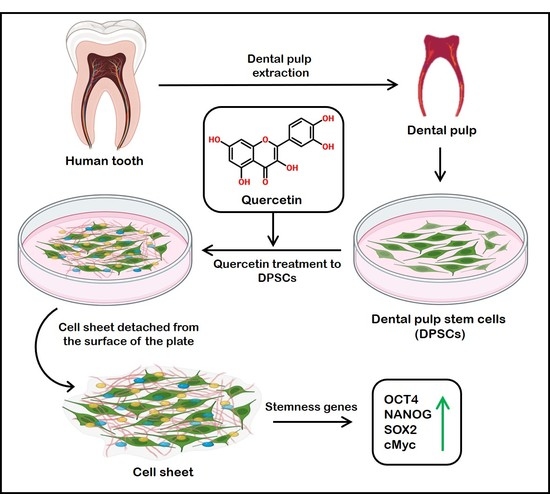
Viability of Quercetin-Induced Dental Pulp Stem Cells in Forming Living Cellular Constructs for Soft Tissue Augmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Culture and Expansion of Human DPSCs
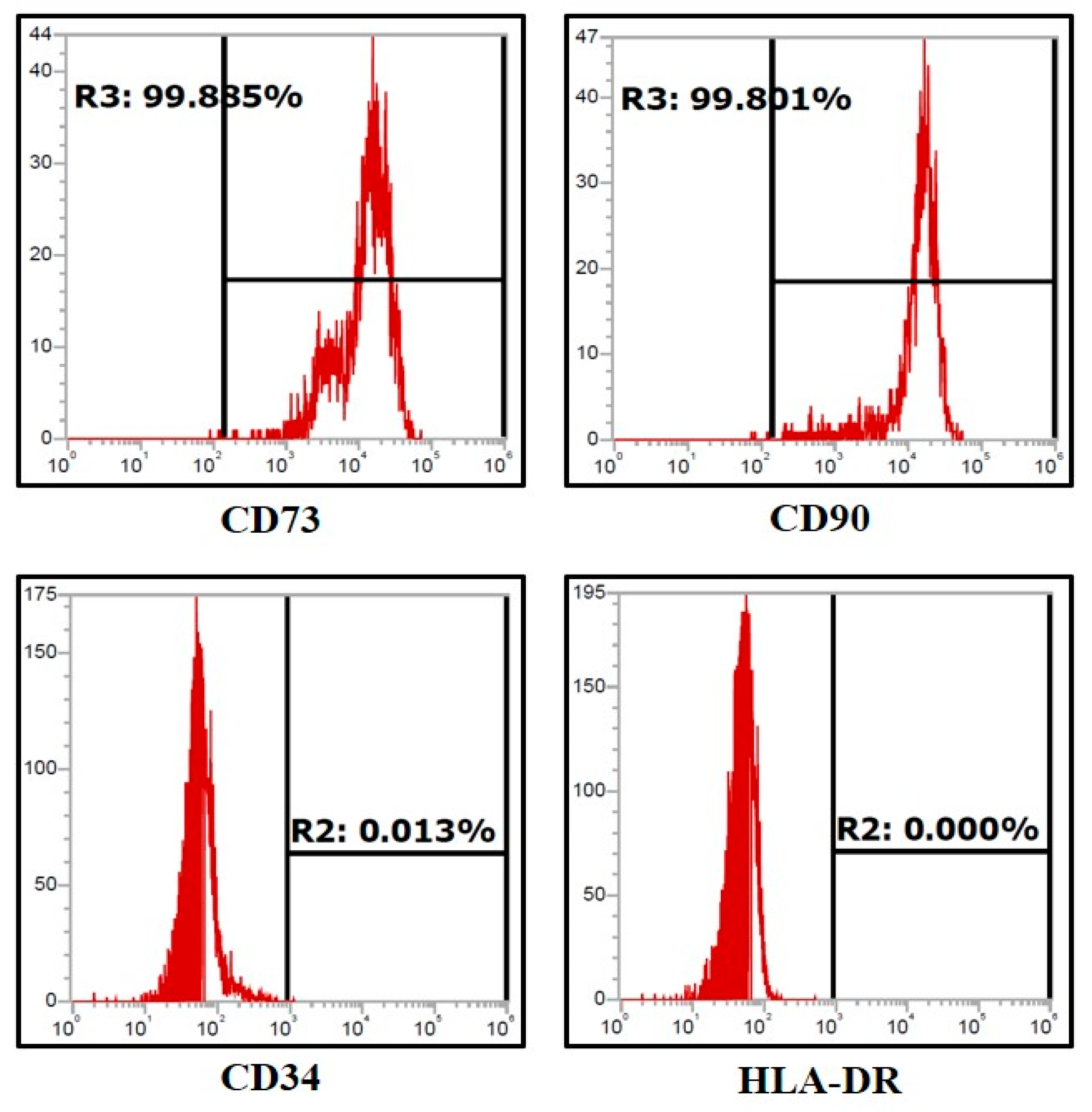
2.3. Characterization of DPSCs Using Flow Cytometry
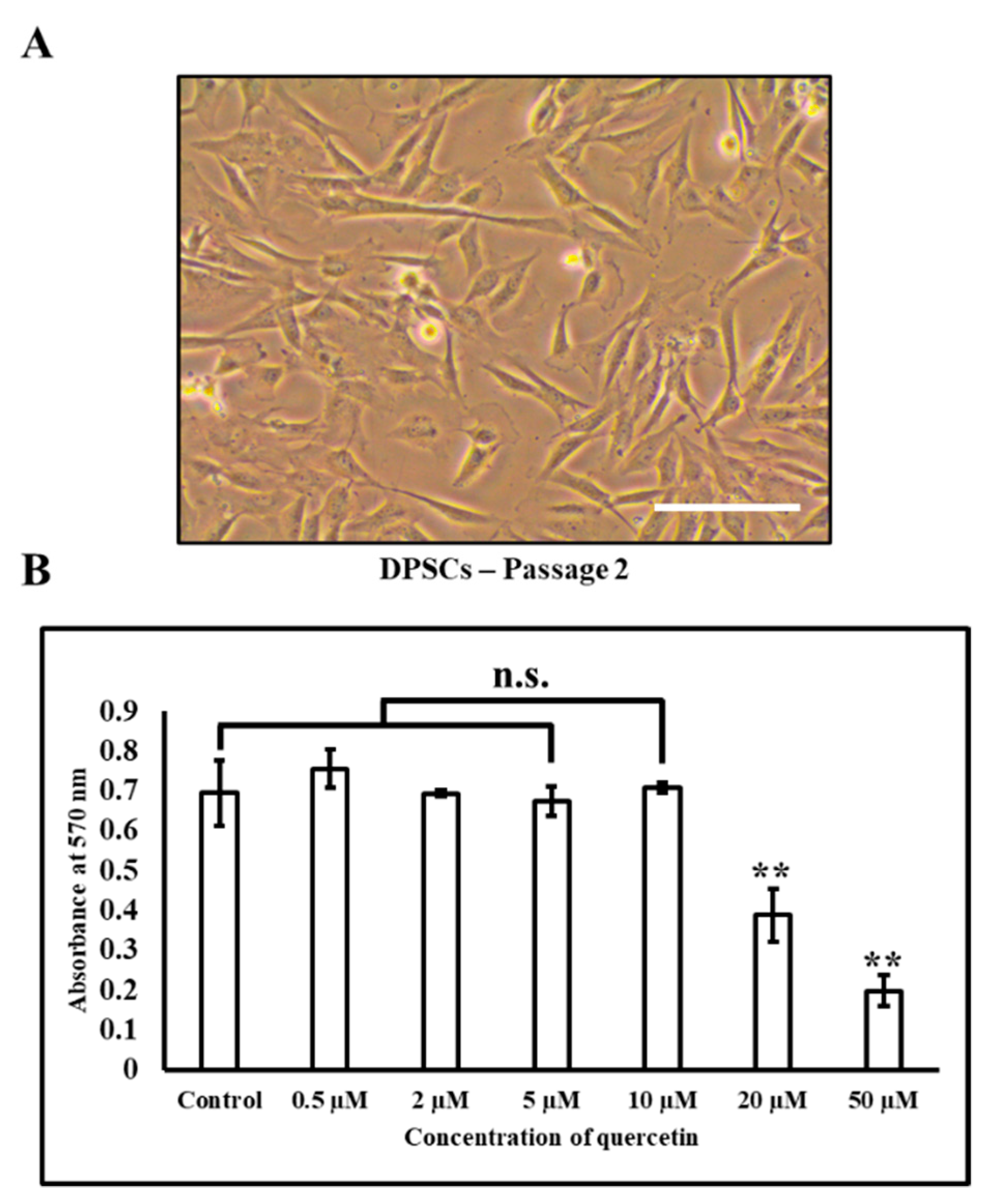
2.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay of DPSC Following Quercetin Treatment
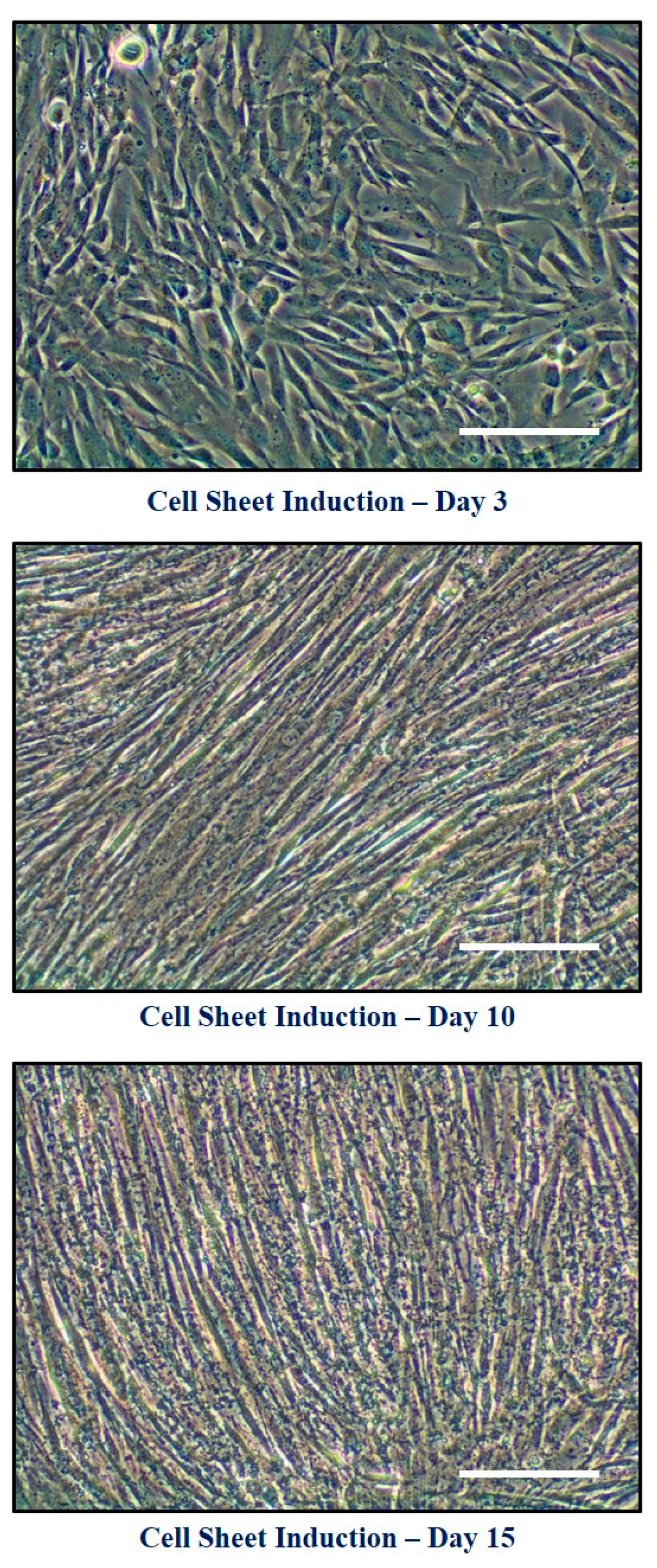
2.5. Formation of Cell Constructs
2.6. Characterization of Living Cell Constructs
2.7. Real-Time PCR for Analysis of Gene Expression
3. Results
3.1. Assessment of Expression of Stem Cell Markers in the Dental Pulp Stem Cells by Flow Cytometry
3.2. Effects of Quercetin Administration on Cell Sheet Characteristics and Physical Properties
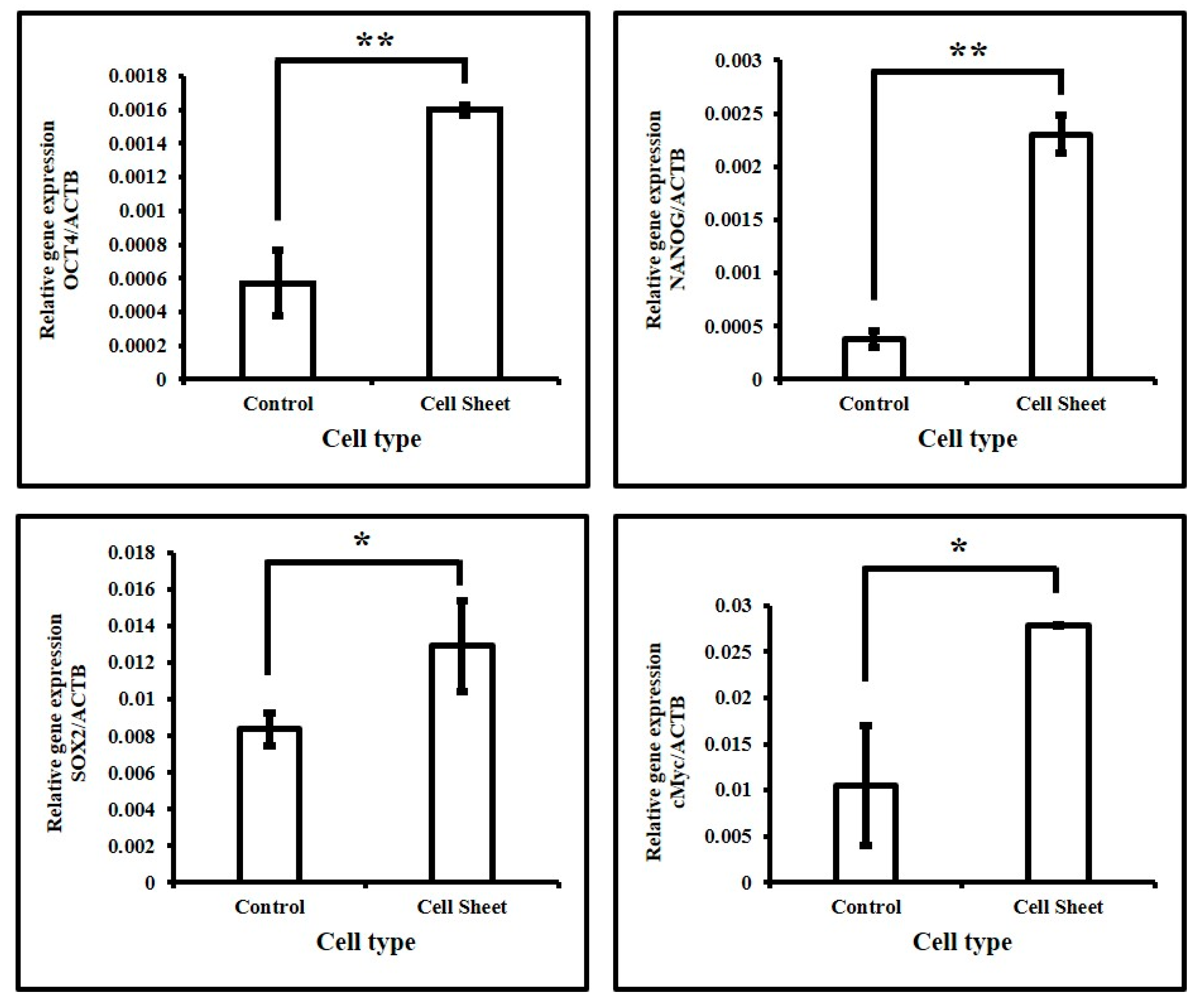
3.3. Assessment of Expression of Stemness-Related Genes in DPSCs with and without Quercetin Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Helderman, W.H.V.P.; Lembariti, B.; Van Der Weijden, G.A.; Hof, M.A.V. T Gingival recession and its association with calculus in subjects deprived of prophylactic dental care. J. Clin. Periodontol. 1998, 25, 106–111. [Google Scholar] [CrossRef]
- Khocht, A.; Simon, G.; Person, P.; Denepitiya, J.L. Gingival Recession in Relation to History of Hard Toothbrush Use. J. Periodontol. 1993, 64, 900–905. [Google Scholar] [CrossRef]
