Malignancy Prediction Capacity and Possible Prediction Model of Circulating Tumor Cells for Suspicious Pulmonary Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Enrollment Criteria
2.2. Tumor Presentation
2.2.1. Macroscopic Presentation
2.2.2. Microscopic Presentation
2.2.3. Cutoff Value of Tumor Presentation
2.3. Pre-Operative Evaluation, Operation, and Surveillance
2.4. Measurement of Circulating Tumor Cells (CTCs)
2.5. Statistics
3. Results
3.1. Characteristics of Cohort
3.2. Presentations between Benign and Malignant Pulmonary Lesions
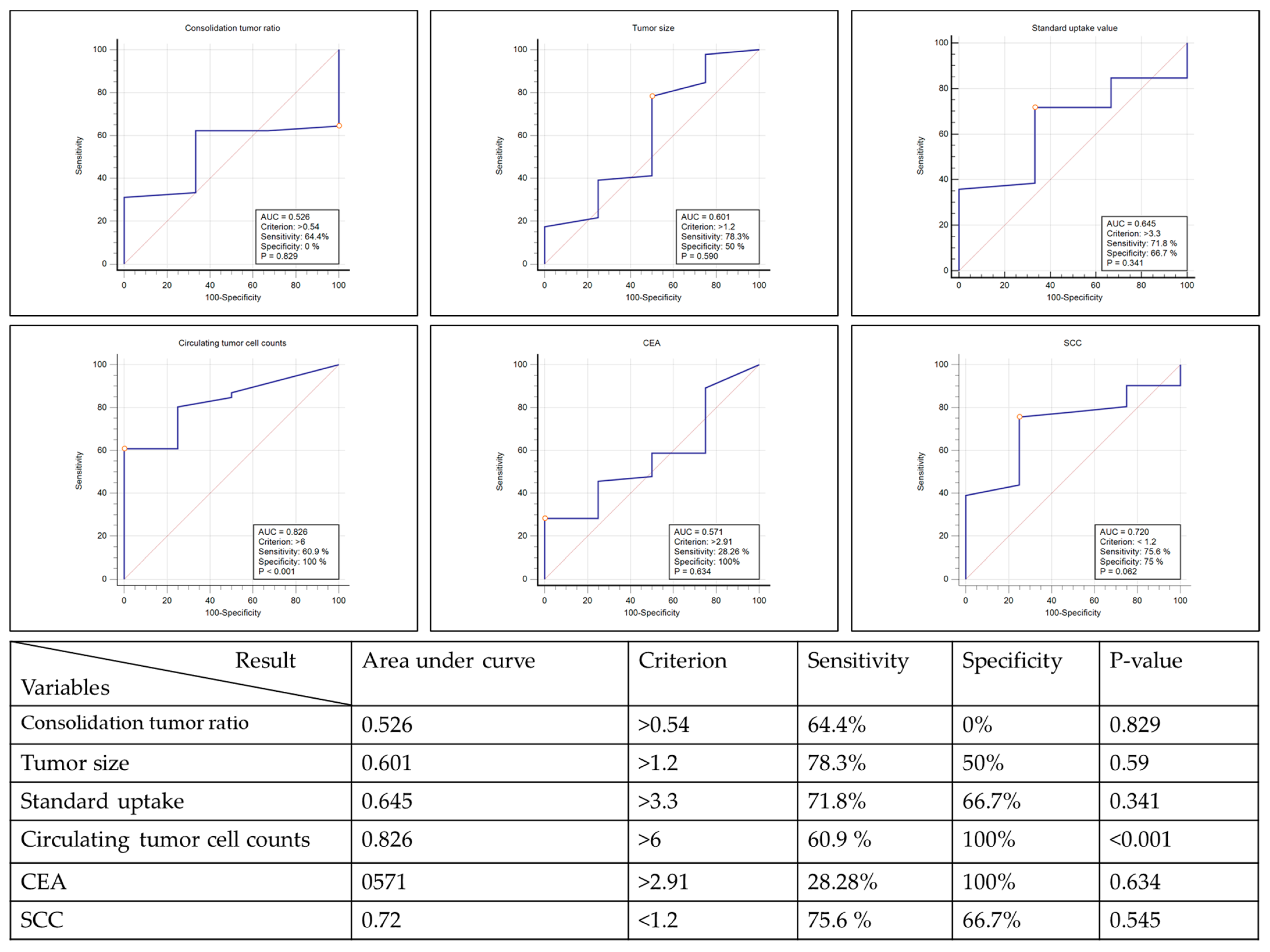
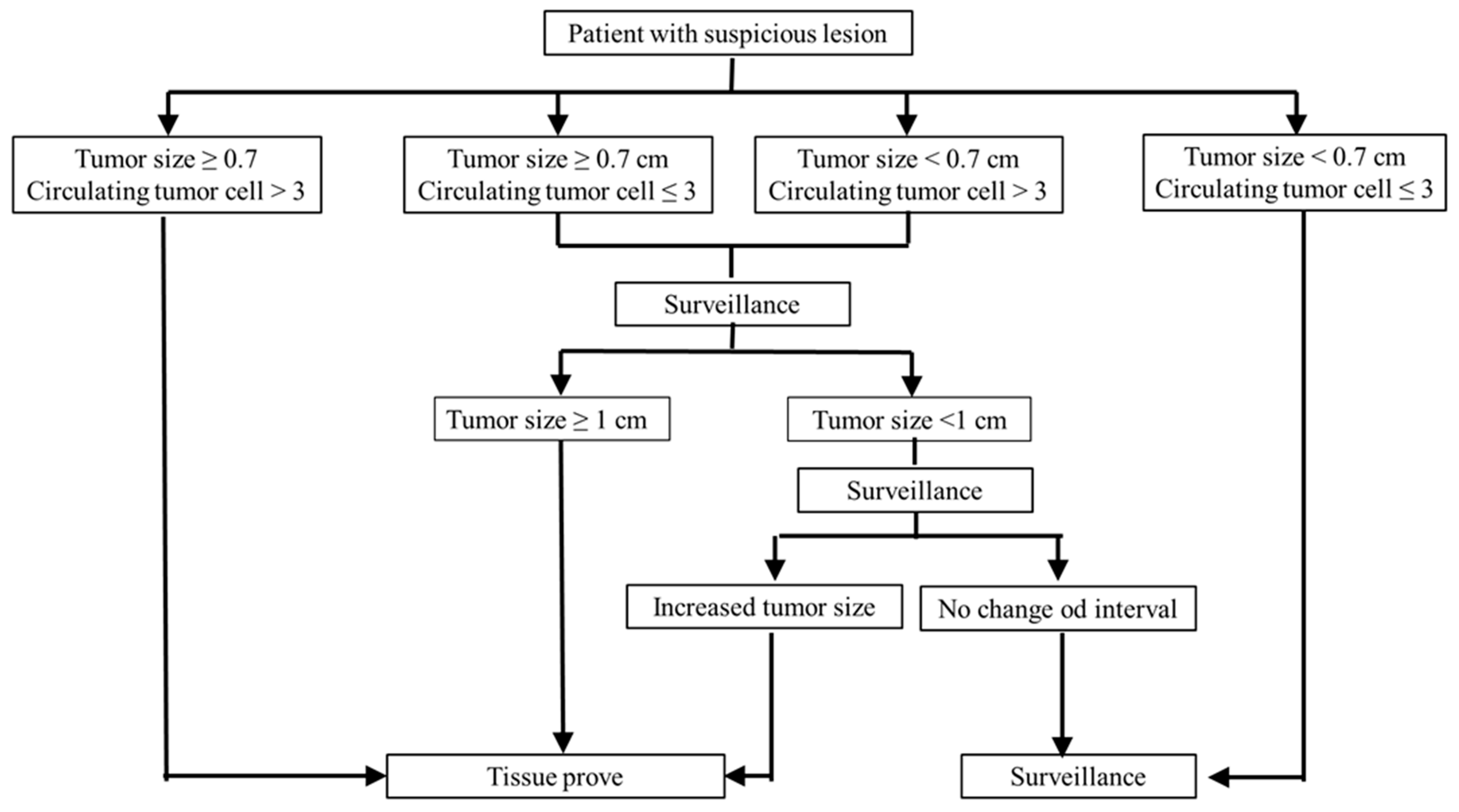
3.3. Malignancy Prediction Capacity of Tumor Presentations and Proposed Prediction Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTC | circulating tumor cells |
| AIC | Akaike information criterion |
| LDCT | low-dose computed tomography |
| C/T ratio | consolidation–tumor ratio |
| SUV | standard uptake value |
| ct DNA | circulating tumor deoxynucleic acid |
| MicroRNA | micro ribonucleic acid |
| IRB | Institutional Review Board |
| CT | computed tomography |
| PET-CT | positron emission tomography–computed tomography |
| PFT | Pulmonary function test |
| PACS | picture archiving and communication system |
| CEA | carcinoembryonic antigen |
| SCC | squamous cell carcinoma antigen |
| MRI | magnetic resonance image |
| mL | milliliters |
| EpCAM | epithelial cell adhesion molecule |
| RBCs | red blood cells |
| SD | standard deviation |
| ROC curve | receiver operating characteristic curve |
| GGO | ground glass opacity |
| AUC | area under curve |
| ECOG | Estern cooperative oncology grouo |
| FEV1 | Forced expiratory volume in 1 s |
| FVC | Forced expiratory capacity |
| A.I.C. | Akaike information criterion |
References
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [PubMed]
- Henschke, C.I.; Yip, R.; Yankelevitz, D.F.; Smith, J.P. Definition of a positive test result in computed tomography screening for lung cancer: A cohort study. Ann. Intern. Med. 2013, 158, 246–252. [Google Scholar] [CrossRef]
