Individual Resonant Frequencies at Low-Gamma Range and Cognitive Processing Speed
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Procedure
2.3. Cognitive Assessment
- Simple reaction time task, in which participants were as asked to detect the presence of a visual stimulus (A letter) as quickly and accurately as possible.
- Two-choice response reaction time task, in which participants had to indicate the direction of the displayed arrow by pressing the left or right button on a keyboard.
- Lexical decision task, in which participants were asked to indicate if the correct word was presented or it contained a mistake.
- Arithmetic decision task, in which subjects were presented with simple arithmetic expressions (simple additions or subtractions) and were asked to indicate whether the displayed outcome was correct or incorrect.
- Semantic categorization task, in which the participants were successively presented with words, and their task was to indicate if the word belonged to the specific category, e.g., furniture, animal, utensils, etc.
- Object judgement task assessing the mental rotation speed by making a decision as to whether two presented abstract waveforms are identical or different.
- Tower of London task (ToL), generally considering tapping at planning and execution speed. In this task, participants had to move the colored disks to achieve the goal configuration in as few moves as possible.
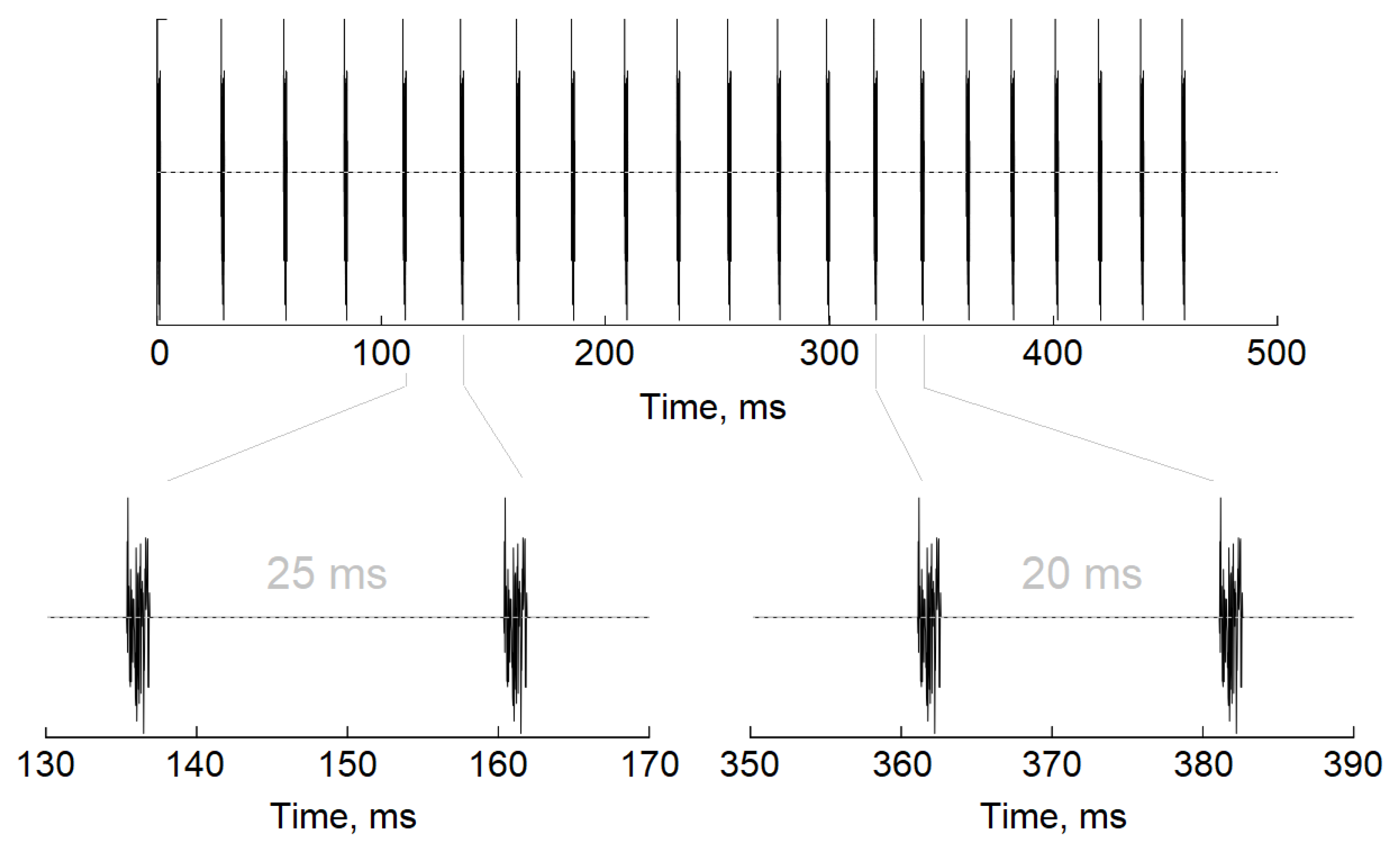
2.4. Auditory Stimulation
2.5. EEG Recording
2.6. EEG Processing
2.7. Statistical Analysis
3. Results
3.1. Cognitive Performance
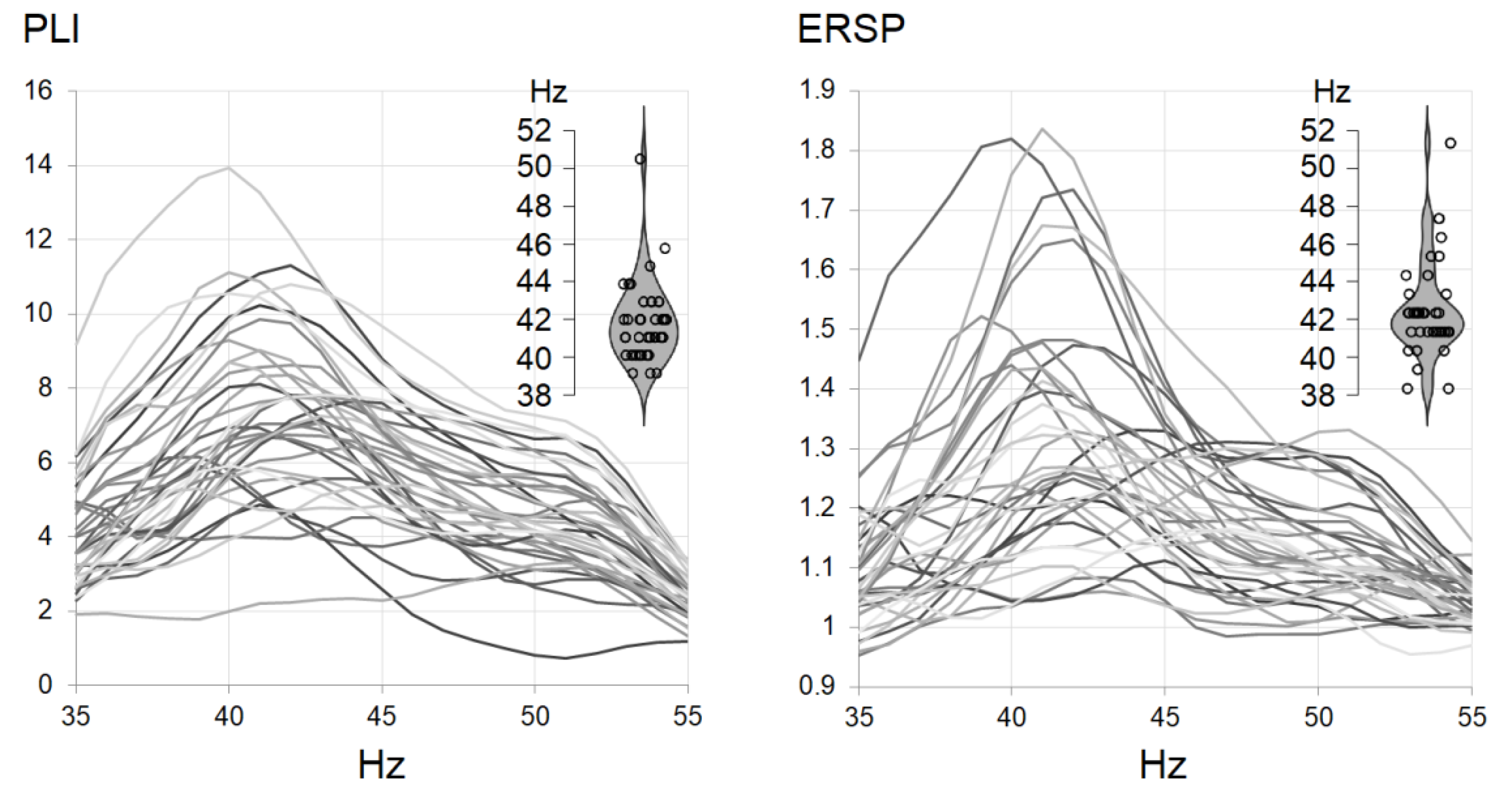
3.2. Envelope Following Responses
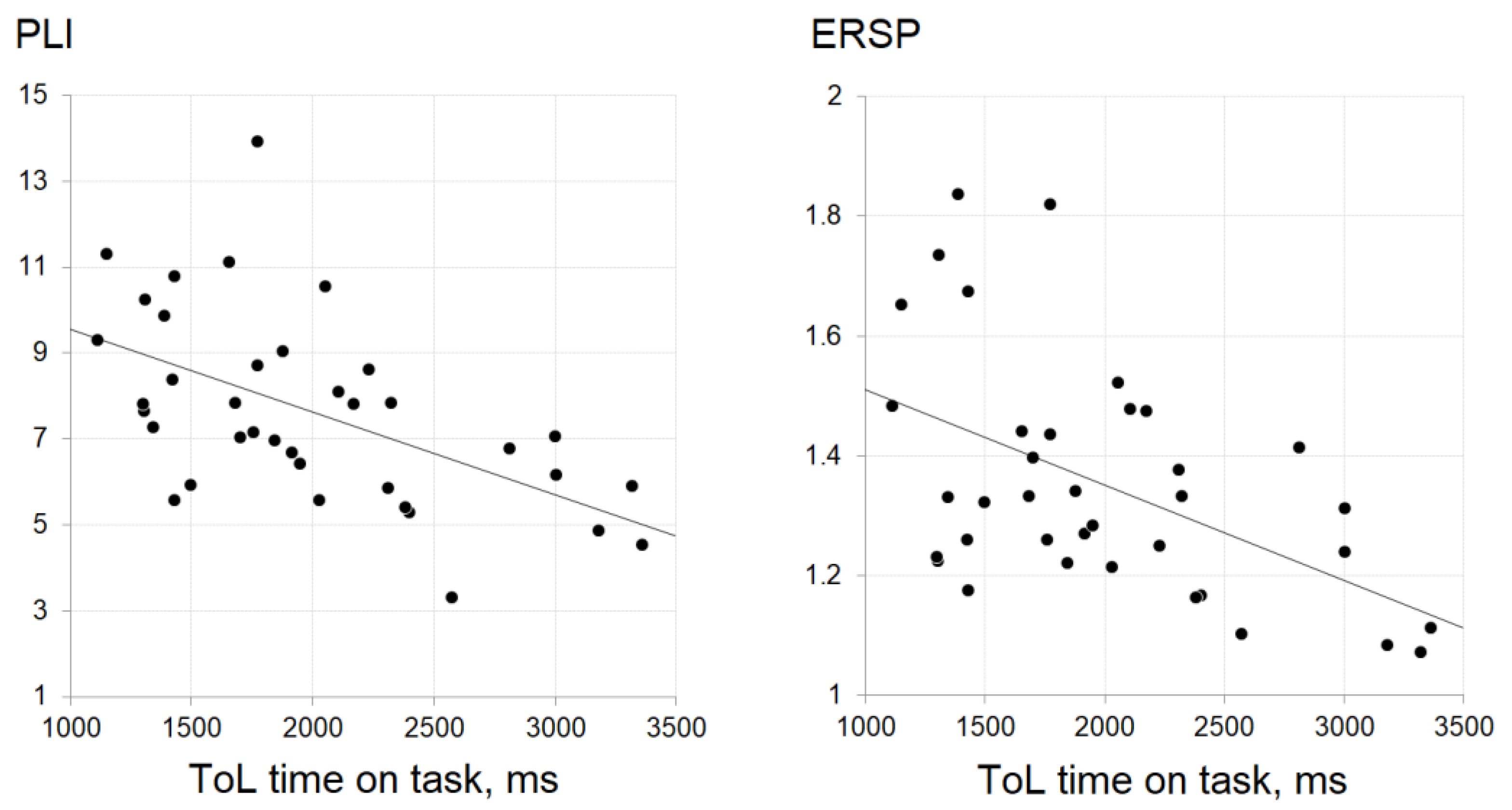
3.3. The Relationship between EEG Measures and Cognitive Processing Speed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Legget, K.T.; Hild, A.K.; Steinmetz, S.E.; Simon, S.T.; Rojas, D.C. MEG and EEG Demonstrate Similar Test-Retest Reliability of the 40 Hz Auditory Steady-State Response. Int. J. Psychophysiol. 2017, 114, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; George, N.; Lachaux, J.-P.; Martinerie, J.; Renault, B.; Varela, F.J. Perception’s Shadow: Long-Distance Synchronization of Human Brain Activity. Nature 1999, 397, 430–433. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O.; Delpuech, C.; Pernier, J. Stimulus Specificity of Phase-Locked and Non-Phase-Locked 40 Hz Visual Responses in Human. J. Neurosci. 