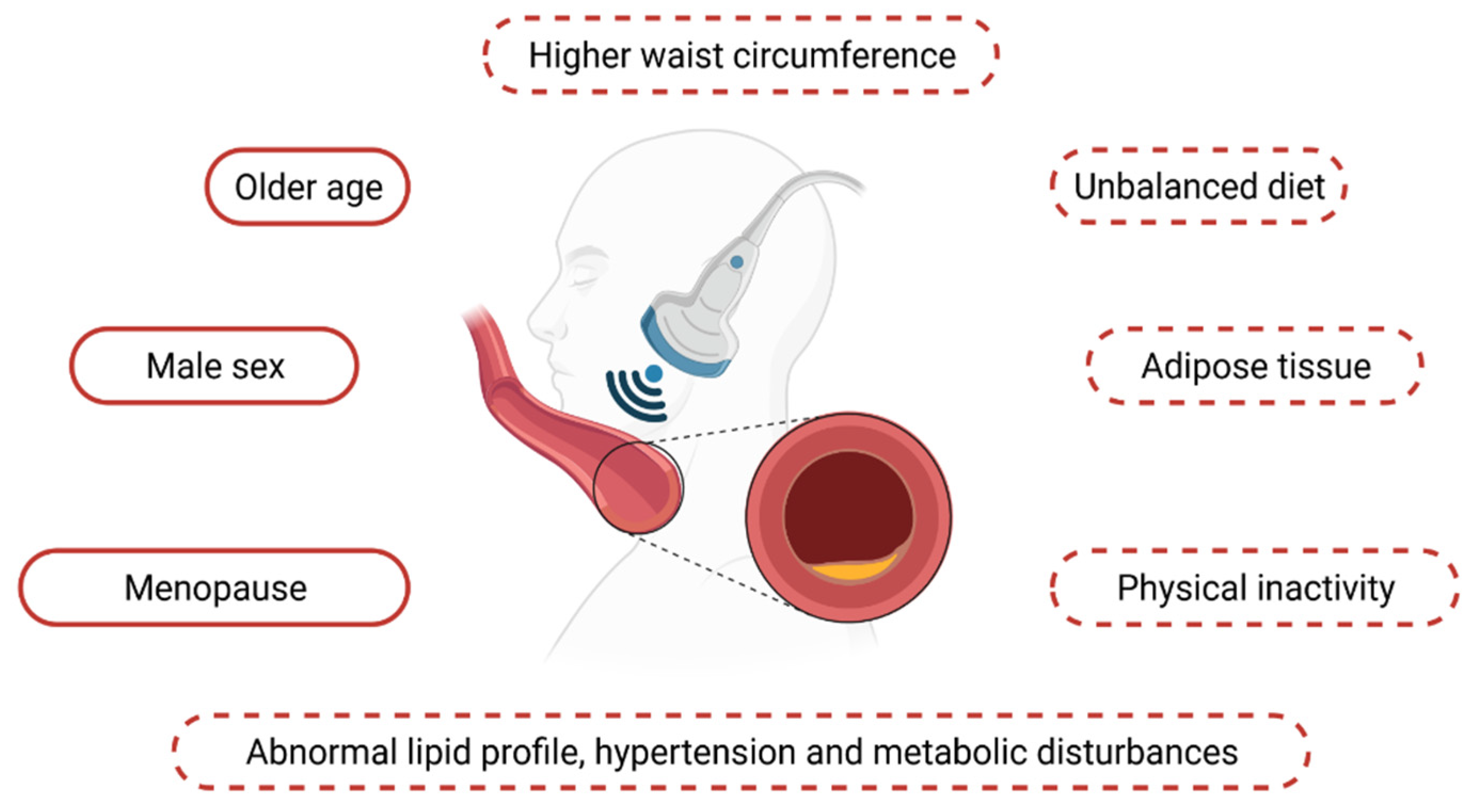
3. Intima-Media Thickness Assessment among Patients with Obesity—Subclinical Atherosclerosis and the Predisposing Factors
4. Reducing cIMT Values—Behavioural and Clinical Factors
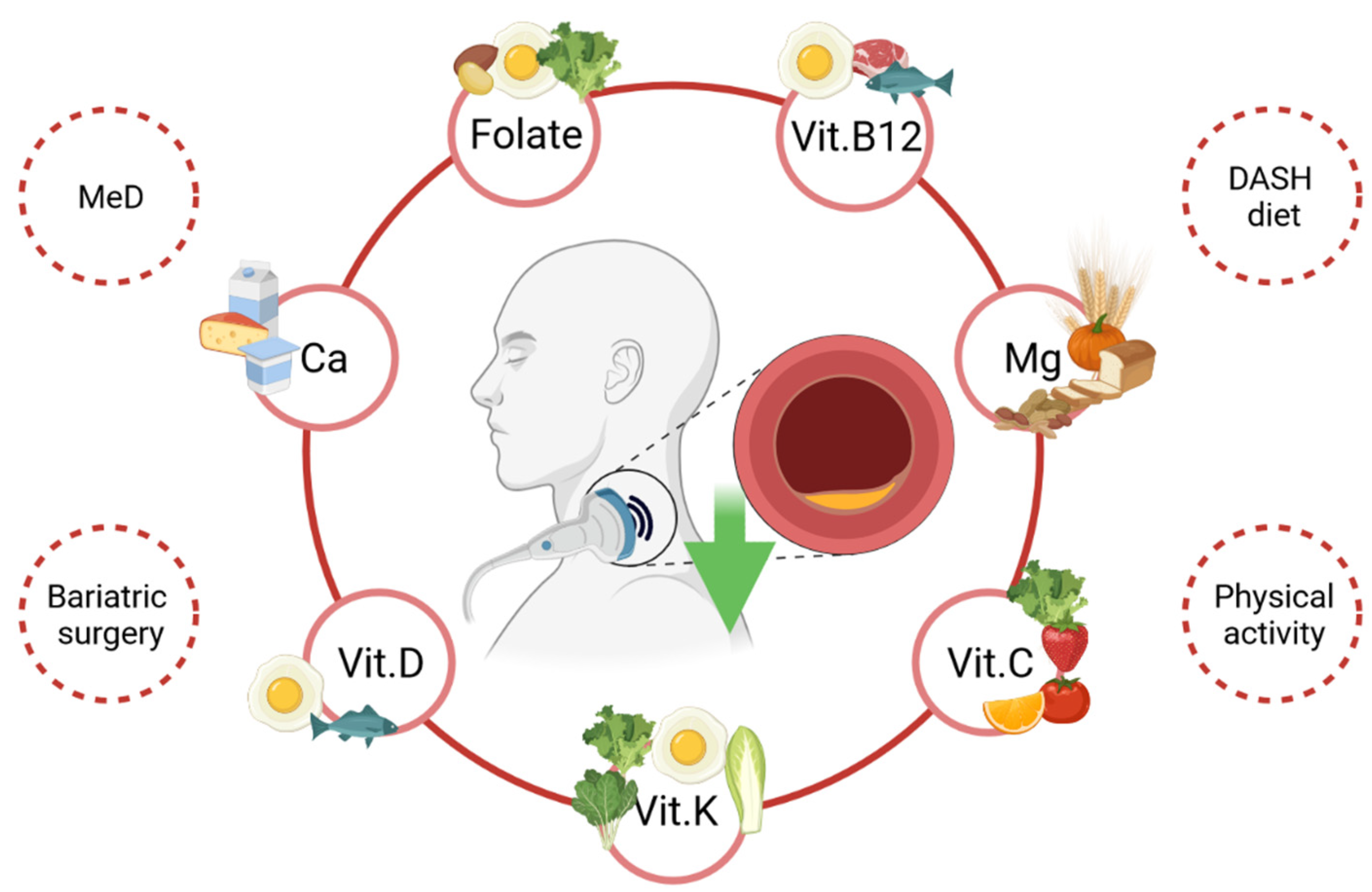
4.1. Diet
4.1.1. Folate, Folic Acid and Vitamin B12
4.1.2. Vitamin D, Vitamin K and Calcium
4.1.3. Magnesium
4.1.4. Mediterranean and the Dietary Approach to Stop Hypertension Diets
4.1.5. Fats and Carbohydrates
4.2. Lifestyle, Physical Activity and Diet Quality Interventions
4.3. Weight Loss Interventions and Bariatric Surgery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, E.S.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 November 2019).
