Evaluation of Drug—Drug Interactions in EGFR-Mutated Non-Small-Cell Lung Cancer Patients during Treatment with Tyrosine-Kinase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Concomitant Medications
- gastric acid suppressant, classified according to their indication as no vs. gastritis/gastroesophageal reflux disease (GERD) versus prophylaxis (e.g., to prevent gastritis due to other concomitant medication), no versus H2 antagonists (such as ranitidine) vs. proton-pump inhibitors;
- statins (yes vs. no);
- other lipid-lowering agents (fibrates, ezetimibe and similar) (yes vs. no);
- aspirin (considered as low-dose daily assumption of aspirin for cardiovascular prevention) (yes vs. no);
- anticoagulants (including new oral anticoagulants, low-molecular weight heparin and cumarinic anticoagulant drugs) (yes vs. no);
- NSAIDs, including COX-2 inhibitors (including both chronic and PRN administration) (yes vs. no);
- ACE inhibitors/angiotensin II receptor blockers (ARBs) (yes vs. no), calcium antagonists (yes vs. no), β-blockers (yes vs. no);
- metformin (yes vs. no) and other oral antidiabetics (yes vs. no);
- opioids (yes vs. no);
- antidepressants/antipsychotics (yes vs. no).
2.3. Drug-PIN
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
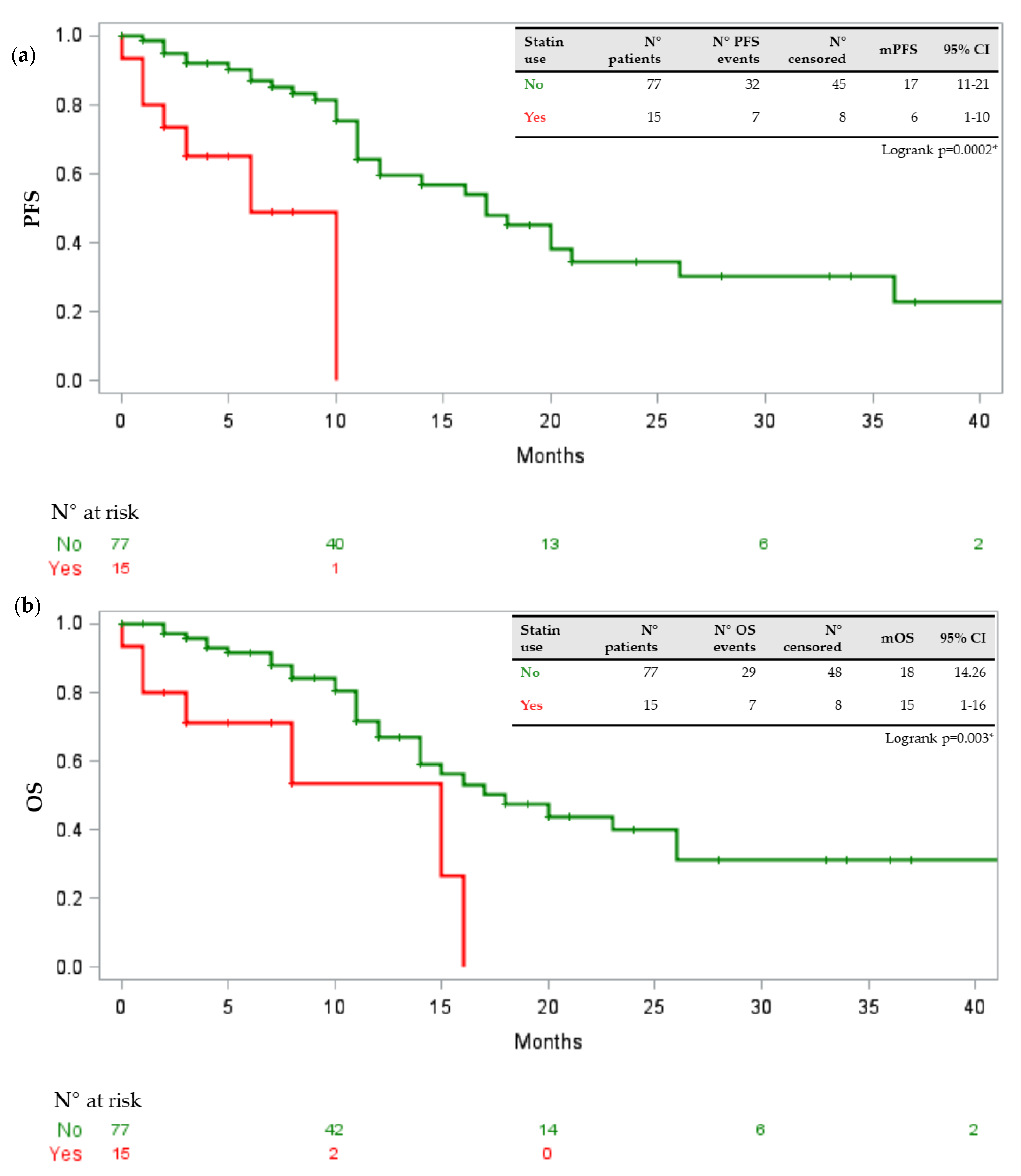
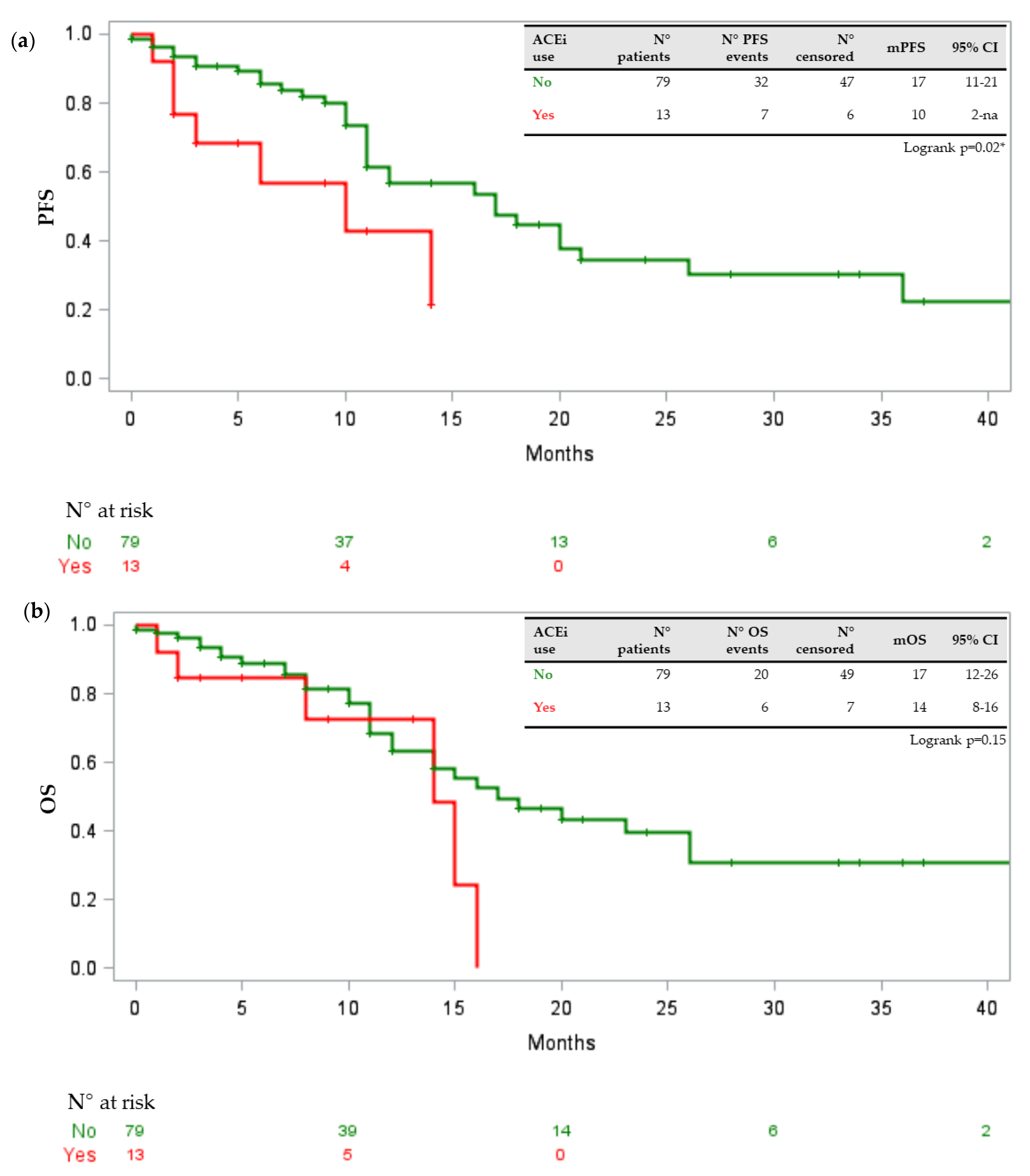
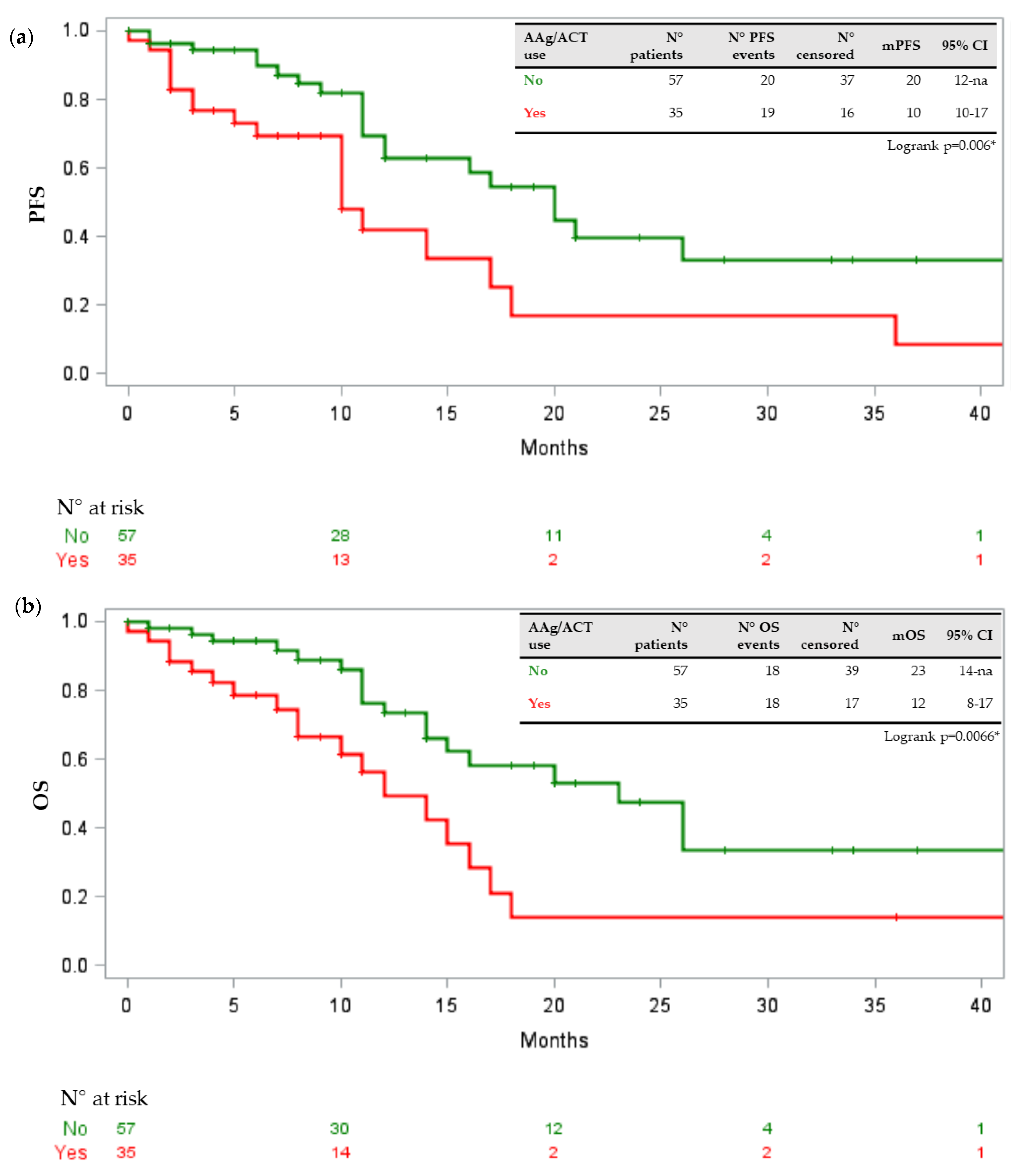
3.2. Drug–Drug Interactions and Toxicities
3.3. Potential Risks Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DDI | drug-drug interaction |
| aNSCLC | advanced non-small-cell lung cancer |
| EGFR | epidermal growth factor receptor |
| TKIs | tyrosine-kinase inhibitors |
| NSCLC | non-small cell lung cancer |
| ADRs | adverse drug reactions |
| OS | overall survival |
| PFS | progression free survival |
| ECOG | Eastern Cooperative Oncology Group |
| ORR | objective response rate |
| GERD | gastroesophageal reflux disease |
References
- Tatro, D. Drug Interaction Facts; J.B. Lippincott: St Louis, MO, USA, 1992. [Google Scholar]