- Horeweg, N.; van Rosmalen, J.; Heuvelmans, M.A.; van der Aalst, C.M.; van der Aalst, C.M.; Vliegenthart, R.; Scholten, E.T.; ten Haaf, K.; Nackaerts, K.; Lammers, J.W.; et al. Lung cancer probability in patients with CT-detectedpulmonary nodules: A prespecified analysis of data from the NELSON trial of low-dose C.T. screening. Lancet Oncol. 2014, 15, 1332–1341. [Google Scholar] [CrossRef]
- Chung, K.; Jacobs, C.; Scholten, E.T.; Goo, J.M.; Prosch, H.; Sverzellati, N.; Ciompi, F.; Mets, O.M.; Gerke, P.K.; Prokop, M.; et al. Lung-RADS Category 4X: Does It Improve Prediction of Malignancy in Su solid Nodules? Radiology 2017, 284, 264–271. [Google Scholar] [CrossRef]
- Chung, K.; Jacobs, C.; Scholten, E.T.; Mets, O.M.; Dekker, I.; Prokop, M.; van Ginneken, B.; Schaefer-Prokop, C.M. Malignancy estimation of Lung-RADS criteria for subsolid nodules on CT: Acuracy of low and high risk spectrum when using NLST nodules. Eur. Radiol. 2017, 27, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef]
- Matsunaga, T.; Suzuki, K.; Takamochi, K.; Oh, S. What is the radiological definition of part-solid tumour in lung cancer? Eur. J. Cardiothorac Surg. 2017, 51, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Tsujikawa, T.; Kondo, C.; Maki, M.; Momose, M.; Nagai, A.; Ohnuki, T.; Nishikawa, T.; Kusakabe, K. Accuracy of P.E.T. for diagnosis of solid pulmonary lesions with 18F-FDG up take below the standardized uptake value of 2.5. J. Nucl. Med. 2006, 47, 426–431. [Google Scholar]
- Lowe, V.J.; Fletcher, J.W.; Gobar, L.; Lawson, M.; Kirchner, P.; Valk, P.; Karis, J.; Hubner, K.; Delbeke, D.; Heiberg, E.V.; et al. Prospective investigation of positron emission tomography in lung nodules. J. Clin. Oncol. 1998, 16, 1075–1084. [Google Scholar] [CrossRef]
- Gould, M.K.; Maclean, C.C.; Kuschner, W.G.; Rydzak, C.E.; Owens, D.K. Accuracy of positron emission tomography for diagnosis of pulmp nary nodules and mass lesions: A meta-analysis. JAMA 2001, 285, 914–924. [Google Scholar] [CrossRef]
- Funama, Y.; Awai, K.; Liu, D.; Oda, S.; Yanaga, Y.; Nakaura, T.; Kawanaka, K.; Shimamura, M.; Yamashita, Y. Detection of nodules showing ground glass opacity in the lungs at low-dose multi-detector computed tomography: Phantom and clinical study. J. Comput. Assist. Tomogr. 2009, 33, 49–53. [Google Scholar] [CrossRef]