1996, 16, 4240–4249. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human Gamma-Frequency Oscillations Associated with Attention and Memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Müller, M.M.; Ray, W.J.; Gruber, T.; Elbert, T. Human Gamma Band Activity and Perception of a Gestalt. J. Neurosci. 1999, 19, 7152–7161. [Google Scholar] [CrossRef] [PubMed]
- Pulvermüller, F.; Lutzenberger, W.; Preißl, H.; Birbaumer, N. Spectral Responses in the Gamma-Band Physiological Signs of Higher Cognitive Processes? Neurorep. Int. J. Rapid Commun. Res. Neurosci. 1995, 6, 2059–2064. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory Gamma Activity in Humans and Its Role in Object Representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Demiralp, T. Human EEG Gamma Oscillations in Neuropsychiatric Disorders. Clin. Neurophysiol. 2005, 116, 2719–2733. [Google Scholar] [CrossRef]
- Mathalon, D.H.; Sohal, V.S. Neural Oscillations and Synchrony in Brain Dysfunction and Neuropsychiatric Disorders: It’s About Time. JAMA Psychiatry 2015, 72, 840–844. [Google Scholar] [CrossRef]
- van Es, M.W.J.; Schoffelen, J.-M. Stimulus-Induced Gamma Power Predicts the Amplitude of the Subsequent Visual Evoked Response. NeuroImage 2019, 186, 703–712. [Google Scholar] [CrossRef]
- Picton, T.W.; John, M.S.; Dimitrijevic, A.; Purcell, D. Human Auditory Steady-State Responses: Respuestas Auditivas de Estado Estable En Humanos. Int. J. Audiol. 2003, 42, 177–219. [Google Scholar] [CrossRef] [PubMed]
- Picton, T. Hearing in Time: Evoked Potential Studies of Temporal Processing. Ear Hear. 2013, 34, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Hsu, J.L.; Hsieh, M.H.; Meyer-Gomes, K.; Sprock, J.; Swerdlow, N.R.; Braff, D.L. Gamma Band Oscillations Reveal Neural Network Cortical Coherence Dysfunction in Schizophrenia Patients. Biol. Psychiatry 2006, 60, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, S.-K.; Kim, D.-W.; Shim, M.; Kim, Y.-W.; Im, C.-H.; Lee, S.-H. Cortical Volume and 40-Hz Auditory-Steady-State Responses in Patients with Schizophrenia and Healthy Controls. NeuroImage Clin. 2019, 22, 101732. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, D.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. A Distributed Frontotemporal Network Underlies Gamma-Band Synchronization Impairments in Schizophrenia Patients. Neuropsychopharmacology 2020, 45, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, D.; Thomas, M.L.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Hierarchical Pathways from Sensory Processing to Cognitive, Clinical, and Functional Impairments in Schizophrenia. Schizophr. Bull. 2020, 47. [Google Scholar] [CrossRef]