- Van Leeuwen, R.W.; Van Gelder, T.; Mathijssen, R.H.; Jansman, F.G. Drug-drug interactions with tyrosine-kinase inhibitors: A clinical perspective. Lancet Oncol. 2014, 15, e315–e326. [Google Scholar] [CrossRef]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013, 18, 601–610. [Google Scholar] [PubMed]
- Doucet, J.; Chassagne, P.; Trivalle, C.; Landrin, I.; Pauty, M.D.; Kadri, N.; Ménard, J.F.; Bercoff, E. Drug-drug interactions related to hospital admissions in older adults: A prospective study of 1000 patients. J. Am. Geriatr. Soc. 1996, 44, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, R.W.; Brundel, D.H.; Neef, C.; Van Gelder, T.; Mathijssen, R.H.J.; Burger, D.M.; Jansman, F.G.A. Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br. J. Cancer 2013, 108, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, R.W.; Swart, E.L.; Boven, E.; Boom, F.A.; Schuitenmaker, M.G.; Hugtenburg, J.G. Potential drug interactions in cancer therapy: A prevalence study using an advanced screening method. Ann. Oncol. 2011, 22, 2334–2341. [Google Scholar] [CrossRef]
- Riechelmann, R.P.; Tannock, I.F.; Wang, L.; Saad, E.D.; Taback, N.A.; Krzyzanowska, M.K. Potential drug interactions and duplicate prescriptions among cancer patients. J. Natl. Cancer Inst. 2007, 99, 592–600. [Google Scholar] [CrossRef]
- Van Leeuwen, R.W.; Jansman, F.G.; Van den Bemt, P.M.; De Man, F.; Piran, F.; Vincenten, I.; Jager, A.; Rijneveld, A.W.; Brugma, J.D.; Mathijssen, R.H.; et al. Drug-drug interactions in patients treated for cancer: A prospective study on clinical interventions. Ann. Oncol. 2015, 26, 992–997. [Google Scholar] [CrossRef]
- Van Oijen, B.; Janknegt, R.; De Wit, H.; Peters, F.; Schouten, H.; Van der Kuy, H. Medication surveillance on intravenous cytotoxic agents: A retrospective study. Int. J. Clin. Pharm. 2013, 35, 554–559. [Google Scholar] [CrossRef]
- Bulsink, A.; Imholz, A.L.T.; Brouwers, J.R.B.J.; Jansman, F.G.A. Characteristics of potential drug-related problems among oncology patients. Int. J. Clin. Pharm. 2013, 35, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Voll, M.L.; Yap, K.D.; Terpstra, W.E.; Crul, M. Potential drug–drug interactions between anti-cancer agents and community pharmacy dispensed drugs. Pharm. World Sci. 2010, 32, 575–580. [Google Scholar] [CrossRef]
