Increased Incidence and Associated Risk Factors of Aspergillosis in Patients with Bronchiectasis
Abstract
:1. Introduction
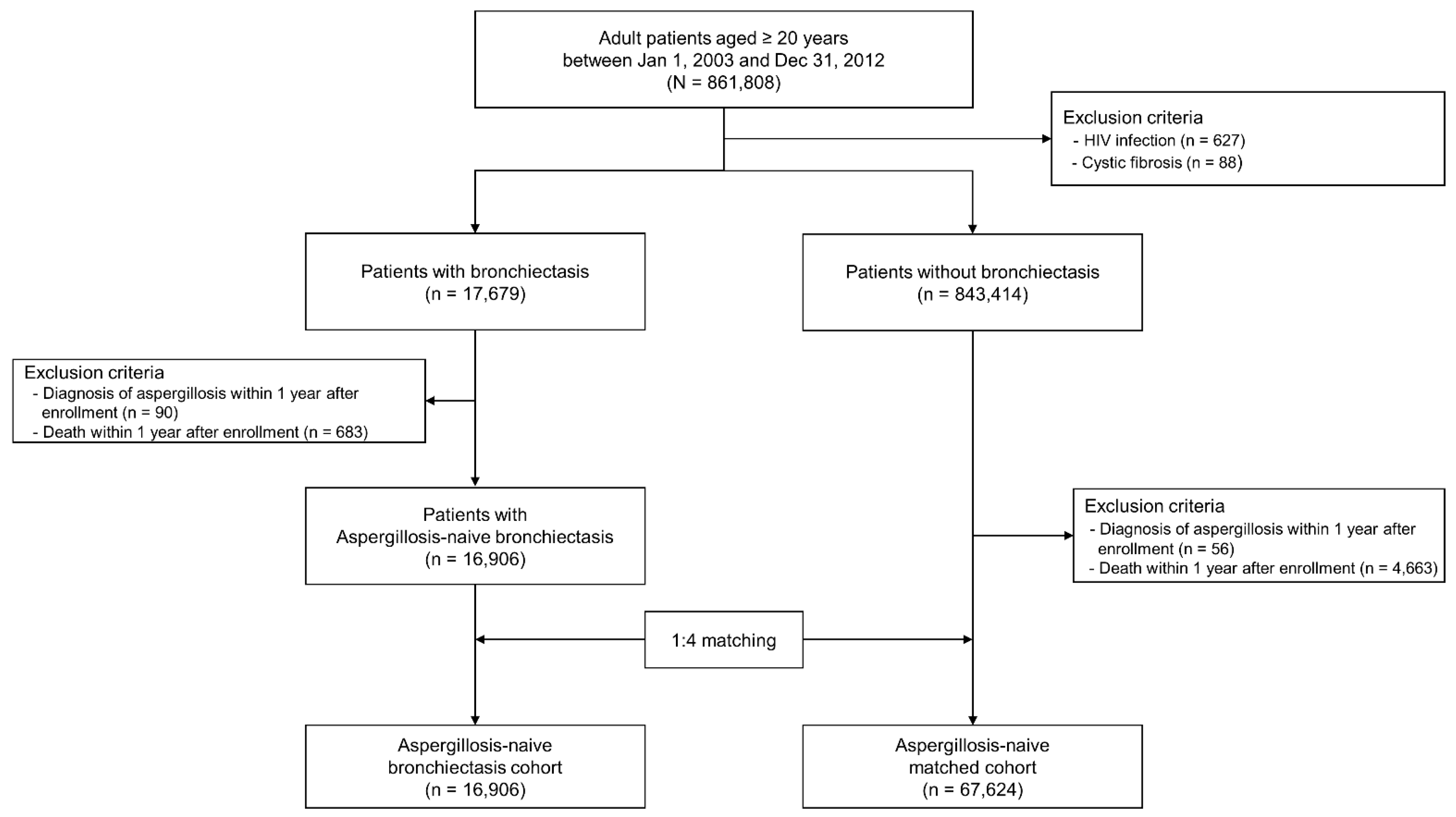
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
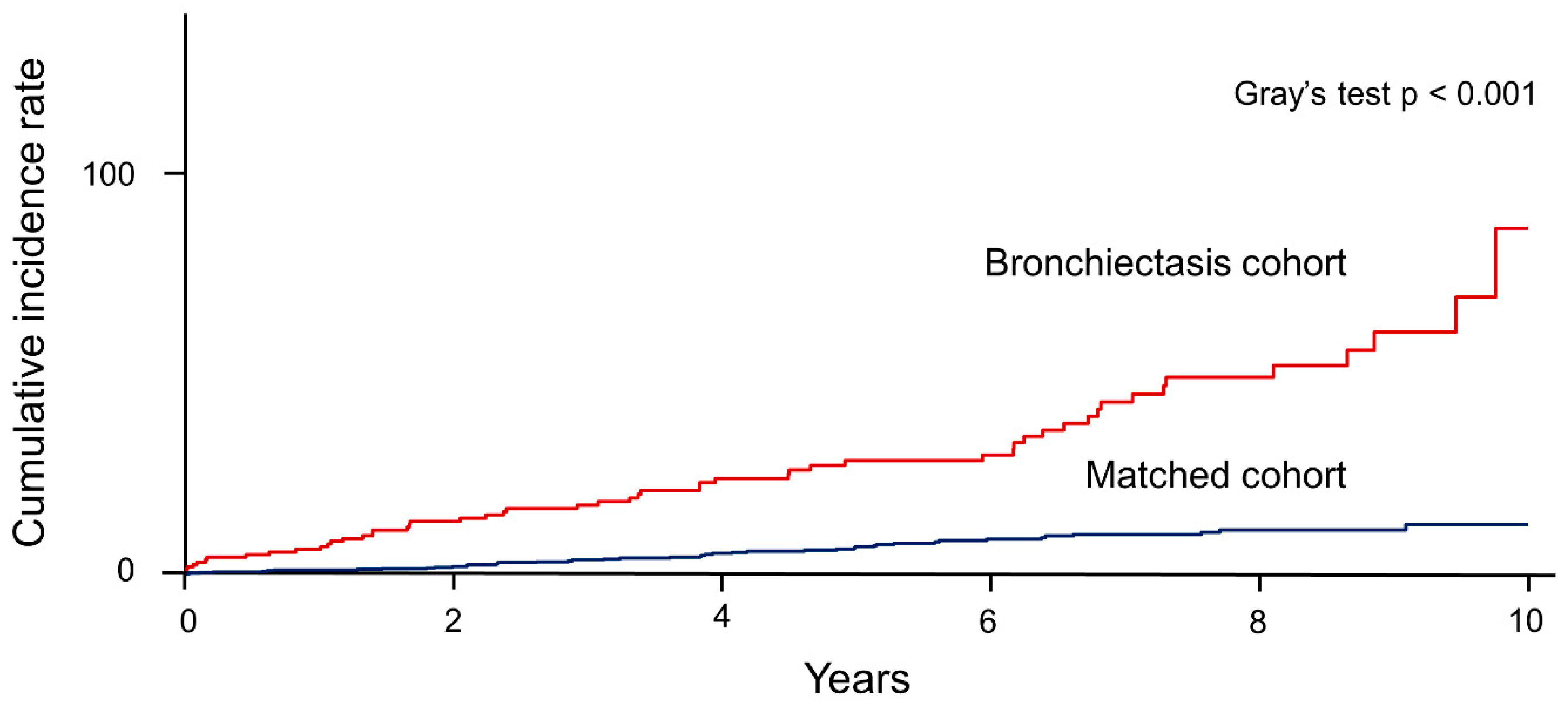
3.2. Incidence Rate and Risk of Aspergillosis in the Bronchiectasis Cohort Versus the Matched Cohort
3.3. Risk Factors of Aspergillosis in the Bronchiectasis Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalmers, J.D.; Chang, A.B.; Chotirmall, S.H.; Dhar, R.; McShane, P.J. Bronchiectasis. Nat. Rev. Dis. Primers 2018, 4, 45. [Google Scholar] [CrossRef]
- King, P.T. The pathophysiology of bronchiectasis. Int. J. Chron. Obs. Pulmon. Dis. 2009, 4, 411–419. [Google Scholar] [CrossRef]
- Máiz, L.; Vendrell, M.; Olveira, C.; Girón, R.; Nieto, R.; Martínez-García, M. Prevalence and factors associated with isolation of Aspergillus and Candida from sputum in patients with non-cystic fibrosis bronchiectasis. Respir. Int. Rev. Thorac. Dis. 2015, 89, 396–403. [Google Scholar] [CrossRef]
- Gashynova, K.; Suska, K.; Dmytrychenko, V. Factors affecting the frequency of exacerbations in adult patients with bronchiectasis. Wiad Lek 2020, 73, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Hadid, W.; Ericsoussi, B.; Rodgers, D.; Sadikot, R.T. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int. J. Infect. Dis 2013, 17, e1000–e1004. [Google Scholar] [CrossRef]