- Koshiyama, D.; Miyakoshi, M.; Thomas, M.L.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Unique Contributions of Sensory Discrimination and Gamma Synchronization Deficits to Cognitive, Clinical, and Psychosocial Functional Impairments in Schizophrenia. Schizophr. Res. 2021, 228, 280–287. [Google Scholar] [CrossRef]
- Parciauskaite, V.; Bjekic, J.; Griskova-Bulanova, I. Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain Sci. 2021, 11, 217. [Google Scholar] [CrossRef]
- Arrondo, G.; Alegre, M.; Sepulcre, J.; Iriarte, J.; Artieda, J.; Villoslada, P. Abnormalities in Brain Synchronization Are Correlated with Cognitive Impairment in Multiple Sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2009, 15, 509–516. [Google Scholar] [CrossRef]
- van Deursen, J.A.; Vuurman, E.F.P.M.; van Kranen-Mastenbroek, V.H.J.M.; Verhey, F.R.J.; Riedel, W.J. 40-Hz Steady State Response in Alzheimer’s Disease and Mild Cognitive Impairment. Neurobiol. Aging 2011, 32, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Lehongre, K.; Ramus, F.; Villiermet, N.; Schwartz, D.; Giraud, A.-L. Altered Low-Gamma Sampling in Auditory Cortex Accounts for the Three Main Facets of Dyslexia. Neuron 2011, 72, 1080–1090. [Google Scholar] [CrossRef]
- Hirtum, T.V.; Ghesquière, P.; Wouters, J. Atypical Neural Processing of Rise Time by Adults with Dyslexia. Cortex 2019, 113, 128–140. [Google Scholar] [CrossRef]
- Puvvada, K.C.; Summerfelt, A.; Du, X.; Krishna, N.; Kochunov, P.; Rowland, L.M.; Simon, J.Z.; Hong, L.E. Delta Vs. Gamma Auditory Steady State Synchrony in Schizophrenia. Schizophr. Bull. 2018, 44, 378–387. [Google Scholar] [CrossRef]
- Rass, O.; Forsyth, J.; Krishnan, G.; Hetrick, W.P.; Klaunig, M.; Breier, A.; O’Donnell, B.F.; Brenner, C.A. Auditory Steady State Response in the Schizophrenia, First-Degree Relatives, and Schizotypal Personality Disorder. Schizophr. Res. 2012, 136, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhou, P.; Wang, C.; Fan, Y.; Tian, Q.; Dong, F.; Zhou, F.; Wang, C. Defects of Gamma Oscillations in Auditory Steady-State Evoked Potential of Schizophrenia. Shanghai Arch. Psychiatry 2018, 30, 27. [Google Scholar]