- Xue, R.; Li, Q.; Geng, Y.; Wang, H.; Wang, F.; Zhang, S. Abdominal obesity and risk of CVD: A dose–response meta-analysis of thirty-one prospective studies. Br. J. Nutr. 2021, 1–31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pignoli, P.; Tremoli, E.; Poli, A.; Oreste, P.; Paoletti, R. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation 1986, 74, 1399–1406. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Mandviwala, T.; Khalid, U.; Deswal, A. Obesity and Cardiovascular Disease: A Risk Factor or a Risk Marker? Curr. Atheroscler. Rep. 2016, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Hussain, S.; Sarwar, S.; Moghal, M.R.; Das, A.; Hossain, M.Z.; Chowdhury, J.A.; Millat, S.; Islam, M.S. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1213–1224. [Google Scholar] [CrossRef]
- Rychter, A.; Skrzypczak-Zielińska, M.; Zielińska, A.; Eder, P.; Souto, E.; Zawada, A.; Ratajczak, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity? Int. J. Mol. Sci. 2020, 21, 5229. [Google Scholar] [CrossRef]
- Kozakova, M.; Natali, A.; Dekker, J.; Beck-Nielsen, H.; Laakso, M.; Nilsson, P.; Balkau, B.; Ferrannini, E. RISC Investigators Insulin Sensitivity and Carotid Intima-Media Thickness: Relationship between Insulin Sensitivity and Cardiovascular Risk Study. Arter. Thromb. Vasc. Biol. 2013, 33, 1409–1417. [Google Scholar] [CrossRef]
- Ahmad, J.; Ahmed, F.; Siddiqui, M.A.; Hameed, B.; Ahmad, I. Inflammation, insulin resistance and carotid IMT in first degree relatives of north Indian type 2 diabetic subjects. Diabetes Res. Clin. Pract. 2006, 73, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xu, A.; Hui, X.; Zhou, P.; Li, X.; Zhong, H.; Tang, W.; Huang, G.; Zhou, Z. Circulating Lipocalin-2 and Retinol-Binding Protein 4 Are Associated with Intima-Media Thickness and Subclinical Atherosclerosis in Patients with Type 2 Diabetes. PLoS ONE 2013, 8, e66607. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Szcześniewska, D.; Szostak-Węgierek, D.; Kwaśniewska, M.; Pająk, A.; Stepaniak, U.; Kozakiewicz, K.; Tykarski, A.; Zdrojewski, T.; Zujko, M.E.; et al. Are dietary habits of the Polish population consistent with the recommendations for prevention of cardiovascular disease?—WOBASZ II project. Kardiol. Polska 2016, 74, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P. Intima-media thickness: Indicator of cardiovascular risk and measure of the extent of atherosclerosis. Vasc. Med. 2004, 9, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Larraya, J.G.; Irimia, P.; Martínez-Vila, E.; Barba, J.; Guembe, M.J.; Varo, N.; Castellano, J.M.; Díez, J. The influence of obesity on the assessment of carotid intima-media thickness. J. Clin. Ultrasound 2012, 40, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L.; Breslau, P.J.; Briët, E.; De Bruyn, A.M.; Van Vliet, H.H.; Ouweland, F.A.V.D.; De Jong, P.T.; Hofman, A.; Grobbee, D.E. Cardiovascular determinants of carotid artery disease. The Rotterdam Elderly Study. Hypertension 1992, 19, 717–720. [Google Scholar] [CrossRef]
- Folsom, A.R.; Kronmal, R.A.; Detrano, R.C.; O’Leary, D.H.; Bild, D.E.; Bluemke, D.A.; Budoff, M.J.; Liu, K.; Shea, S.; Szklo, M.; et al. Coronary Artery Calcification Compared With Carotid Intima-Media Thickness in the Prediction of Cardiovascular Disease IncidenceThe Multi-Ethnic Study of Atherosclerosis (MESA). Arch. Intern. Med. 2008, 168, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Bild, D.E.; Bluemke, D.A.; Burke, G.L.; Detrano, R.; Roux, A.V.D.; Folsom, A.R.; Greenland, P.; Jacobs, D.R., Jr.; Kronmal, R.A.; Liu, K.; et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am. J. Epidemiol. 2002, 156, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; von Kegler, S.; Steinmetz, H.; Markus, H.S.; Sitzer, M. Carotid Intima-Media Thickening Indicates a Higher Vascular Risk Across a Wide Age Range: Prospective Data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006, 37, 87–92. [Google Scholar] [CrossRef]
- Baigent, C.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; Backer, G.G.D.; Delgado, V.; Ference, B.A.; Graham, I.M.; Halliday, A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar]