- Gunsolley, J.; Quinn, S.; Tew, J.; Gooss, C.; Brooks, C.; Schenkein, H. The Effect of Smoking on Individuals with Minimal Periodontal Destruction. J. Periodontol. 1998, 69, 165–170. [Google Scholar] [CrossRef]
- Honjo, K.-I.; Yamamoto, T.; Adachi, T.; Amemiya, T.; Mazda, O.; Kanamura, N.; Kita, M. Evaluation of a dental pulp-derived cell sheet cultured on amniotic membrane substrate. Bio-Med. Mater. Eng. 2015, 25, 203–212. [Google Scholar] [CrossRef]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin rescued TNF-alpha-induced impairments in bone marrow-derived mesenchymal stem cell osteogenesis and improved osteoporosis in rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar] [PubMed]
- Li, Y.; Wang, J.; Chen, G.; Feng, S.; Wang, P.; Zhu, X.; Zhang, R. Quercetin promotes the osteogenic differentiation of rat mesenchymal stem cells via mitogen-activated protein kinase signaling. Exp. Ther. Med. 2015, 9, 2072–2080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pedroni, A.C.F.; Sarra, G.; De Oliveira, N.K.; Moreira, M.S.; Deboni, M.C.; Marques, M.M. Cell sheets of human dental pulp stem cells for future application in bone replacement. Clin. Oral Investig. 2018, 23, 2713–2721. [Google Scholar] [CrossRef]
- Patil, V.R.; Kharat, A.H.; Kulkarni, D.G.; Kheur, S.M.; Bhonde, R.R. Long term explant culture for harvesting homogeneous population of human dental pulp stem cells. Cell Biol. Int. 2018, 42, 1602–1610. [Google Scholar] [CrossRef]