- Tada, M.; Nagai, T.; Kirihara, K.; Koike, S.; Suga, M.; Araki, T.; Kobayashi, T.; Kasai, K. Differential Alterations of Auditory Gamma Oscillatory Responses Between Pre-Onset High-Risk Individuals and First-Episode Schizophrenia. Cereb. Cortex 2016, 26, 1027–1035. [Google Scholar] [CrossRef]
- Baltus, A.; Herrmann, C.S. Auditory Temporal Resolution Is Linked to Resonance Frequency of the Auditory Cortex. Int. J. Psychophysiol. 2015, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Baltus, A.; Herrmann, C.S. The Importance of Individual Frequencies of Endogenous Brain Oscillations for Auditory Cognition—A Short Review. Brain Res. 2016, 1640, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Baltus, A.; Wagner, S.; Wolters, C.H.; Herrmann, C.S. Optimized Auditory Transcranial Alternating Current Stimulation Improves Individual Auditory Temporal Resolution. Brain Stimulat. 2018, 11, 118–124. [Google Scholar] [CrossRef]
- Zaehle, T.; Lenz, D.; Ohl, F.W.; Herrmann, C.S. Resonance Phenomena in the Human Auditory Cortex: Individual Resonance Frequencies of the Cerebral Cortex Determine Electrophysiological Responses. Exp. Brain Res. 2010, 203, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and Criteria Changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Dolphin, W.F. The Envelope Following Response to Multiple Tone Pair Stimuli. Hear. Res. 1997, 110, 1–14. [Google Scholar] [CrossRef]
- Purcell, D.W.; John, S.M.; Schneider, B.A.; Picton, T.W. Human Temporal Auditory Acuity as Assessed by Envelope Following Responses. J. Acoust. Soc. Am. 2004, 116, 3581–3593. [Google Scholar] [CrossRef] [PubMed]
- Griskova-Bulanova, I.; Voicikas, A.; Dapsys, K.; Melynyte, S.; Andruskevicius, S.; Pipinis, E. Envelope Following Response to 440 Hz Carrier Chirp-Modulated Tones Show Clinically Relevant Changes in Schizophrenia. Brain Sci. 2021, 11, 22. [Google Scholar] [CrossRef]
- Artieda, J.; Valencia, M.; Alegre, M.; Olaziregi, O.; Urrestarazu, E.; Iriarte, J. Potentials Evoked by Chirp-Modulated Tones: A New Technique to Evaluate Oscillatory Activity in the Auditory Pathway. Clin. Neurophysiol. 2004, 115, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Arnfred, S.M.; Raballo, A.; Morup, M.; Parnas, J. Self-Disorder and Brain Processing of Proprioception in Schizophrenia Spectrum Patients: A Re-Analysis. Psychopathology 2015, 48, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Parciauskaite, V.; Voicikas, A.; Jurkuvenas, V.; Tarailis, P.; Kraulaidis, M.; Pipinis, E.; Griskova-Bulanova, I. 40-Hz Auditory Steady-State Responses and the Complex Information Processing: An Exploratory Study in Healthy Young Males. PLoS ONE 2019, 14, e0223127. [Google Scholar] [CrossRef]