- Stein, J.H.; Korcarz, C.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- Touboul, P.; Hennerici, M.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Ebrahim, S.; Hernandez, R.H.; Jaff, M.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011): An Update on Behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar]
- Wang, Z.; Li, W.; Tian, J. Gender is a determinant of carotid artery stiffness independent of age and blood pressure. Br. J. Radiol. 2021, 94, 20200796. [Google Scholar] [CrossRef]
- Ojima, S.; Kubozono, T.; Kawasoe, S.; Kawabata, T.; Miyahara, H.; Tokushige, K.; Ohishi, M. Gender differences in the risk factors associated with atherosclerosis by carotid intima-media thickness, plaque score, and pulse wave velocity. Heart Vessel. 2021, 1–11. [Google Scholar] [CrossRef]
- Simon, A.; Gariepy, J.; Chironi, G.; Megnien, J.-L.; Levenson, J. Intima–media thickness: A new tool for diagnosis and treatment of cardiovascular risk. J. Hypertens. 2002, 20, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jarauta, E.; Mateo-Gallego, R.; Bea, A.; Burillo, E.; Calmarza, P.; Civeira, F. Carotid Intima-Media Thickness in Subjects With No Cardiovascular Risk Factors. Rev. Esp. Cardiol. (Engl. Ed.) 2010, 63, 97–102. [Google Scholar] [CrossRef]
- Mil, S.R.; Biter, L.U.; Geijn, G.J.M.; Birnie, E.; Dunkelgrun, M.; Ijzermans, J.N.M.; Meulen, N.; Mannaerts, G.H.H.; Cabezas, M.C. The effect of sex and menopause on carotid intima-media thickness and pulse wave velocity in morbid obesity. Eur. J. Clin. Investig. 2019, 49, e13118. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Chiarello, P.; Buscemi, C.; Corleo, D.; Massenti, M.F.; Barile, A.M.; Rosafio, G.; Maniaci, V.; Settipani, V.; Cosentino, L.; et al. Characterization of Metabolically Healthy Obese People and Metabolically Unhealthy Normal-Weight People in a General Population Cohort of the ABCD Study. J. Diabetes Res. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Romagnolli, C.; Bensenor, I.M.; Santos, I.S.; Lotufo, P.A.; Bittencourt, M.S. Impact of metabolically healthy obesity on carotid intima-media thickness—The Brazilian Longitudinal Study of Adult Health. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Farcas, A.D.; Vonica, C.L.; Golea, A. Non-alcoholic fatty liver disease, bulb carotid intima-media thickness and obesity phenotypes: Results of a prospective observational study. Med. Ultrason. 2017, 19, 265. [Google Scholar] [CrossRef]
- Jae, S.Y.; Franklin, B.; Choi, Y.-H.; Fernhall, B. Metabolically Healthy Obesity and Carotid Intima-Media Thickness. Mayo Clin. Proc. 2015, 90, 1217–1224. [Google Scholar] [CrossRef]
- Talavera-Garcia, E.; Delgado-Lista, J.; Garcia-Rios, A.; Delgado-Casado, N.; Gomez-Luna, P.; Gomez-Garduño, A.; Delgado, F.G.; Alcala-Diaz, J.F.; Yubero-Serrano, E.; Marin, C.; et al. Influence of Obesity and Metabolic Disease on Carotid Atherosclerosis in Patients with Coronary Artery Disease (CordioPrev Study). PLoS ONE 2016, 11, e0153096. [Google Scholar] [CrossRef]
- Lin, A.; Lacy, M.E.; Eaton, C.; Correa, A.; Wu, W.-C. Inflammatory Obesity Phenotypes, Gender Effects and Subclinical Atherosclerosis in African Americans: The Jackson Heart Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2431–2438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B. Carotid-Wall Intima–Media Thickness and Cardiovascular Events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100,667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Talari, H.R.; Rafiee, M.; Farrokhian, A.; Raygan, F.; Bahmani, F.; Mofrad, M.D.; Hamidian, Y.; Tamtaji, O.R.; Karamali, F.; Asemi, Z. The Effects of Folate Supplementation on Carotid Intima-Media Thickness and Metabolic Status in Patients with Metabolic Syndrome. Ann. Nutr. Metab. 2016, 69, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Savopoulos, C.; Karamitsos, D.; Economou, I.; Destanis, E.; Chryssogonidis, I.; Pidonia, I.; Zebekakis, P.; Polatides, C.; Sion, M.; et al. The effect of folic acid supplementation on carotid intima-media thickness in patients with cardiovascular risk: A randomized, placebo-controlled trial. Int. J. Cardiol. 2010, 143, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Karamali, M.; Esmaillzadeh, A. Metabolic response to folate supplementation in overweight women with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Mol. Nutr. Food Res. 2014, 58, 1465–1473. [Google Scholar] [CrossRef]