- Boedeker, K.L.; Cooper, V.N.; McNitt-Gray, M.F. Application of the noise power spectrum in modern diagnostic MDCT: Part I. Measurement of noise power spectra and noise equivalent quanta. Phys. Med. Biol. 2007, 52, 4027–4046. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Nicolini, S.; Caroli, P.; Nanni, C.; Massaro, A.; Marzola, M.C.; Rubello, D.; Fanti, S. PET/CT imaging in different types of lung cancer: An overview. Eur. J. Radiol. 2012, 81, 988–1001. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, P.; Wu, R.; Lu, K.; Zhou, H. Identifying the Best Marker Combination in C.E.A., CA125, CY211, NSE, and S.C.C. for Lung Cancer Screening by Combining R.O.C. Curve and Logistic Regression Analyses: Is It Feasible? Dis. Markers. 2018, 2018, 2082840. [Google Scholar] [CrossRef]
- Tas, F.; Aydiner, A.; Topuz, E.; Yasasever, V.; Karadeniz, A.; Saip, P. Utility of the serum tumor markers: CYFRA 21.1, carcinoembryonic antigen (C.E.A.), and squamous cell carcinoma antigen (S.C.C.) in squamous cell lung cancer. J. Exp. Clin. Cancer Res. 2000, 19, 477–481. [Google Scholar] [PubMed]
- Pastor, A.; Menéndez, R.; Cremades, M.J.; Pastor, V.; Llopis, R.; Aznar, J. Diagnostic value of SCC, CEA and CYFRA 21.1 in lung cancer: A Bayesian analysis. Eur. Respir. J. 1997, 10, 603–609. [Google Scholar] [PubMed]
- Molina, R.; Marrades, R.M.; Augé, J.M.; Escudero, J.M.; Viñolas, N.; Reguart, N.; Ramirez, J.; Filella, X.; Molins, L.; Agustí, A. Assessment of a Combined Panel of Six Serum Tumor Markers for Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 427–437. [Google Scholar] [CrossRef]
- Ma, S.; Wang, W.; Xia, B.; Zhang, S.; Yuan, H.; Jiang, H.; Meng, W.; Zheng, X.; Wang, X. Multiplexed Serum Biomarkers for the Detection of Lung Cancer. EBioMedicine 2016, 11, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Chen, K.Z.; Lou, F.; Yang, F.; Zhang, J.B.; Ye, H.; Chen, W.; Guan, T.; Zhao, M.Y.; Su, X.X.; Shi, R.; et al. Circulating tumor D.N.A. detection in early-stage non-small cell lung cancer patients by targeted sequencing. Sci. Rep. 2016, 6, 31985. [Google Scholar] [CrossRef]