- Miladpour, B.; Rasti, M.; Owji, A.A.; Mostafavipour, Z.; Khoshdel, Z.; Noorafshan, A.; Zal, F. Quercetin potentiates transdifferentiation of bone marrow mesenchymal stem cells into the beta cells in vitro. J. Endocrinol. Investig. 2016, 40, 513–521. [Google Scholar] [CrossRef]
- Pang, X.-G.; Cong, Y.; Bao, N.-R.; Li, Y.-G.; Zhao, J.-N. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. BioMed Res. Int. 2018, 2018, 4178021. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Pariyar, R.; Kim, J.; Lee, H.-S.; Seo, J. Quercetin-3-O-glucuronide promotes the proliferation and migration of neural stem cells. Neurobiol. Aging 2017, 52, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, B.; Gao, Z.; Xu, J.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Regeneration characteristics of different dental derived stem cell sheets. J. Oral Rehabil. 2020, 47, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, A.C.; Diniz, I.M.; Abe, G.L.; Moreira, M.S.; Sipert, C.R.; Marques, M.M. Photobiomodulation therapy and vitamin C on longevity of cell sheets of human dental pulp stem cells. J. Cell. Physiol. 2018, 233, 7026–7035. [Google Scholar] [CrossRef]
- Itoh, Y.; Sasaki, J.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2016, 10, 261–270. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T.; Nevins, M.L.; Neiva, R.; Cochran, D.L.; Mellonig, J.T.; Giannobile, W.V.; Bates, D. Living Cellular Construct for Increasing the Width of Keratinized Gingiva: Results from a Randomized, Within-Patient, Controlled Trial. J. Periodontol. 2011, 82, 1414–1423. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T.; Nunn, M.E.; Lavin, P.T. A Pilot Study to Evaluate a Tissue-Engineered Bilayered Cell Therapy as an Alternative to Tissue from the Palate. J. Periodontol. 2008, 79, 1847–1856. [Google Scholar] [CrossRef]
- Parasuraman, S.; David, A.V.A.; Arulmoli, R. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin Suppresses Cyclooxygenase-2 Expression and Angiogenesis through Inactivation of P300 Signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef]
- Rigano, D.; Formisano, C.; Basile, A.; Lavitola, A.; Senatore, F.; Rosselli, S.; Bruno, M. Antibacterial activity of flavonoids and phenylpropanoids from Marrubium globosumssp. libanoticum. Phytother. Res. 2007, 21, 395–397. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, G.; Le, Y.; Ju, J.; Zhang, P.; Wan, D.; Zhao, Q.; Jin, G.; Su, H.; Liu, J.; et al. Quercetin promotes human epidermal stem cell proliferation through the estrogen receptor/β-catenin/c-Myc/cyclin A2 signaling pathway. Acta Biochim. Biophys. Sin. 2020, 52, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Zuhr, O.; Bäumer, D.; Hürzeler, M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J. Clin. Periodontol. 