- Qin, X.; Xu, M.; Zhang, Y.; Li, J.; Xu, X.; Wang, X.; Xu, X.; Huo, Y. Effect of folic acid supplementation on the progression of carotid intima-media thickness: A meta-analysis of randomized controlled trials. Atherosclerosis 2012, 222, 307–313. [Google Scholar] [CrossRef]
- Appel, L.J. Beyond (or back to) traditional risk factors: Preventing cardiovascular disease in patients with chronic kidney disease. Ann. Intern. Med. 2004, 140, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.H.; Chaitanya, V.; Suchitra, M.M.; Rao, P.V.L.N.S.; Lakshmi, B.V.; Kumar, V.S. Osteopontin, cardiovascular risk factors and carotid intima-Media thickness in chronic kidney disease. Indian J. Nephrol. 2018, 28, 358–364. [Google Scholar] [CrossRef]
- Yu, F.-P.; Zhao, Y.-C.; Gu, B.; Hu, J.; Yang, Y.-Y. Chronic Kidney Disease and Carotid Atherosclerosis in Patients With Acute Stroke. Neurologist 2015, 20, 23–26. [Google Scholar] [CrossRef]
- Kajitani, N.; Uchida, H.A.; Suminoe, I.; Kakio, Y.; Kitagawa, M.; Sato, H.; Wada, J. Chronic kidney disease is associated with carotid atherosclerosis and symptomatic ischaemic stroke. J. Int. Med. Res. 2018, 46, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Yilmaz, M.I.; Siriopol, D.; Unal, H.U.; Saglam, M.; Karaman, M.; Gezer, M.; Sonmez, A.; Eyileten, T.; Aydin, I.; et al. Thyroid function and cardiovascular events in chronic kidney disease patients. J. Nephrol. 2016, 30, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, Y.; Jiang, Y.; Zhu, M. The Association between Serum Retinol-Binding Protein 4 Levels and Cardiovascular Events in Patients with Chronic Kidney Disease. Lab. Med. 2020, 51, 491–497. [Google Scholar] [CrossRef]
- Ito, S.; Nagasawa, T.; Abe, M.; Mori, T. Strain vessel hypothesis: A viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens. Res. 2009, 32, 115–121. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Li, X.-J.; Zhang, W.; Liu, C.-Q.; Zhang, H.-J.; Lin, J.-R.; Yan, B.; Yu, Y.-X.; Shi, X.-L.; Li, C.-D.; et al. Chinese Lacto-Vegetarian Diet Exerts Favorable Effects on Metabolic Parameters, Intima-Media Thickness, and Cardiovascular Risks in Healthy Men. Nutr. Clin. Pract. 2012, 27, 392–398. [Google Scholar] [CrossRef]
- Kwok, T.; Chook, P.; Qiao, M.; Tam, L.; Poon, Y.K.P.; Ahuja, A.T.; Woo, J.; Celermajer, D.S.; Woo, K.S. Vitamin B-12 supplementation improves arterial function in vegetarians with subnormal vitamin B-12 status. J. Nutr. Health Aging 2012, 16, 569–573. [Google Scholar] [CrossRef]
- Petchey, W.G.; Hickman, I.J.; Duncan, E.; Prins, J.B.; Hawley, C.M.; Johnson, D.W.; Barraclough, K.; Isbel, N.M. The role of 25-hydroxyvitamin D deficiency in promoting insulin resistance and inflammation in patients with Chronic Kidney Disease: A randomised controlled trial. BMC Nephrol. 2009, 10, 41–46. [Google Scholar] [CrossRef]
- Asemi, Z.; Karamali, M.; Esmaillzadeh, A. Effects of calcium–vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: A randomised placebo-controlled trial. Diabetologia 2014, 57, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Raygan, F.; Bahmani, F.; Rezavandi, Z.; Talari, H.R.; Rafiee, M.; Poladchang, S.; Mofrad, M.D.; Taheri, S.; Mohammadi, A.A.; et al. The effects of vitamin D, K and calcium co-supplementation on carotid intima-media thickness and metabolic status in overweight type 2 diabetic patients with CHD. Br. J. Nutr. 2016, 116, 286–293. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3–5. Pol. Arch. Intern. Med. 2015, 125, 631–640. [Google Scholar] [CrossRef]
- Li, S.; Na, L.; Li, Y.; Gong, L.; Yuan, F.; Niu, Y.; Zhao, Y.; Sun, C. Long-term calcium supplementation may have adverse effects on serum cholesterol and carotid intima-media thickness in postmenopausal women: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2013, 98, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Mason, B.; Horne, A.; Ames, R.; Clearwater, J.; Bava, U.; Orr-Walker, B.; Wu, F.; Evans, M.C.; Gamble, G.D. Effects of Calcium Supplementation on Serum Lipid Concentrations in Normal Older Women: A Randomized Controlled Trial. Am. J. Med. 2002, 112, 343–347. [Google Scholar] [CrossRef]
