Fertility, Pregnancy and Lactation Considerations for Women with CF in the CFTR Modulator Era
Abstract
1. Introduction
2. Fertility
2.1. Possible Causes of Infertility in Women with CF
2.2. Unexpected Pregnancies and Improved Fertility with Modulators
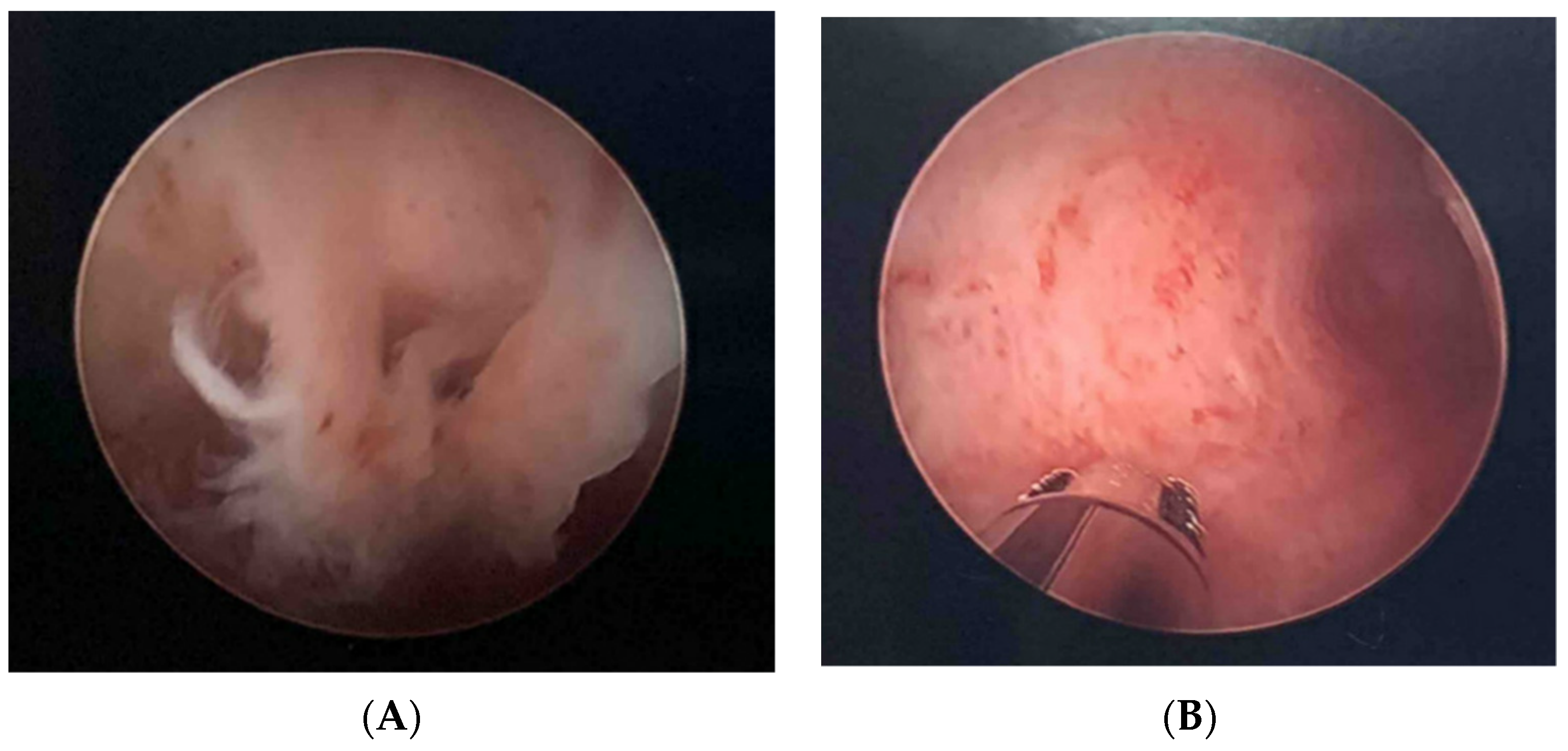
2.3. Fertility Case Example
3. Pregnancy
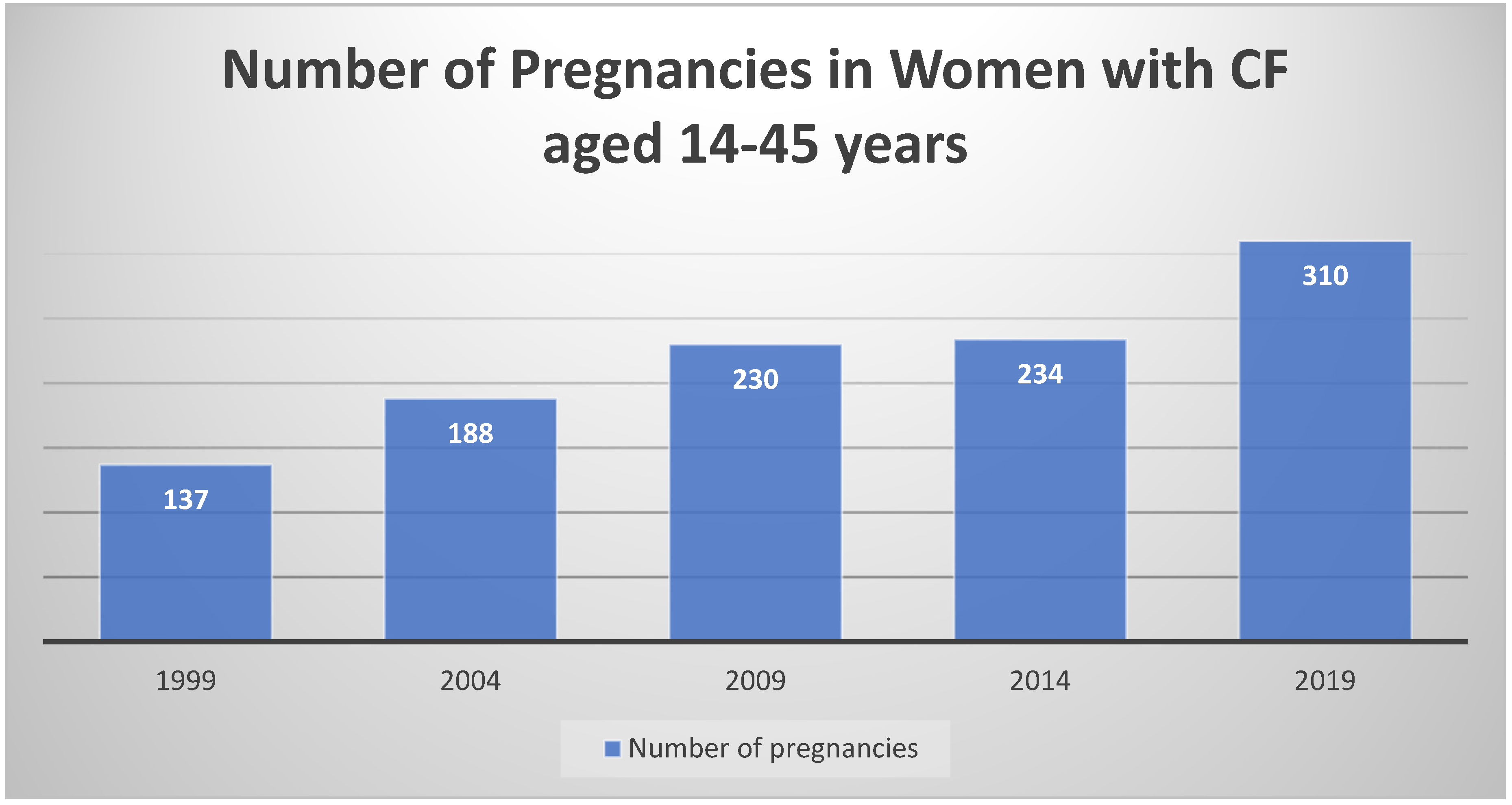
3.1. History of Pregnancy in Women with CF
3.2. Pregnancy Case Example
3.3. Data from Animal Reproductive Models Following CFTR Modulator Administration
3.4. Data in Pregnant Women Exposed to CFTR Modulators during Pregnancy
3.5. Considerations for Infants Exposed to CFTR Modulators during Pregnancy
4. Lactation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gan, K.H.; Geus, W.P.; Bakker, W.; Lamers, C.B.; Heijerman, H.G. Genetic and clinical features of patients with cystic fibrosis diagnosed after the age of 16 years. Thorax 1995, 50, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, M.; Redmond, A.; Hill, A.; Elborn, J. Clinical features associated with a delayed diagnosis of cystic fibrosis. Respiration 2000, 67, 402–407. [Google Scholar] [CrossRef]
- Registry, Cystic Fibrosis Foundation. 2019 Annual Data Report. Available online: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-Patient-Registry-Annual-Data-Report.pdf (accessed on 18 April 2021).