- Lopez-Martin, C.; Siles, M.G.; Alcaide-Garcia, J.; Felipe, V.F. Role of clinical pharmacists to prevent drug interactions in cancer outpatients: A single-centre experience. Int. J. Clin. Pharm. 2014, 36, 1251–1259. [Google Scholar] [CrossRef]
- Rompelman, F.M.; Smit, A.A.; Franssen, E.J.; Crul, M. Drug–drug interactions of cytostatics with regular medicines in lung cancer patients. J. Oncol. Pharm. Pr. 2016, 23, 483–490. [Google Scholar] [CrossRef]
- Peters, S.; Zimmermann, S.; Adjei, A.A. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: Comparative pharmacokinetics and drug–drug interactions. Cancer Treat. Rev. 2014, 40, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.-Y.; Li, J.-L. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. OncoTargets Ther. 2019, 12, 5467–5484. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-M.; Lai, C.-H.; Chang, H.-C.; Chao, T.-Y.; Tseng, C.-C.; Fang, W.-F.; Wang, C.-C.; Chung, Y.-H.; Wang, Y.-H.; Su, M.-C.; et al. Antacid use and de novo brain metastases in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who were treated using first-line first-generation epidermal growth factor receptor tyrosine kinase inhibitors. PLoS ONE 2016, 11, e0149722. [Google Scholar] [CrossRef] [PubMed]
- Kumarakulasinghe, N.B.; Syn, N.; Soon, Y.Y.; Asmat, A.; Zheng, H.; Loy, E.Y.; Pang, B.; Soo, R.A. EGFR kinase inhibitors and gastric acid suppressants in EGFR-mutant NSCLC: A retrospective database analysis of potential drug interaction. Oncotarget 2016, 7, 85542–85550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Credible Meds Website. Available online: http://crediblemeds.org (accessed on 19 February 2021).