2014, 41, S123–S142. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Tavelli, L.; McGuire, M.K.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J. Periodontol. 2019, 91, 9–16. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.; Nibali, L.; Pilipchuk, S.; Berglundh, T.; Giannobile, W. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2013, 59, 117–130. [Google Scholar] [CrossRef]
- Nunery, W.R. Risk of Prion Transmission with the Use of Xenografts and Allografts in Surgery. Ophthalmic Plast. Reconstr. Surg. 2001, 17, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.G.; Serafim, R.C.; Melo, G.B.; Silva, M.C.P.; Lizier, N.F.; Maranduba, C.M.C.; Smith, R.L.; Kerkis, A.; Cerruti, H.; Gomes, J.A.P.; et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.H.; Hu, L.; Zhou, Y.; Ge, Z.; Wang, H.; Wu, C.-T.; Jin, J. A Sandwich Structure of Human Dental Pulp Stem Cell Sheet, Treated Dentin Matrix, and Matrigel for Tooth Root Regeneration. Stem Cells Dev. 2020, 29, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.-I.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef]
- Monteiro, N.; Smith, E.E.; Angstadt, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Dental cell sheet biomimetic tooth bud model. Biomaterials 2016, 106, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.; Pedroni, A.; Cury, D.; Moreira, M.; Rosin, F.; Sarra, G.; Marques, M. Effects of photobiomodulation therapy on