- Rass, O.; Krishnan, G.; Brenner, C.A.; Hetrick, W.P.; Merrill, C.C.; Shekhar, A.; O’Donnell, B.F. Auditory Steady State Response in Bipolar Disorder: Relation to Clinical State, Cognitive Performance, Medication Status, and Substance Disorders. Bipolar Disord. 2010, 12, 793–803. [Google Scholar] [CrossRef]
- Deary, I.J.; Johnson, W.; Starr, J.M. Are Processing Speed Tasks Biomarkers of Cognitive Aging? Psychol. Aging 2010, 25, 219–228. [Google Scholar] [CrossRef]
- Lu, P.H.; Lee, G.J.; Tishler, T.A.; Meghpara, M.; Thompson, P.M.; Bartzokis, G. Myelin Breakdown Mediates Age-Related Slowing in Cognitive Processing Speed in Healthy Elderly Men. Brain Cogn. 2013, 81, 131–138. [Google Scholar] [CrossRef]
- Salthouse, T.A. The Processing-Speed Theory of Adult Age Differences in Cognition. Psychol. Rev. 1996, 103, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Friedova, L.; Rusz, J.; Motyl, J.; Srpova, B.; Vodehnalova, K.; Andelova, M.; Novotna, K.; Novotny, M.; Ruzickova, H.; Tykalova, T.; et al. Slowed Articulation Rate Is Associated with Information Processing Speed Decline in Multiple Sclerosis: A Pilot Study. J. Clin. Neurosci. 2019, 65, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Karbasforoushan, H.; Duffy, B.; Blackford, J.U.; Woodward, N.D. Processing Speed Impairment in Schizophrenia Is Mediated by White Matter Integrity. Psychol. Med. 2015, 45, 109–120. [Google Scholar] [CrossRef] [PubMed]
- McKenna, B.S.; Theilmann, R.J.; Sutherland, A.N.; Eyler, L.T. Fusing Functional MRI and Diffusion Tensor Imaging Measures of Brain Function and Structure to Predict Working Memory and Processing Speed Performance among Inter-Episode Bipolar Patients. J. Int. Neuropsychol. Soc. 2015, 21, 330–341. [Google Scholar] [CrossRef]
- Patrick, K.E.; Schultheis, M.T.; Agate, F.T.; McCurdy, M.D.; Daly, B.P.; Tarazi, R.A.; Chute, D.L.; Hurewitz, F. Executive Function “Drives” Differences in Simulated Driving Performance between Young Adults with and without Autism Spectrum Disorder. Child Neuropsychol. 2020, 26, 1–17. [Google Scholar] [CrossRef]