- Cystic Fibrosis Mutation Database. Available online: http://www.genet.sickkids.on.ca/Home.html (accessed on 3 May 2021).
- UK Cystic Fibrosis Registry. Annual Data Repot 2019; UK Trust: 2021. Available online: https://www.cysticfibrosis.org.uk/sites/default/files/202012/2019%20Registry%20Annual%20Data%20report_Sep%202020.pdf (accessed on 18 April 2021).
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Dřevínek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; Van Der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Rowe, S.M.; Daines, C.; Ringshausen, F.C.; Kerem, E.; Wilson, J.; Tullis, E.; Nair, N.; Simard, C.; Han, L.; Ingenito, E.P.; et al. Tezacaftor–Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N. Engl. J. Med. 2017, 377, 2024–2035. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Hughan, K.S.; Daley, T.; Rayas, M.S.; Kelly, A.; Roe, A. Female reproductive health in cystic fibrosis. J. Cyst. Fibros. 2019, 18 (Suppl. 2), S95–S104. [Google Scholar] [CrossRef]
- Münster, E.; Letzel, S.; Passet-Wittig, J.; Schneider, N.F.; Schuhrke, B.; Seufert, R.; Zier, U. Who is the gate keeper for treatment in a fertility clinic in Germany? -baseline results of a prospective cohort study (PinK study). BMC Pregnancy Childbirth 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shteinberg, M.; Ben Lulu, A.; Downey, D.G.; Blumenfeld, Z.; Rousset-Jablonski, C.; Perceval, M.; Colombo, A.; Stein, N.; Livnat, G.; Gur, M.; et al. Failure to conceive in women with CF is associated with pancreatic insufficiency and advancing age. J. Cyst. Fibros. 2019, 18, 525–529. [Google Scholar] [CrossRef]
- Tournier, A.; Murris, M.; Prevotat, A.; Fanton, A.; Bettiol, C.; Parinaud, J. Fertility of women with cystic fibrosis: A French survey. Reprod. Biomed. Online 2019, 39, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Shwachman, H.; Perlmutter, A.D.; Rule, A.; Khaw, K.-T.; Holsclaw, D.S. Reproductive Failure in Males with Cystic Fibrosis. N. Engl. J. Med. 1968, 279, 65–69. [Google Scholar] [CrossRef]
- Neinstein, L.S.; Stewart, D.; Wang, C.-I.; Johnson, I. Menstrual dysfunction in cystic fibrosis. J. Adolesc. Health Care 1983, 4, 153–157. [Google Scholar] [CrossRef]
- Stead, R.J.; Hodson, M.E.; Batten, J.C.; Adams, J.; Jacobs, H.S. AMENORRHOEA IN CYSTIC FIBROSIS. Clin. Endocrinol. 1987, 26, 187–195. [Google Scholar] [CrossRef]
- Sutton, S.; Rosenbluth, D.; Raghavan, D.; Zheng, J.; Jain, R. Effects of puberty on cystic fibrosis related pulmonary exacerbations in women versus men. Pediatr. Pulmonol. 2014, 49, 28–35. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.H.; Lu, Y.C.; Ding, G.L.; Yu, M.K.; Tsang, L.L.; Fok, K.L.; Liu, X.M.; Zhang, X.H.; Chung, Y.W.; et al. Impaired CFTR-Dependent Amplification of FSH-Stimulated Estrogen Production in Cystic Fibrosis and PCOS. J. Clin. Endocrinol. Metab. 2012, 97, 923–932. [Google Scholar] [CrossRef]
- Schram, C.; Stephenson, A.; Hannam, T.; Tullis, E. Cystic fibrosis (cf) and ovarian reserve: A cross-sectional study examining serum anti-mullerian hormone (amh) in young women. J. Cyst. Fibros. 2015, 14, 398–402. [Google Scholar] [CrossRef]
- Tizzano, E.F.; Silver, M.M.; Chitayat, D.; Benichou, J.C.; Buchwald, M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. Am. J. Pathol. 1994, 144, 906–914. [Google Scholar] [PubMed]