the extracellular matrix of human dental pulp cell sheets. J. Photochem. Photobiol. B Biol. 2019, 194, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Anter, J.; Dorado, G.; Quesada-Gómez, J.M. Effects of quercetin, a natural phenolic compound, in the differentiation of human mesenchymal stem cells (MSC) into adipocytes and osteoblasts. J. Nutr. Biochem. 2016, 32, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Terashi, H.; Marcelo, C.; Feinberg, S. Development and characterization of a tissue-engineered human oral mucosa equivalent produced in a serum-free culture system. J. Dent. Res. 2000, 79, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Takacs, G.; Terashi, H.; Feinberg, S.E. Ex vivo development of a composite human oral mucosal equivalent. J. Oral Maxillofac. Surg. 1999, 57, 571–577. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| NANOG | 5′-TTT GTG GGC CTG AAG AAA ACT-3′ | 5′-AGG GCT GTC CTG AAT AAG CAG-3′ |
| OCT4 | 5′-GTG GAG GAA GCT GAC AAC AA-3′ | 5′-ATT CTC CAG GTT GCC TCT CA-3′ |
| cMyc | 5′-AGA AAT GTC CTG AGC AAT CAC C-3′ | 5′-AAG GTT GTG AGG TTG CAT TTG A-3′ |
| SOX2 | 5′-CCA GCA GAC TTC ACA TGT CC-3′ | 5′-ACA TGT GTG AGA GGG GCA GT-3′ |
| ACTIN | 5′-AGA GCT ACG AGC TGC CTG AC-3′ | 5′-AGC ACT GTG TTG GCG TAC AG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fageeh, H.N.; Bhandi, S.; Mashyakhy, M.; Kahtani, A.A.; Badran, Z.; Mehta, D.; Fageeh, H.I.; Balaji, T.M.; Baeshen, H.A.; Varadarajan, S.; et