- Zhang, Y.; Xu, J.; Lou, Y.; Hu, S.; Yu, K.; Li, R.; Zhang, X.; Jin, B.; Han, B. Pretreatment direct bilirubin and total cholesterol are significant predictors of overall survival in advanced non-small-cell lung cancer patients with EGFR mutations. Int. J. Cancer 2017, 140, 1645–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, C.J.; Takimoto, C.H.; Latz, J.E.; Baker, S.D.; Murry, D.J.; Krull, J.H.; Fife, K.; Battiato, L.; Cleverly, A.; Chaudhary, A.K.; et al. Two drug interaction studies evaluating the pharmacokinetics and toxicity of pemetrexed when coadministered with aspirin or Ibuprofen in patients with advanced cancer. Clin. Cancer Res. 2006, 12, 536–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenia, M.L.; Long, K.S.; Hartshorn, E.A. Methotrexate and non steroidal anti-inflammatory drug interactions. Ann. Pharmacother. 1992, 26, 234–237. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The role of cholesterol in cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, A.; Gabitova, L.; Astsaturov, I. Regulation of cholesterol biosynthesis and cancer signaling. Curr. Opin. Pharmacol. 2012, 12, 710–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar]
- Mills, E.J.; Rachlis, B.; Wu, P.; Devereaux, P.J.; Arora, P.; Perri, D. Primary prevention of cardiovascular mortality and events with statin treatments: A network meta-analysis involving more than 65,000 patients. J. Am. Coll. Cardiol. 2008, 52, 1769–1781. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.-E.; Na, K.-S.; Park, D.-S.; Choi, K.-H.; Kim, B.-R.; Shim, H.; Jeong, E.-T.; Kim, H.-R. Apoptotic induction by simvastatin in human lung cancer A549 cells via Akt signaling dependent down-regulation of survivin. Investig. N. Drugs 2010, 29, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Hanai, J.-I.; Doro, N.; Sasaki, A.T.; Kobayashi, S.; Cantley, L.C.; Seth, P.; Sukhatme, V.P. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein Kinase (MAPK) and Phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J. Cell. Physiol. 2011, 227, 1709–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Liu, B.; Yuan, J.; Yang, J.; Zhang, J.; An, Y.; Tie, L.; Pan, Y.; Li, X. Atorvastatin reduces vascular endothelial growth factor (VEGF) expression in human non-small cell lung carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS) production. Mol. Oncol. 