- Yang, M.; Shen, H.; Qiu, C.; Ni, Y.; Wang, L.; Dong, W.; Liao, Y.; Du, J. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. Eur. J. Cancer 2013, 49, 604–615. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lee, C.L.; Wu, C.F.; Fu, J.Y.; Yang, C.T.; Wen, C.T.; Liu, Y.H.; Liu, H.P.; Hsieh, J.C. Circulating Tumor Cells as a Tool of Minimal Residual Disease Can Predict Lung Cancer Recurrence: A longitudinal, Prospective Trial. Diagnostics 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Wu, Y.L.; Lee, J.S.; Yu, C.J.; Sriuranpong, V.; Sandoval-Tan, J.; Ladrera, G.; Thongprasert, S.; Srimuninnimit, V.; Liao, M.; et al. Detection and dynamic changes of EGFR mutations from circulating tumor D.N.A. as a predictor of survival outcomes in NSCLC patients treated with first-line intercalated erlotinib and chemotherapy. Clin. Cancer Res. 2015, 21, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Tsai, Y.M.; Lien, C.T.; Kuo, P.L.; Hung, J.Y. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef] [PubMed]
- Maly, V.; Maly, O.; Kolostova, K.; Bobek, V. Circulating Tumor Cells in Diagnosis and Treatment of Lung Cancer. In Vivo 2019, 33, 1027–1037. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Gao, L.; Jiang, X.; Fu, R.; Zhang, T.; Ren, T.; Hu, P.; Wu, Y.; Zhao, P.; et al. Clinical significance of circulating tumor cells and tumor markers in the diagnosis of lung cancer. Cancer Med. 2019, 8, 3782–3792. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, F.; Li, X.; Yang, G.; Zhang, L.; Ren, S.; Zhao, C.; Deng, Q.; Li, W.; Gao, G.; et al. Folate Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1163–1171. [Google Scholar] [CrossRef]
- Zhou, Q.; Geng, Q.; Wang, L.; Huang, J.; Liao, M.; Li, Y.; Ding, Z.; Yang, S.; Zhao, H.; Shen, Q.; et al. Value of folate receptor-positive circulating tumour cells in the clinical management of indeterminate lung nodules: A non-invasive biomarker for predicting malignancy and tumour invasiveness. EBioMedicine 2019, 41, 236–243. [Google Scholar] [CrossRef]
- Pedersen, J.H.; Saghir, Z.; Wille, M.M.; Thomsen, L.H.; Skov, B.G.; Ashraf, H. Ground-Glass Opacity Lung Nodules in the Era of Lung Cancer CT Screening: Radiology, Pathology, and Clinical Management. Oncology 2016, 30, 266–274. [Google Scholar]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xu, G.B. Effect of circulating tumor cells combined with negative enrichment and CD45-FISH identification in diagnosis, therapy monitoring and prognosis of primary lung cancer. Med. Oncol. 2014, 31, 240. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Falchini, M.; Maddau, C.; Salvianti, F.; Nistri, M.; Bertelli, E.; Sali, L.; Zuccherelli, S.; Vella, A.; Matucci, M.; et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J. Cancer Res. Clin. Oncol. 2016, 142, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Su, P.J.; Wu, M.H.; Wang, H.M.; Lee, C.L.; Huang, W.K.; Wu, C.E.; Chang, H.K.; Chao, Y.K.; Tseng, C.K.; Chiu, T.K.; et al. Circulating Tumour Cells as an Independent Prognostic Factor in Patients with Advanced Oesophageal Squamous Cell Carcinoma Undergoing Chemoradiotherapy. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.; Bhagat, A.A.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Wan, Y.L.; Huang, T.Y.; Wu, C.F.; Liu, Y.H.; Hsieh, M.J.; Wu, Y.C.; Wu, C.Y. Correlation between image characteristics and pathologic findings in non small cell lung cancer patients after anatomic resection. PLoS ONE 2018, 13, e0206386. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean ± SD(%) | Characteristic | Mean ± SD (%) |
|---|---|---|---|
| Case number | 50 | PET-CT presentation | |
| Age (years) | 64.0 ± 12.4 | Dose | 10.1 ± 0.6 |
| Sex (M:F) | Blood sugar | 99.2 ± 21.3 | |
| Male | 27 (54%) | Tumor SUV | 6.7 ± 5.3 |
| Female | 23 (46%) | Biochemical data | |
| ECOG scroe | Albumin | 4.3 ± 0.3 | |
| 0 | 48 (96%) | Albumin/Total protein | 0.6 ± 0.1 |
| 1 | 2 (4%) | White blood cells | 9010.0 ± 16,737.2 |
| Smoking | 16 (32%) | Seg (%) | 59.9 ± 10.8 |
| Packets per day | 0.4 ± 0.8 | Tumor marker (ng/mL) | |
| Smoking years | 11.8 ± 18.5 | SCC | 1.1 ± 0.6 |
| Packet years | 15.9 ± 30.2 | CEA | 3.1 ± 3.3 |
| PFT | CTC counts (cells/mL) | 12.1 ± 14.8 | |
| FEV1 | 2.1 ± 0.7 | Operation method | |
| FVC | 2.6 ± 0.8 | Lobectomy | 29 (58%) |
| FEV1/FVC (%) | 79.9 ± 9.1 | Segmentectomy | 17 (34%) |
| C.T. presentation | Wedge resection | 4 (8%) | |
| Tumor location | Operation times (min) | 224.0 ± 63.0 | |
| Left lower lobe | 5 (10%) | Blood loss (ml) | 61.1 ± 83.2 |
| Left upper lobe | 11 (22%) | Pathology | |
| Right lower lobe | 14 (28%) | Benign | 4 (8%) |
| Right middle lobe | 6 (12%) | Malignant | |
| Right upper lobe | 14 (28%) | Lung primary | 41 (82%) |
| Maximal tumor size | 2.3 ± 1.2 | Adenocarcinoma | 33 (66%) |