al. Viability of Quercetin-Induced Dental Pulp Stem Cells in Forming Living Cellular Constructs for Soft Tissue Augmentation. J. Pers. Med. 2021, 11, 430. https://doi.org/10.3390/jpm11050430
Fageeh HN, Bhandi S, Mashyakhy M, Kahtani AA, Badran Z, Mehta D, Fageeh HI, Balaji TM, Baeshen HA, Varadarajan S, et al. Viability of Quercetin-Induced Dental Pulp Stem Cells in Forming Living Cellular Constructs for Soft Tissue Augmentation. Journal of Personalized Medicine. 2021; 11(5):430. https://doi.org/10.3390/jpm11050430
Chicago/Turabian StyleFageeh, Hytham N., Shilpa Bhandi, Mohammed Mashyakhy, Ahmed Al Kahtani, Zahi Badran, Deepak Mehta, Hammam Ibrahim Fageeh, Thodur Madapusi Balaji, Hosam Ali Baeshen, Saranya Varadarajan, and et al. 2021. "Viability of Quercetin-Induced Dental Pulp Stem Cells in Forming Living Cellular Constructs for Soft Tissue Augmentation" Journal of Personalized Medicine 11, no. 5: 430. https://doi.org/10.3390/jpm11050430
APA StyleFageeh, H. N., Bhandi, S., Mashyakhy, M., Kahtani, A. A., Badran, Z., Mehta, D., Fageeh, H. I., Balaji, T. M., Baeshen, H. A., Varadarajan, S., Raj, A. T., Patil, V. R., Vyas, N., Zanza, A., Testarelli, L., & Patil, S. (2021). Viability of Quercetin-Induced Dental Pulp Stem Cells in Forming Living Cellular Constructs for Soft Tissue Augmentation. Journal of Personalized Medicine, 11(5), 430. https://doi.org/10.3390/jpm11050430