- Mueller, S.T.; Piper, B.J. The Psychology Experiment Building Language (PEBL) and PEBL Test Battery. J. Neurosci. Methods 2014, 222, 250–259. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Mørup, M.; Hansen, L.K.; Arnfred, S.M. ERPWAVELAB: A Toolbox for Multi-Channel Analysis of Time-Frequency Transformed Event Related Potentials. J. Neurosci. Methods 2007, 161, 361–368. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Verhagen, J.; Marsman, M.; Gronau, Q.F.; Jamil, T.; Smira, M.; Epskamp, S.; Wild, A.; Ly, A.; et al. Software to Sharpen Your Stats. APS Obs. 2015, 28, 27–29. [Google Scholar]
- Lee, M.D.; Wagenmakers, E.-J. Bayesian Cognitive Modeling: A Practical Course; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-1-107-65391-7. [Google Scholar]
- Oda, Y.; Onitsuka, T.; Tsuchimoto, R.; Hirano, S.; Oribe, N.; Ueno, T.; Hirano, Y.; Nakamura, I.; Miura, T.; Kanba, S. Gamma Band Neural Synchronization Deficits for Auditory Steady State Responses in Bipolar Disorder Patients. PLoS ONE 2012, 7, e39955. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.F.; Vohs, J.L.; Krishnan, G.P.; Rass, O.; Hetrick, W.P.; Morzorati, S.L. The Auditory Steady-State Response (ASSR): A Translational Biomarker for Schizophrenia. Suppl. Clin. Neurophysiol. 2013, 62, 101–112. [Google Scholar] [PubMed]
- Thuné, H.; Recasens, M.; Uhlhaas, P.J. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-Analysis. JAMA Psychiatry 2016, 73, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Gransier, R.; Hofmann, M.; van Wieringen, A.; Wouters, J. Stimulus-Evoked Phase-Locked Activity along the Human Auditory Pathway Strongly Varies across Individuals. Sci. Rep. 2021, 11, 143. [Google Scholar] [CrossRef]
- Parker, D.A.; Hamm, J.P.; McDowell, J.E.; Keedy, S.K.; Gershon, E.S.; Ivleva, E.I.; Pearlson, G.D.; Keshavan, M.S.; Tamminga, C.A.; Sweeney, J.A.; et al. Auditory Steady-State EEG Response across the Schizo-Bipolar Spectrum. Schizophr. Res. 2019, 209, 218–226. [Google Scholar] [CrossRef]
- Cazalis, F.; Valabrègue, R.; Pélégrini-Issac, M.; Asloun, S.; Robbins, T.W.; Granon, S. Individual Differences in Prefrontal Cortical Activation on the Tower of London Planning Task: Implication for Effortful Processing. Eur. J. Neurosci. 2003, 17, 2219–2225. [Google Scholar] [CrossRef]
- Pulos, S.; Denzine, G. Individual Differences in Planning Behavior and Working Memory: A Study of the Tower of London. Individ. Differ. Res. 2005, 3, 99–104. [Google Scholar]