- Bostick, R.M.; Fosdick, L.; Grandits, G.A.; Grambsch, P.; Gross, M.; Louis, T.A. Effect of Calcium Supplementation on Serum Cholesterol and Blood Pressure: A Randomized, Double-blind, Placebo-Controlled, Clinical Trial. Arch. Fam. Med. 2000, 9, 31–38. [Google Scholar] [CrossRef]
- Gannagé-Yared, M.H.; Azoury, M.; Mansour, I.; Baddoura, R.; Halaby, G.; Naaman, R. Effects of a short-term calcium and vitamin D treatment on serum cytokines, bone markers, insulin and lipid concentrations in healthy post-menopausal women. J. Endocrinol. Investig. 2003, 26, 748–753. [Google Scholar] [CrossRef]
- Reid, I.R. Cardiovascular Effects of Calcium Supplements. Nutrients 2013, 5, 2522–2529. [Google Scholar] [CrossRef]
- Talari, H.R.; Zakizade, M.; Soleimani, A.; Bahmani, F.; Ghaderi, A.; Mirhosseini, N.; Eslahi, M.; Babadi, M.; Mansournia, M.A.; Asemi, Z. Effects of magnesium supplementation on carotid intima–media thickness and metabolic profiles in diabetic haemodialysis patients: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2019, 121, 809–817. [Google Scholar] [CrossRef]
- Silva, A.P. Magnesium and Mortality in Patients with Diabetes and Early Chronic Kidney Disease. J. Diabetes Metab. 2014, 5. [Google Scholar] [CrossRef]
- Tzanakis, I.; Virvidakis, K.; Tsomi, A.; Mantakas, E.; Girousis, N.; Karefyllakis, N.; Papadaki, A.; Kallivretakis, N.; Mountokalakis, T. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes. Res. 2004, 17, 102–108. [Google Scholar] [PubMed]
- Chacko, S.A.; Sul, J.; Song, Y.; Li, X.; Leblanc, J.; You, Y.; Butch, A.; Liu, S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: A randomized, double-blind, controlled, crossover trial in overweight individuals. Am. J. Clin. Nutr. 2010, 93, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Wright, C.B.; Gu, Y.; Demmer, R.T.; Boden-Albala, B.; Elkind, M.S.V.; Sacco, R.L.; Scarmeas, N. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: The Northern Manhattan Study. Am. J. Clin. Nutr. 2011, 94, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Rychter, A.M.; Ratajczak, A.E.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Non-Systematic Review of Diet and Nutritional Risk Factors of Cardiovascular Disease in Obesity. Nutrients 2020, 12, 814. [Google Scholar] [CrossRef]
- Gardener, H.; Wright, C.; Cabral, D.; Scarmeas, N.; Gu, Y.; Cheung, K.; Elkind, M.S.; Sacco, R.L.; Rundek, T. Mediterranean diet and carotid atherosclerosis in the Northern Manhattan Study. Atherosclerosis 2014, 234, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Murie-Fernandez, M.; Irimia, P.; Toledo, E.; Martínez-Vila, E.; Buil-Cosiales, P.; Serrano-Martínez, M.; Ruíz-Gutierrez, V.; Ros, E.; Estruch, R.; Martínez-González, M. Ángel Carotid intima-media thickness changes with Mediterranean diet: A randomized trial (PREDIMED-Navarra). Atherosclerosis 2011, 219, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Romero-Mamani, E.-S.; Gilabert, R.; Núñez, I.; De La Torre, R.; Corella, L.; Ruiz-Gutiérrez, V.; López-Sabater, M.C.; Pintó, X.; Rekondo, J.; et al. Changes in Ultrasound-Assessed Carotid Intima-Media Thickness and Plaque With a Mediterranean Diet: A Substudy of the PREDIMED Trial. Arter. Thromb. Vasc. Biol. 2014, 34, 439–445. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Salas-Salvadó, J.; Ros, E.; Estruch, R.; Corella, D.; Fitó, M.; Martínez-González, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. The PREDIMED trial, Mediterranean diet and health outcomes: How strong is the evidence? Nutr. Metab. Cardiovasc. Dis. 2017, 27, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Ros, E. The PREDIMED study. Endocrinol. Diabetes Nutr. 2017, 64, 63–66. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Scappaticcio, L.; Giugliano, D.; Esposito, K. Mediterranean diet cools down the inflammatory milieu in type 2 diabetes: The MÉDITA randomized controlled trial. Endocrine 2016, 54, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Gicchino, M.; Caputo, M.; Giugliano, D.; Esposito, K. Effect of a Mediterranean diet on endothelial progenitor cells and carotid intima-media thickness in type 2 diabetes: Follow-up of a randomized trial. Eur. J. Prev. Cardiol. 2017, 24, 399–408. [Google Scholar] [CrossRef]