- Yang, B.; Ryu, J.; Kim, T.; Jo, Y.S.; Kim, Y.; Park, H.Y.; Kang, Y.A.; Lee, S.J.; Lee, O.-J.; Moon, J.-Y.; et al. Impact of bronchiectasis on incident NTM pulmonary disease: A 10-year national cohort study. CHEST 2020. [Google Scholar] [CrossRef]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef]
- De Soyza, A.; Aliberti, S. Bronchiectasis and Aspergillus: How are they linked? Med. Mycol. 2017, 55, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yang, B.; Nam, H.; Kyoung, D.S.; Sim, Y.S.; Park, H.Y.; Lee, J.S.; Lee, S.W.; Oh, Y.M.; Ra, S.W.; et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Lee, M.R.; Huang, H.L.; Chen, L.C.; Yang, H.C.; Ko, J.C.; Cheng, M.H.; Chong, I.W.; Lee, L.N.; Wang, J.Y.; Dimopoulos, G. Seroprevalence of Aspergillus IgG and disease prevalence of chronic pulmonary aspergillosis in a country with intermediate burden of tuberculosis: A prospective observational study. Clin. Microbiol. Infect. 2020, 26, e1091–e1097. [Google Scholar] [CrossRef]
- Andrejak, C.; Nielsen, R.; Thomsen, V.O.; Duhaut, P.; Sorensen, H.T.; Thomsen, R.W. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013, 68, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kunst, H.; Wickremasinghe, M.; Wells, A.; Wilson, R. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur. Respir. J. 2006, 28, 352–357. [Google Scholar] [CrossRef]