| Consolidation–tumor ratio (C/T ratio) | 0.7 ± 0.4 | Invasive mucinous adenocarcinoma | 3 (6%) |
| Tumor composition | Squamous cell carcinoma | 3 (6%) | |
| Pure GGO (C/T ratio:0) | 9 (18%) | Other | 2 (4%) |
| GGO predominant (CT ratio 1~50%) | 3 (6%) | Metastatic | 5 (10%) |
| Solid predominant (CT ratio: 51~99%) | 26 (52%) | Tumor size (cm) | 2.3 ± 1.2 |
| Pure solid (C/Tratio:1) | 10 (20%) | Hospital stay (Days) | 4.2 ± 2.1 |
| Cavitary lesion | 2 (4%) |
| Etiology | Benign (4) | Malignant (46) | p-Value * | Benign (4) | Malignant (46) | p-Value # | ||
|---|---|---|---|---|---|---|---|---|
| Factor | Metastatic (5) | Lung Cancer (41) | ||||||
| Consolidation–tumor ratio | 0.7 ± 0.1 (3) 1 | 0.9 ± 0.2 (5) | 0.6 ± 0.4 (40) 3 | 0.22 | 0.7 ± 0.2 (3) 1 | 0.6 ± 0.4 (45) 3 | 0.88 | |
| Tumor size | 1.9 ± 1.1 (4) | 2.8 ± 1.1 (5) | 2.2 ± 1.2 (41) | 0.40 | 1.9 ± 1.3 (4) | 2.3 ± 1.2 (46) | 0.51 | |
| Tumor SUV | 4.2 ± 2.7 (3) 1 | 10.7 ± 5.9 (5) | 6.3 ± 4.9 (35) 4 | 0.22 | 4.2 ± 3.3 (3) 1 | 6.9 ± 5.3 (40) 4 | 0.38 | |
| CTC | 2.0 ± 2.5 (4) | 17.4 ± 16.2 (5) | 12.5 ± 14.7 (41) | 0.09 | 2.0 ± 2.8 (4) | 13.0 ± 15.1 (46) | 0.03 | |
| CEA | 1.6 ± 1.0 (4) | 2.9 ± 3.2(5) | 2.9 ± 3.3 (41) | 0.87 | 1.7 ± 1.0 (4) | 2.9 ± 3.3 (46) | 0.64 | |
| SCC | 1.4 ± 0.4 (4) | 0.9 ± 0.2 (4) 2 | 1.1 ± 0.6 (37) 5 | 0.35 | 1.4 ± 0.4 (4) | 1.0 ± 0.6 (41) 2,5 | 0.15 | |
| Marker | C/T Ratio | Tumor Size | Tumor SUV | CTC | CEA | SCC |
|---|---|---|---|---|---|---|
| Cutoff value | >50% | ≥0.7 cm | >2.5 | >3 cells/mL | >3.4 ng/mL | >3.5 ng/mL |
| Sensitivity (95% CI) | 0.73 (0.60~0.86) | 0.96 (0.84~0.99) | 0.78 (0.61~0.89) | 0.70 (0.56~0.83) | 0.78 (0.63~0.89) | 1.00 (1.00~1.00) |
| Specificity (95% CI) | 0 (0~0.69) | 0.25 (0.01~0.78) | 0.33 (0.02~0.87) | 0.75 (0.33~1.0000) | 0 (0~0.60) | 0 (0~0.60) |
| Positive likelihood ratio (95% CI) | 0.73 (0.60~0.86) | 1.28 (0.72~2.25) | 1.16 (0.51~2.63) | 2.78 (0.50~15.36) | 0.78 (0.67~0.91) | 1 (1.00~1.00) |
| Negative likelihood ratio (95% CI) | Infinity | 0.17 (0.01~2.93) | 0.68 (0.10~4.43) | 0.41 (0.22~0.74) | Infinity | 0 |
| Positive predictive value (95% CI) | 0.92 (0.83~1.00) | 0.94 (0.81~0.98) | 0.94 (0.78~0.99) | 0.97 (0.91~1.00) | 0.90 (0.75~0.97) | 0.91 (0.83~0.99) |