- Hayslip, C.; Hao, E.; Usala, S.J. The cystic fibrosis transmembrane regulator gene is expressed in the human endocervix throughout the menstrual cycle. Fertil. Steril. 1997, 67, 636–640. [Google Scholar] [CrossRef]
- Ismail, N.; Giribabu, N.; Muniandy, S.; Salleh, N. Estrogen and progesterone differentially regulate the levels of cystic fibrosis transmembrane regulator (CFTR), adenylate cyclase (AC), and cyclic adenosine mono-phosphate (cAMP) in the rat cervix. Mol. Reprod. Dev. 2015, 82, 463–474. [Google Scholar] [CrossRef]
- Edenborough, F.P. Respiratory diseases in pregnancy bullet 4: Women with cystic fibrosis and their potential for reproduction. Thorax 2001, 56, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ahmed, A.; Patrizio, P. Cystic fibrosis and fertility. Curr. Opin. Obstet. Gynecol. 2013, 25, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.C.; Shi, Q.X.; Zhou, C.X.; Wang, X.F.; Xu, W.M.; Chen, W.Y.; Chen, A.J.; Ni, Y.; Yuan, Y.Y. Critical role of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Mol. Cell. Endocrinol. 2006, 250, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, A.; Grzelewski, T.; Jerzyńska, J.; Majak, P.; Sołoniewicz, A.; Stelmach, W.; Stelmach, I. Sexual and Reproductive Health Knowledge in Cystic Fibrosis Female Patients and Their Parents. J. Sex. Med. 2009, 6, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Gage, L.A. What deficits in sexual and reproductive health knowledge exist among women with cystic fibrosis? A systematic review. Health Soc. Work 2012, 37, 29–36. [Google Scholar] [CrossRef]
- Kazmerski, T.M.; Gmelin, T.; Slocum, B.; Borrero, S.; Miller, E. Attitudes and Decision Making Related to Pregnancy Among Young Women with Cystic Fibrosis. Matern. Child Health J. 2016, 21, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.M.; Phelan, P.D.; Bowes, G. Reproductive health in young women with cystic fibrosis: Knowledge, behavior and attitudes. J. Adolesc. Health 1995, 17, 46–50. [Google Scholar] [CrossRef]
- Ladores, S.; Kazmerski, T.M.; Rowe, S.M. A Case Report of Pregnancy During Use of Targeted Therapeutics for Cystic Fibrosis. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ladores, S.; Bray, L.A.; Brown, J. Two Unanticipated Pregnancies While on Cystic Fibrosis Gene-Specific Drug Therapy. J. Patient Exp. 2020, 7, 4–7. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.E.; Goodwin, D.L.; Nesmith, A.; Garcia, B.; Mingora, C.; Ladores, S.L.; Rowe, S.M.; Krick, S.; Solomon, G.M. Elexacafator/tezacaftor/ivacaftor resolves subfertility in females with CF: A two center case series. J. Cyst. Fibros. 2021. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Jain, R. Maternal and fetal outcomes following elexacaftor-tezacaftor-ivacaftor use during pregnancy and lactation. J. Cyst. Fibros. 2021. [Google Scholar] [CrossRef]
- Siegel, B.; Siegel, S. Pregnancy and Delivery in a Patient with Cystic Fibrosis of the Pancreas: Report of a case. Obstet. Gynecol. 1960, 16, 438–440. [Google Scholar]
- Patel, E.M.; Swamy, G.K.; Heine, R.P.; Kuller, J.A.; James, A.H.; Grotegut, C.A. Medical and obstetric complications among pregnant women with cystic fibrosis. Am. J. Obstet. Gynecol. 2015, 212, 98–e1. [Google Scholar] [CrossRef] [PubMed]
- Jelin, A.C.; Sharshiner, R.; Caughey, A.B. Maternal co-morbidities and neonatal outcomes associated with cystic fibrosis*. J. Matern. Neonatal Med. 2016, 30, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, A.; Chapman, S.J.; MacKillop, L. The outcome of pregnancy in women with cystic fibrosis: A UK population-based descriptive study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1696–1703. [Google Scholar] [CrossRef]