- Dhar, R.; Singh, S.; Talwar, D.; Mohan, M.; Tripathi, S.K.; Swarnakar, R.; Trivedi, S.; Rajagopala, S.; D’Souza, G.; Padmanabhan, A.; et al. Bronchiectasis in India: Results from the European multicentre bronchiectasis audit and research collaboration (EMBARC) and respiratory research network of India registry. Lancet Glob. Health 2019, 7, e1269–e1279. [Google Scholar] [CrossRef]
- Visser, S.K.; Bye, P.T.P.; Fox, G.J.; Burr, L.D.; Chang, A.B.; Holmes-Liew, C.L.; King, P.; Middleton, P.G.; Maguire, G.P.; Smith, D.; et al. Australian adults with bronchiectasis: The first report from the Australian bronchiectasis registry. Respir. Med. 2019, 155, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Shteinberg, M.; Aliberti, S.; Goeminne, P.C.; Hill, A.T.; Fardon, T.C.; Obradovic, D.; Stone, G.; Trautmann, M.; Davis, A.; et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Salzer, H.J.; Heyckendorf, J.; Kalsdorf, B.; Rolling, T.; Lange, C. Characterization of patients with chronic pulmonary aspergillosis according to the new ESCMID/ERS/ECMM and IDSA guidelines. Mycoses 2017, 60, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Irfan, M.; Zubairi, A.B.; Jabeen, K.; Awan, S.; Khan, J.A. Clinical manifestations and outcomes of pulmonary aspergillosis: Experience from Pakistan. BMJ Open Respir. Res. 2016, 3, e000155. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2021 Report). Available online: https://goldcopd.org/2021-gold-reports (accessed on 5 March 2021).