- Luciana, M.; Collins, P.F.; Olson, E.A.; Schissel, A.M. Tower of London Performance in Healthy Adolescents: The Development of Planning Skills and Associations With Self-Reported Inattention and Impulsivity. Dev. Neuropsychol. 2009, 34, 461–475. [Google Scholar] [CrossRef]
- Phillips, L.H. The Role of Memory in the Tower of London Task. Memory 1999, 7, 209–231. [Google Scholar] [CrossRef]
- Unterrainer, J.M.; Rahm, B.; Kaller, C.P.; Leonhart, R.; Quiske, K.; Hoppe-Seyler, K.; Meier, C.; Müller, C.; Halsband, U. Planning Abilities and the Tower of London: Is This Task Measuring a Discrete Cognitive Function? J. Clin. Exp. Neuropsychol. 2004, 26, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.D.; Carpenter, P.A.; Varma, S.; Just, M.A. Frontal and Parietal Participation in Problem Solving in the Tower of London: FMRI and Computational Modeling of Planning and High-Level Perception. Neuropsychologia 2003, 41, 1668–1682. [Google Scholar] [CrossRef]
- Binder, M.; Górska, U.; Griskova-Bulanova, I. 40Hz Auditory Steady-State Responses in Patients with Disorders of Consciousness: Correlation between Phase-Locking Index and Coma Recovery Scale-Revised Score. Clin. Neurophysiol. 2017, 128, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Górska, U.; Pipinis, E.; Voicikas, A.; Griskova-Bulanova, I. Auditory Steady-State Response to Chirp-Modulated Tones: A Pilot Study in Patients with Disorders of Consciousness. NeuroImage Clin. 2020, 27, 102261. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.L.; Thomas, M.L.; Joshi, Y.B.; Hochberger, W.C.; Koshiyama, D.; Nungaray, J.A.; Cardoso, L.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; et al. Gamma Oscillations Predict Pro-Cognitive and Clinical Response to Auditory-Based Cognitive Training in Schizophrenia. Transl. Psychiatry 2020, 10, 405. [Google Scholar] [CrossRef] [PubMed]

| Task | RT (ms) | Principal Component Loadings | |||
|---|---|---|---|---|---|
| Mean | SD | Component 1 | Component 2 | Component 3 | |
| Simple reaction time task | 294.38 | 50.75 | 0.65 * | 0.59 | −0.33 |
| Two-choice response time task | 378.16 | 61.19 | 0.76 * | 0.51 | - |
| Arithmetic decision task | 1116.12 | 327.84 | 0.72 * | - | 0.31 |
| Lexical decision task | 1248.64 | 357.79 | 0.63 * | −0.62 | −0.40 |
| Semantic categorization task | 751.97 | 209.61 | 0.84 * | −0.44 | - |
| Object judgement task | 814.68 | 188.15 | 0.61 * | - | 0.41 |
| Tower of London task | 1997.52 | 626.12 | 0.51 | - | 0.62 * |
| 40-Hz EFR | IGF-EFR | t-Test | IGF | ||