- da Luz, P.; Coimbra, S.; Favarato, D.; Albuquerque, C.; Mochiduky, R.; Rochitte, C.; Hojaij, E.; Gonsalves, C.; Laurindo, F. Coronary artery plaque burden and calcium scores in healthy men adhering to long-term wine drinking or alcohol abstinence. Braz. J. Med. Biol. Res. 2014, 47, 697–705. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, K.; Moreira, T.M.; Belfort, G.P.; Silva, C.F.D.M.D.; Padilha, P.D.C.; De Barros, D.C.; Saunders, C. Adaptação da dieta DASH (Dietary Approaches to Stop Hypertension) para cuidado nutricional no período pós-parto, no âmbito da Atenção Básica. Rev. Bras. Epidemiol. 2019, 22, e190035. [Google Scholar] [CrossRef]
- Maddock, J.; Ziauddeen, N.; Ambrosini, G.L.; Wong, A.; Hardy, R.; Ray, S. Adherence to a Dietary Approaches to Stop Hypertension (DASH)-type diet over the life course and associated vascular function: A study based on the MRC 1946 British birth cohort. Br. J. Nutr. 2018, 119, 581–589. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.A.; Kovacs, C.; Moreira, P.; Magnoni, D.; Saleh, M.H.; Faintuch, J. Unsaturated Fatty Acids Improve Atherosclerosis Markers in Obese and Overweight Non-diabetic Elderly Patients. Obes. Surg. 2017, 27, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Angerer, P.; Kothny, W.; Störk, S.; von Schacky, C. Effect of dietary supplementation with ω-3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovasc. Res. 2002, 54, 183–190. [Google Scholar] [CrossRef][Green Version]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.-P.; Voutilainen, S. Dietary Fatty Acids and Risk of Coronary Heart Disease in Men: The Kuopio Ischemic Heart Disease Risk Factor Study. Arter. Thromb. Vasc. Biol. 2014, 34, 2679–2687. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Huang, W.-S.; Chen, H.-C.; Chang, C.-H.; Lee, L.-T.; Chen, H.-S.; Kang, Y.-D.; Chie, W.-C.; Jan, C.-F.; Wang, W.-D.; et al. Effect of a 90 g/day low-carbohydrate diet on glycaemic control, small, dense low-density lipoprotein and carotid intima-media thickness in type 2 diabetic patients: An 18-month randomised controlled trial. PLoS ONE 2020, 15, e0240158. [Google Scholar] [CrossRef]
- Petersen, K.; Keogh, J.; Lister, N.; Clifton, P. Dietary quality and carotid intima media thickness in type 1 and type 2 diabetes: Follow-up of a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 830–838. [Google Scholar] [CrossRef]
- Petersen, K.S.; Clifton, P.M.; Blanch, N.; Keogh, J.B. Effect of improving dietary quality on carotid intima media thickness in subjects with type 1 and type 2 diabetes: A 12-mo randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 771–779. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Mitchell, S.; Sahye-Pudaruth, S.; Coveney, J.; Olowoyeye, O.; Patel, D.; De Souza, R.J.; Augustin, L.S.; et al. Cross-sectional associations between dietary intake and carotid intima media thickness in type 2 diabetes: Baseline data from a randomised trial. BMJ Open 2017, 7, e015026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.; Ling, W.; Feng, D.; Wei, X.; Yang, C.; Ma, J. Fruit Consumption Is Associated with Lower Carotid Intima-Media Thickness and C-Reactive Protein Levels in Patients with Type 2 Diabetes Mellitus. J. Am. Diet. Assoc. 2011, 111, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Blekkenhorst, L.C.; Bondonno, C.P.; Lewis, J.R.; Woodman, R.; Devine, A.; Bondonno, N.P.; Lim, W.H.; Zhu, K.; Beilin, L.J.; Thompson, P.L.; et al. Cruciferous and Total Vegetable Intakes Are Inversely Associated With Subclinical Atherosclerosis in Older Adult Women. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Blekkenhorst, L.C.; Prince, R.L.; Ivey, K.L.; Lewis, J.R.; Devine, A.; Woodman, R.J.; Lundberg, J.O.; Croft, K.D.; Thompson, P.L.; et al. Association of Vegetable Nitrate Intake With Carotid Atherosclerosis and Ischemic Cerebrovascular Disease in Older Women. Stroke 2017, 48, 1724–1729. [Google Scholar] [CrossRef]