- Shahi, M.; Ayatollahi Mousavi, S.A.; Nabili, M.; Aliyali, M.; Khodavaisy, S.; Badali, H. Aspergillus colonization in patients with chronic obstructive pulmonary disease. Curr. Med. Mycol. 2015, 1, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Waqas, S.; Dunne, K.; Talento, A.F.; Wilson, G.; Martin-Loeches, I.; Keane, J.; Rogers, T.R. Prospective observational study of respiratory Aspergillus colonization or disease in patients with various stages of chronic obstructive pulmonary disease utilizing culture versus nonculture techniques. Med. Mycol. 2020. [Google Scholar] [CrossRef]
- Saraceno, J.L.; Phelps, D.T.; Ferro, T.J.; Futerfas, R.; Schwartz, D.B. Chronic necrotizing pulmonary aspergillosis: Approach to management. Chest 1997, 112, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Camuset, J.; Nunes, H.; Dombret, M.C.; Bergeron, A.; Henno, P.; Philippe, B.; Dauriat, G.; Mangiapan, G.; Rabbat, A.; Cadranel, J. Treatment of chronic pulmonary aspergillosis by voriconazole in nonimmunocompromised patients. Chest 2007, 131, 1435–1441. [Google Scholar] [CrossRef]
- Tuberculosis Association. Aspergilloma and residual tuberculous cavities—The results of a resurvey. Tubercle 1970, 51, 227–245. [Google Scholar] [CrossRef]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Dhooria, S.; Prasad, K.T.; Muthu, V.; Aggarwal, A.N.; Rawat, A.; Pal, A.; Bal, A.; Garg, M.; Chakrabarti, A.; et al. Sensitization to A.fumigatus in subjects with non-cystic fibrosis bronchiectasis. Mycoses 2020. [Google Scholar] [CrossRef]
- Page, I.D.; Byanyima, R.; Hosmane, S.; Onyachi, N.; Opira, C.; Richardson, M.; Sawyer, R.; Sharman, A.; Denning, D.W. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur. Respir. J. 2019, 53, 1801184. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef]
- Ritz, N.; Ammann, R.A.; Aebischer, C.C.; Schoeni-Affolter, F.; Schoeni, M.H. Risk factors for allergic bronchopulmonary aspergillosis and sensitisation to Aspergillus fumigatus in patients with cystic fibrosis. Eur. J. Pediatrics 2005, 164, 577–582. [Google Scholar] [CrossRef]
- Noni, M.; Katelari, A.; Dimopoulos, G.; Kourlaba, G.; Spoulou, V.; Alexandrou-Athanassoulis, H.; Doudounakis, S.-E.; Tzoumaka-Bakoula, C. Inhaled corticosteroids and Aspergillus fumigatus isolation in cystic fibrosis. Med. Mycol. 2014, 52, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Psoter, K.J.; Jennings, M.T.; Merlo, C.A.; Boyle, M.P.; Hadjiliadis, D.; Kawut, S.M.; Lechtzin, N. Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Alby, K.; Ng, S.C.W.; Fleck, V.; Kubrak, C.; Rubenstein, R.C.; Dorgan, D.J.; Kawut, S.M.; Hadjiliadis, D. The presence of Aspergillus fumigatus is associated with worse respiratory quality of life in cystic fibrosis. J. Cyst. Fibros. 2020, 19, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Welham, S.A.; Sullivan, A.L.; Loebinger, M.R. Updated BTS adult bronchiectasis guideline 2018: A multidisciplinary approach to comprehensive care. Thorax 2019, 74, 1–3. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 84,530) | Bronchiectasis Cohort (n = 16,906) | Matched Cohort (n = 67,624) | p-Value | |