2012, 6, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-Y.; Li, C.-H.; Lin, C.-L.; Liang, J.-A. Long-term statin use in patients with lung cancer and dyslipidemia reduces the risk of death. Oncotarget 2016, 7, 42208–42215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardwell, C.R.; Mc Menamin, Ú.; Hughes, C.M.; Murray, L.J. Statin use and survival from lung cancer: A population- based cohort study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 833–841. [Google Scholar] [CrossRef] [Green Version]
- Hung, M.-S.; Chen, I.-C.; Lee, C.-P.; Huang, R.-J.; Chen, P.-C.; Tsai, Y.-H.; Yang, Y.-H. Statin improves survival in patients with EGFR-TKI lung cancer: A nationwide population-based study. PLoS ONE 2017, 12, e0171137. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.-E.; Kwon, S.-J.; Kim, Y.-S.; Park, D.-S.; Kim, B.-R.; Yoon, K.-H.; Jeong, E.-T.; Kim, H.-R. Effect of simvastatin on the resistance to EGFR tyrosine kinase inhibitors in a non-small cell lung cancer with the T790M mutation of EGFR. Exp. Cell Res. 2014, 323, 288–296. [Google Scholar] [CrossRef]
- Fiala, O.; Pesek, M.; Fínek, J.; Minarik, M.; Benesova, L.; Bortlíček, Z.; Topolčan, O. Statins augment efficacy of EGFR-TKIs in patients with advanced-stage non-small cell lung cancer harbouring KRAS mutation. Tumor Biol. 2015, 36, 5801–5805. [Google Scholar] [CrossRef]
- Ali, A.; Levantini, E.; Fhu, C.W.; Teo, J.T.; Clohessy, J.G.; Goggi, J.L.; Wu, C.-S.; Chen, L.; Chin, T.M.; Tenen, D.G. CAV1-GLUT3 signaling is important for cellular energy and can be targeted by Atorvastatin in Non-Small Cell Lung Cancer. Theranostics 2019, 9, 6157–6174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, Z.; Zhao, M.; Wang, Q.; Qiao, C.; Miao, L.; Ding, X. High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J. Cell. Physiol. 2018, 233, 6722–6732. [Google Scholar] [CrossRef]