- Zou, Z.-Y.; Xu, X.-R.; Lin, X.-M.; Zhang, H.-B.; Xiao, X.; Ouyang, L.; Huang, Y.-M.; Wang, X.; Liu, Y.-Q. Effects of lutein and lycopene on carotid intima–media thickness in Chinese subjects with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2014, 111, 474–480. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, K.; Chen, C.; Wang, P.; Zhang, B.; Zhou, Q.; Mei, F.; Su, Y. Soya isoflavone consumption in relation to carotid intima–media thickness in Chinese equol excretors aged 40–65 years. Br. J. Nutr. 2012, 108, 1698–1704. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Zahedmehr, A.; Mohammad-Zadeh, A.; Sanati, H.-R.; Shakerian, F.; Firouzi, A.; Kiani, R.; Nasrollahzadeh, J. Effect of garlic powder tablet on carotid intima-media thickness in patients with coronary artery disease: A Preliminary Randomized Controlled Trial. Nutr. Health 2013, 22, 143–155. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.N.; Columbus, M.L.; Shields, K.J.; Asubonteng, J.; Meyer, M.L.; Sutton-Tyrrell, K.; Goodpaster, B.H.; Delany, J.P.; Jakicic, J.M.; Barinas-Mitchell, E. Effects of an intensive behavioral weight loss intervention consisting of caloric restriction with or without physical activity on common carotid artery remodeling in severely obese adults. Metabolism 2012, 61, 1589–1597. [Google Scholar] [CrossRef]
- Vamvakis, A.; Gkaliagkousi, E.; Lazaridis, A.; Grammatikopoulou, M.G.; Triantafyllou, A.; Nikolaidou, B.; Koletsos, N.; Anyfanti, P.; Tzimos, C.; Zebekakis, P.; et al. Impact of Intensive Lifestyle Treatment (Diet Plus Exercise) on Endothelial and Vascular Function, Arterial Stiffness and Blood Pressure in Stage 1 Hypertension: Results of the HINTreat Randomized Controlled Trial. Nutrients 2020, 12, 1326. [Google Scholar] [CrossRef]
- Elkoustaf, R.A.; Aldaas, O.M.; Batiste, C.D.; Mercer, A.; Robinson, M.; Newton, D.; Burchett, R.; Cornelius, C.; Patterson, H.; Ismail, M.H. Lifestyle Interventions and Carotid Plaque Burden: A Comparative Analysis of Two Lifestyle Intervention Programs in Patients with Coronary Artery Disease. Perm. J. 2019, 23, 18.196. [Google Scholar] [CrossRef]
- Marshall, D.; Walizer, E.; Vernalis, M. The Effect of a One-Year Lifestyle Intervention Program on Carotid Intima Media Thickness. Mil. Med. 2011, 176, 798–804. [Google Scholar] [CrossRef]
- Lundby-Christensen, L.; Tarnow, L.; Hansen, D.L.; Worm, D.; Naver, L.S.; Hvolris, L.E.; Wiinberg, N.; Vaag, A.A.; Almdal, T.P. Carotid intima-media thickness is reduced 12 months after gastric bypass surgery in obese patients with type 2 diabetes or impaired glucose tolerance. J. Diabetes Complicat. 2014, 28, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Lima, M.M.D.O.; Felici, A.C.; Pareja, J.C.; Vasques, A.C.J.; Novaes, F.S.; Rodovalho, S.; Hirsch, F.F.P.; Matos-Souza, J.R.; Chaim, É.A.; et al. Early Regression of Carotid Intima-Media Thickness after Bariatric Surgery and Its Relation to Serum Leptin Reduction. Obes. Surg. 2017, 28, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Martin, J.M.; Aracil, E.; Galindo, J.; Escobar-Morreale, H.F.; Balsa, J.A.; Botella-Carretero, J.I. Improvement in cardiovascular risk in women after bariatric surgery as measured by carotid intima-media thickness: Comparison of sleeve gastrectomy versus gastric bypass. Surg. Obes. Relat. Dis. 2017, 13, 848–854. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychter, A.M.; Naskręt, D.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Can We Change in Diet and Behaviour in Order to Decrease Carotid Intima-Media Thickness in Patients with Obesity? J. Pers. Med. 2021, 11, 505. https://doi.org/10.3390/jpm11060505
Rychter AM, Naskręt D, Zawada A, Ratajczak AE, Dobrowolska A, Krela-Kaźmierczak I. What Can We Change in Diet and Behaviour in Order to Decrease Carotid Intima-Media Thickness in Patients with Obesity? Journal of Personalized Medicine. 2021; 11(6):505. https://doi.org/10.3390/jpm11060505
Chicago/Turabian StyleRychter, Anna Maria, Dariusz Naskręt, Agnieszka Zawada, Alicja Ewa Ratajczak, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "What Can We Change in Diet and Behaviour in Order to Decrease Carotid Intima-Media Thickness in Patients with Obesity?" Journal of Personalized Medicine 11, no. 6: 505. https://doi.org/10.3390/jpm11060505
APA StyleRychter, A. M., Naskręt, D., Zawada, A., Ratajczak, A. E., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). What Can We Change in Diet and Behaviour in Order to Decrease Carotid Intima-Media Thickness in Patients with Obesity? Journal of Personalized Medicine, 11(6), 505. https://doi.org/10.3390/jpm11060505