|---|---|---|---|---|
| Age, years | >0.999 | |||
| 20–29 | 3860 (4.6) | 772 (4.6) | 3088 (4.6) | |
| 30–39 | 7840 (9.3) | 1568 (9.3) | 6272 (9.3) | |
| 40–49 | 13,530 (16.0) | 2706 (16.0) | 10,824 (16.0) | |
| 50–59 | 19,000 (22.5) | 3800 (22.5) | 15,200 (22.5) | |
| 60–69 | 20,560 (24.3) | 4112 (24.3) | 16,448 (24.3) | |
| ≥70 | 19,740 (23.3) | 3948 (23.3) | 15,792 (23.3) | |
| Sex | >0.999 | |||
| Male | 39,930 (47.2) | 7986 (47.2) | 31,944 (47.2) | |
| Female | 44,600 (52.8) | 8920 (52.8) | 35,680 (52.8) | |
| Type of insurance | <0.001 | |||
| Self-employed health insurance | 32,097 (38.0) | 6426 (38.0) | 25,671 (38.0) | |
| Employee health insurance | 50,384 (59.6) | 9993 (59.1) | 40,391 (59.7) | |
| Medical aid | 2049 (2.4) | 487 (2.9) | 1562 (2.3) | |
| Pulmonary comorbidities | ||||
| Asthma | 11,831 (14.0) | 5819 (34.4) | 6012 (8.9) | <0.001 |
| COPD | 8205 (9.7) | 4679 (27.7) | 3526 (5.2) | <0.001 |
| Previous pulmonary tuberculosis | 3776 (4.5) | 2035 (12.0) | 1741 (2.6) | <0.001 |
| NTM pulmonary disease | 21 (0.0) | 19 (0.1) | 2 (<0.1) | <0.001 |
| Extra-pulmonary comorbidities | ||||
| Diabetes mellitus | 14,134 (16.7) | 3442 (20.4) | 10,692 (15.8) | <0.001 |
| Rheumatologic disease | 3346 (4.0) | 1022 (6.1) | 2324 (3.4) | <0.001 |
| Malignancy | ||||
| Lung cancer | 843 (1.0) | 639 (3.8) | 204 (0.3) | <0.001 |
| Other cancers | 2119 (2.5) | 632 (3.7) | 1487 (2.2) | <0.001 |
| Total (N = 84,675) | Male (n = 40,015) | Female (n = 44,660) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | IR | sdHR | 95% CI | Case | IR | sdHR | 95% CI | Case | IR | sdHR | 95% CI | |
| Overall | ||||||||||||
| Matched | 45 | 10.9 | Ref | 27 | 14.2 | Ref | 18 | 8.1 | Ref | |||
| Bronchiectasis | 51 | 50.2 | 4.53 | 3.25–6.32 | 27 | 57.9 | 4.00 | 2.57–6.23 | 24 | 43.7 | 5.33 | 3.21–8.87 |
| Age group | ||||||||||||
| <60 years | ||||||||||||
| Matched | 22 | 9.5 | Ref | 16 | 15.0 | Ref | 6 | 4.8 | Ref | |||
| Bronchiectasis | 27 | 46.8 | 4.91 | 3.07–7.84 | 13 | 49.1 | 3.25 | 1.77–5.97 | 14 | 44.9 | 9.33 | 4.11–21.22 |
| ≥60 years | ||||||||||||
| Matched | 23 | 12.8 | Ref | 11 | 13.1 | Ref | 12 | 12.5 | Ref | |||
| Bronchiectasis | 24 | 54.7 | 4.17 | 2.60–6.71 | 14 | 69.3 | 5.09 | 2.64–9.82 | 10 | 42.2 | 3.33 | 1.66–6.69 |
| Numbers at Risk (N = 16,906) | Aspergillosis (n = 51) | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | Adjusted HR | 95% CI | |||
| Age | ||||||
| ≤39 | 2340 (13.8) | 2/2340 (0.1) | Ref | Ref | Ref | Ref |
| 40–49 | 2706 (16.0) | 11/2706 (0.4) | 4.91 | 1.09–22.16 | 4.43 | 0.98–20.01 |
| 50–59 | 3800 (22.5) | 14/3800 (0.4) | 5.30 | 1.20–23.34 | 4.01 | 0.90–17.81 |
| 60–69 | 4112 (24.3) | 13/4112 (0.3) | 4.57 | 1.03–20.27 | 2.77 | 0.61–12.52 |