- Irwin, M.E.; Mueller, K.L.; Bohin, N.; Ge, Y.; Boerner, J.L. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell. Physiol. 2010, 226, 2316–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Shimamura, T.; Perera, S.; Carlson, N.E.; Cai, D.; Shapiro, G.I.; Wong, K.K.; Letai, A. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007, 67, 11867–11875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, K.; Ito, F. EGF receptor in relation to tumor development: Molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2009, 277, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Ascilar, M.; De Jong, F.A.; Verweij, J.; Mathijssen, R.H.J. Complementary and alternative medicine during cancer treatment: Beyond innocence. Oncologist 2006, 11, 732–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Metabolyzed by CYP | Can Inhibit | Can Induce | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3A4 | 3A5 | 2D6 | 1A1 | 1A2 | 1B1 | 2C8 | 2C9 | 2C19 | 2E1 | |||
| Erlotinib | +++ | +++ | + | + | ++ | + | + | + | - | - | CYP3A4 (m) | CYP1A1 CYP1A2 |
| CYP2C8 (m) | ||||||||||||
| CYP1A1 (s) | ||||||||||||
| Gefitinib | +++ | ++ | +++ | ++ | + | - | - | - | - | - | CYP2C19 (w) CYP2D6 (w) | - |
| Afatinib | - | - | - | - | - | - | - | - | - | - | - | - |
| Osimertinib | +++ | +++ | - | - | - | - | - | - | - | - | - | CYP3A (w) |
| Study Population | N° | % |
|---|---|---|
| Age | ||
| <70 | 50 | 54 |
| ≥70 | 42 | 46 |
| Sex | ||
| Male | 31 | 34 |
| Female | 61 | 66 |
| ECOG PS | ||
| 0–1 | 82 | 89 |
| ≥2 | 10 | 11 |
| BMI (kg/m2) | ||
| Median | 22 | |
| (16.4–36.5) | ||
| Treatments | ||
| Gefitinib | 6 | 6 |
| Afatinib | 13 | 14 |
| Osimertinib | 73 | 80 |
| Disease burden | ||
| 0–2 sites | 34 | 37 |
| 3 and more than 3 | 58 | 63 |
| * including primitive lesion | ||
| Concomitant medications | ||
| Up to 2 | 14 | 15 |
| 3 and more than 3 | 78 | 85 |
| Comorbidities | ||
| Up to 2 | 60 | 65 |
| 3 and up | 18 | 20 |
| Statins | ||
| Yes | 15 | 16 |
| No | 77 | 84 |
| ACE inhibitors | ||
| Yes | 13 | 14 |
| No | 79 | 86 |
| Sartans | ||
| Yes | 14 | 15 |
| No | 78 | 85 |
| Calcium antagonists | ||
| Yes | 10 | 11 |
| No | 82 | 89 |
| B-blockers | ||
| Yes | 24 | 26 |
| No | 68 | 74 |
| Anticoagulants/aspirin | ||