| Negative predictive value (95% CI) | 0 (0~0.30) | 0.33 (0.02~0.87) | 0.10 (0.01~0.46) | 0.18 (0.00~0.36 | 0 (0~0.34) | 0 |
| Accuracy (95% CI) | 0.69 (0.56~0.82) | 0.90 (0.82~0.98) | 0.74 (0.61~0.87) | 0.70 (0.57~0.83) | 0.72 (0.60~0.84) | 0.91 (0.83~0.99) |
| a. Comparison of curve fitting criteria (tumor size/circulating tumor cell/combination) | |||||
|---|---|---|---|---|---|
| Factors | Tumor Size ≥ 0.7 cm | Circulating Tumor Cell > 3 | Combined | ||
| Curve Fitting Criteria | |||||
| Akaike information criterion (AIC) | 29.21 | 26.74 | 26.73 | ||
| b. Logistic regression for model selection | |||||
| Model Selection | Odds Ratio (95% Confidence Interval) | Chi-Square (p Value) | |||
| Tumor ≥ 0.7 cm | 15.00 (0.74~303.74) | 0.077 | |||
| Tumor < 0.7 cm | 1 | ||||
| CTC > 3 | 12.33 (1.14~132.93) | 0.038 | |||
| CTC ≤ 3 | 1 | ||||
| Tumor ≥ 0.7 cm (controlled CTC > 3) | 13.58 (0.38~484.48) | 0.152 | |||
| CTC > 3 (controlled tumor size ≥ 0.7 cm) | 11.85 (0.98~143.22) | 0.051 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Y.; Fu, J.-Y.; Wu, C.-F.; Hsieh, M.-J.; Liu, Y.-H.; Liu, H.-P.; Hsieh, J.C.-H.; Peng, Y.-T. Malignancy Prediction Capacity and Possible Prediction Model of Circulating Tumor Cells for Suspicious Pulmonary Lesions. J. Pers. Med. 2021, 11, 444. https://doi.org/10.3390/jpm11060444
Wu C-Y, Fu J-Y, Wu C-F, Hsieh M-J, Liu Y-H, Liu H-P, Hsieh JC-H, Peng Y-T. Malignancy Prediction Capacity and Possible Prediction Model of Circulating Tumor Cells for Suspicious Pulmonary Lesions. Journal of Personalized Medicine. 2021; 11(6):444. https://doi.org/10.3390/jpm11060444
Chicago/Turabian StyleWu, Ching-Yang, Jui-Ying Fu, Ching-Feng Wu, Ming-Ju Hsieh, Yun-Hen Liu, Hui-Ping Liu, Jason Chia-Hsun Hsieh, and Yang-Teng Peng. 2021. "Malignancy Prediction Capacity and Possible Prediction Model of Circulating Tumor Cells for Suspicious Pulmonary Lesions" Journal of Personalized Medicine 11, no. 6: 444. https://doi.org/10.3390/jpm11060444
APA StyleWu, C.-Y., Fu, J.-Y., Wu, C.-F., Hsieh, M.-J., Liu, Y.-H., Liu, H.-P., Hsieh, J. C.-H., & Peng, Y.-T. (2021). Malignancy Prediction Capacity and Possible Prediction Model of Circulating Tumor Cells for Suspicious Pulmonary Lesions. Journal of Personalized Medicine, 11(6), 444. https://doi.org/10.3390/jpm11060444