| ≥70 | 3948 (23.4) | 11/3948 (0.3) | 5.32 | 1.17–24.06 | 3.16 | 0.68–14.66 |
| Sex | ||||||
| Female | 7986 (47.2) | 27/7986 (0.3) | Ref | Ref | ||
| Male | 8920 (52.8) | 24/8920 (0.3) | 1.35 | 0.78–2.34 | ||
| Type of insurance | ||||||
| Self-employed health insurance | 6426 (38.0) | 19/6426 (0.3) | Ref | Ref | ||
| Employee health insurance | 9993 (59.1) | 31/9993 (0.3) | 1.16 | 0.66–2.06 | ||
| Medical aid | 487 (2.9) | 1/487 (0.2) | 1.73 | 0.23–13.02 | ||
| Comorbidities | ||||||
| COPD | 4679 (27.7) | 27/4679 (0.6) | 3.09 | 1.78–5.35 | 1.95 | 1.07–3.57 |
| Asthma | 5819 (34.4) | 28/5819 (0.5) | 2.27 | 1.31–3.93 | 1.27 | 0.68–2.37 |
| Previous pulmonary tuberculosis | 2035 (12.0) | 19/2305 (0.8) | 4.83 | 2.74–8.53 | 3.67 | 2.03–6.64 |
| NTM pulmonary disease | 19 (0.1) | 1/19 (5.3) | 28.73 | 3.96–208.41 | 11.25 | 1.49–85.18 |
| Diabetes mellitus | 3442 (20.4) | 11/3442 (0.3) | 1.32 | 0.68–2.58 | ||
| Rheumatologic disease | 1022 (6.1) | 3/1022 (0.3) | 0.93 | 0.29–2.99 | ||
| Lung cancer | 639 (3.8) | 3/639 (0.5) | 1.65 | 0.52–5.31 | ||
| Medication | ||||||
| Use of ICS | ||||||
| No use | 13,149 (77.8) | 27/13,149 (0.2) | Ref | Ref | Ref | Ref |
| <1 year | 2540 (15.0) | 14/2540 (0.6) | 2.50 | 1.31–4.77 | 1.83 | 0.92–3.63 |
| ≥1 year | 1217 (7.2) | 10/1217 (0.8) | 3.39 | 1.64–7.01 | 1.69 | 0.74–3.89 |
| Systemic corticosteroids * | ||||||
| No use | 3602 (21.3) | 5/3602 (0.1) | Ref | Ref | Ref | Ref |
| <10 mg/day | 12,764 (75.5) | 41/12,764 (0.3) | 1.43 | 0.56–3.65 | 1.29 | 0.50–3.31 |
| ≥10 mg/day or more | 540 (3.2) | 5/540 (0.9) | 3.60 | 1.04–12.50 | 2.15 | 0.59–7.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Kim, T.; Ryu, J.; Park, H.Y.; Hwangbo, B.; Kong, S.-Y.; Kwon, Y.-S.; Lee, S.J.; Ra, S.W.; Oh, Y.-M.; et al. Increased Incidence and Associated Risk Factors of Aspergillosis in Patients with Bronchiectasis. J. Pers. Med. 2021, 11, 422. https://doi.org/10.3390/jpm11050422
Yang B, Kim T, Ryu J, Park HY, Hwangbo B, Kong S-Y, Kwon Y-S, Lee SJ, Ra SW, Oh Y-M, et al. Increased Incidence and Associated Risk Factors of Aspergillosis in Patients with Bronchiectasis. Journal of Personalized Medicine. 2021; 11(5):422. https://doi.org/10.3390/jpm11050422
Chicago/Turabian StyleYang, Bumhee, Taehee Kim, Jiin Ryu, Hye Yun Park, Bin Hwangbo, Sun-Young Kong, Yong-Soo Kwon, Seung Jun Lee, Seung Won Ra, Yeon-Mok Oh, and et al. 2021. "Increased Incidence and Associated Risk Factors of Aspergillosis in Patients with Bronchiectasis" Journal of Personalized Medicine 11, no. 5: 422. https://doi.org/10.3390/jpm11050422
APA StyleYang, B., Kim, T., Ryu, J., Park, H. Y., Hwangbo, B., Kong, S.-Y., Kwon, Y.-S., Lee, S. J., Ra, S. W., Oh, Y.-M., Sohn, J. W., Choe, K. H., Choi, H., & Lee, H. (2021). Increased Incidence and Associated Risk Factors of Aspergillosis in Patients with Bronchiectasis. Journal of Personalized Medicine, 11(5), 422. https://doi.org/10.3390/jpm11050422