|---|---|---|---|---|---|
| PLI | Mean | 7.07 | 7.63 | −6.534, p < 0.001 | 41.89 |
| SD | 2.39 | 2.20 | 2.27 | ||
| ERSP | Mean | 1.29 | 1.35 | −6.849, p < 0.001 | 42.19 |
| SD | 0.20 | 0.20 | 2.57 |
| Task | PLI | ERSP | |||||
|---|---|---|---|---|---|---|---|
| 40-Hz EFR | IGF-EFR | IGF | 40-Hz EFR | IGF-EFR | IGF | ||
| Simple reaction time task | r | 0.05 | 0.04 | −0.03 | 0.03 | 0.03 | 0.01 |
| p | 0.79 | 0.83 | 0.86 | 0.86 | 0.85 | 0.94 | |
| Two-choice response time task | r | 0.08 | 0.02 | −0.18 | 0.09 | 0.03 | −0.20 |
| p | 0.62 | 0.93 | 0.29 | 0.60 | 0.87 | 0.23 | |
| Arithmetic decision task | r | −0.13 | −0.15 | −0.06 | −0.12 | −0.16 | −0.12 |
| p | 0.46 | 0.36 | 0.71 | 0.47 | 0.34 | 0.49 | |
| Lexical decision task | r | −0.10 | −0.11 | −0.02 | −0.03 | −0.07 | −0.18 |
| p | 0.58 | 0.52 | 0.90 | 0.87 | 0.70 | 0.28 | |
| Semantic categorization task | r | −0.20 | −0.23 | −0.04 | −0.15 | −0.18 | −0.10 |
| p | 0.23 | 0.18 | 0.80 | 0.39 | 0.28 | 0.55 | |
| Mental rotation task | r | −0.16 | −0.16 | 0.21 | −0.10 | −0.12 | 0.10 |
| p | 0.35 | 0.34 | 0.21 | 0.54 | 0.47 | 0.57 | |
| Tower of London task | r | −0.50 | −0.55 | 0.08 | −0.49 | −0.51 | 0.09 |
| p | 0.002 | <0.001 | 0.65 | 0.002 | 0.001 | 0.60 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parciauskaite, V.; Pipinis, E.; Voicikas, A.; Bjekic, J.; Potapovas, M.; Jurkuvenas, V.; Griskova-Bulanova, I. Individual Resonant Frequencies at Low-Gamma Range and Cognitive Processing Speed. J. Pers. Med. 2021, 11, 453. https://doi.org/10.3390/jpm11060453
Parciauskaite V, Pipinis E, Voicikas A, Bjekic J, Potapovas M, Jurkuvenas V, Griskova-Bulanova I. Individual Resonant Frequencies at Low-Gamma Range and Cognitive Processing Speed. Journal of Personalized Medicine. 2021; 11(6):453. https://doi.org/10.3390/jpm11060453
Chicago/Turabian StyleParciauskaite, Vykinta, Evaldas Pipinis, Aleksandras Voicikas, Jovana Bjekic, Mindaugas Potapovas, Vytautas Jurkuvenas, and Inga Griskova-Bulanova. 2021. "Individual Resonant Frequencies at Low-Gamma Range and Cognitive Processing Speed" Journal of Personalized Medicine 11, no. 6: 453. https://doi.org/10.3390/jpm11060453
APA StyleParciauskaite, V., Pipinis, E., Voicikas, A., Bjekic, J., Potapovas, M., Jurkuvenas, V., & Griskova-Bulanova, I. (2021). Individual Resonant Frequencies at Low-Gamma Range and Cognitive Processing Speed. Journal of Personalized Medicine, 11(6), 453. https://doi.org/10.3390/jpm11060453