| Si | 35 | 38 |
| No | 57 | 62 |
| Gastric acid suppressant | ||
| Yes | 64 | 70 |
| No | 28 | 30 |
| Metformin | ||
| Yes | 7 | 8 |
| No | 85 | 92 |
| Insulinotherapy | ||
| Yes | 1 | 1 |
| No | 0 | 99 |
| Other oral antidiabetics | ||
| Yes | 3 | 3 |
| No | 89 | 97 |
| Antidepressants/antipsychotics | ||
| Yes | 23 | 25 |
| No | 69 | 75 |
| Oppioids | ||
| Yes | 18 | 20 |
| No | 74 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Occhipinti, M.; Brambilla, M.; Galli, G.; Manglaviti, S.; Giammaruco, M.; Prelaj, A.; Ferrara, R.; De Toma, A.; Proto, C.; Beninato, T.; et al. Evaluation of Drug—Drug Interactions in EGFR-Mutated Non-Small-Cell Lung Cancer Patients during Treatment with Tyrosine-Kinase Inhibitors. J. Pers. Med. 2021, 11, 424. https://doi.org/10.3390/jpm11050424
Occhipinti M, Brambilla M, Galli G, Manglaviti S, Giammaruco M, Prelaj A, Ferrara R, De Toma A, Proto C, Beninato T, et al. Evaluation of Drug—Drug Interactions in EGFR-Mutated Non-Small-Cell Lung Cancer Patients during Treatment with Tyrosine-Kinase Inhibitors. Journal of Personalized Medicine. 2021; 11(5):424. https://doi.org/10.3390/jpm11050424
Chicago/Turabian StyleOcchipinti, Mario, Marta Brambilla, Giulia Galli, Sara Manglaviti, Maristella Giammaruco, Arsela Prelaj, Roberto Ferrara, Alessandro De Toma, Claudia Proto, Teresa Beninato, and et al. 2021. "Evaluation of Drug—Drug Interactions in EGFR-Mutated Non-Small-Cell Lung Cancer Patients during Treatment with Tyrosine-Kinase Inhibitors" Journal of Personalized Medicine 11, no. 5: 424. https://doi.org/10.3390/jpm11050424
APA StyleOcchipinti, M., Brambilla, M., Galli, G., Manglaviti, S., Giammaruco, M., Prelaj, A., Ferrara, R., De Toma, A., Proto, C., Beninato, T., Zattarin, E., Lo Russo, G., Gelibter, A. J., Simmaco, M., Preissner, R., Garassino, M. C., De Braud, F., & Marchetti, P. (2021). Evaluation of Drug—Drug Interactions in EGFR-Mutated Non-Small-Cell Lung Cancer Patients during Treatment with Tyrosine-Kinase Inhibitors. Journal of Personalized Medicine, 11(5), 424. https://doi.org/10.3390/jpm11050424