- Cohen-Cymberknoh, M.; Reiss, B.G.; Reiter, J.; Lechtzin, N.; Melo, J.; Pérez, G.; Blau, H.; Mussaffi, H.; Levine, H.; Bentur, L.; et al. Baseline Cystic fibrosis disease severity has an adverse impact on pregnancy and infant outcomes, but does not impact disease progression. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gabbay-Benziv, R. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World J. Diabetes 2015, 6, 481–488. [Google Scholar] [CrossRef]
- Schechter, M.S.; Quittner, A.L.; Konstan, M.W.; Millar, S.J.; Pasta, D.J.; McMullen, A. Long-term Effects of Pregnancy and Motherhood on Disease Outcomes of Women with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2013, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Bessonova, L.; Volkova, N.; Higgins, M.; Bengtsson, L.; Tian, S.; Simard, C.; Konstan, M.W.; Sawicki, G.S.; Sewall, A.; Nyangoma, S.; et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax 2018, 73, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Pregnancy and Lactation Labeling Rule; Food and Drug Administration: Silver Spring, MD, USA, 2016. Available online: https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule (accessed on 19 April 2021).
- Ivacaftor (Kalydeco). United States Prescribing Information. Available online: https://pi.vrtx.com/files/uspi_ivacaftor.pdf (accessed on 19 April 2021).
- Lumacaftor/ivacaftor (Orkambi) United States Prescribing Information. Available online: https://pi.vrtx.com/files/uspi_lumacaftor_ivacaftor.pdf (accessed on 19 April 2021).
- Elexacaftor/tezacaftor/ivacaftor (Trikafta) United States Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212273s000lbl.pdf (accessed on 19 April 2021).
- Tezacaftor/ivacaftor (Symdeko) United States Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210491lbl.pdf (accessed on 19 April 2021).
- Trimble, A.; McKinzie, C.; Terrell, M.; Stringer, E.; Esther, C.R. Measured fetal and neonatal exposure to Lumacaftor and Ivacaftor during pregnancy and while breastfeeding. J. Cyst. Fibros. 2018, 17, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Kroon, M.; Akkerman-Nijland, A.; Rottier, B.; Koppelman, G.; Akkerman, O.; Touw, D. Drugs during pregnancy and breast feeding in women diagnosed with Cystic Fibrosis-An update. J. Cyst. Fibros. 2018, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Panchaud, A.; Di Paolo, E.R.; Koutsokera, A.; Winterfeld, U.; Weisskopf, E.; Baud, D.; Sauty, A.; Csajka, C. Safety of Drugs during Pregnancy and Breastfeeding in Cystic Fibrosis Patients. Respiration 2016, 91, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; Nazareth, D. A successful uncomplicated CF pregnancy while remaining on Ivacaftor. J. Cyst. Fibros. 2016, 15, 133–134. [Google Scholar] [CrossRef]
- Jones, G.H.; Walshaw, M.J. Potential impact on fertility of new systemic therapies for cystic fibrosis. Paediatr. Respir. Rev. 2015, 16 (Suppl. 1), 25–27. [Google Scholar] [CrossRef]
- Vekaria, S.; Popowicz, N.; White, S.W.; Mulrennan, S. To be or not to be on CFTR modulators during pregnancy: Risks to be considered. J. Cyst. Fibros. 2020, 19, e7–e8. [Google Scholar] [CrossRef]
- Mainz, J.G.; Michl, R.K.; Beiersdorf, N.; Lorenz, M.; Schneider, U.; Groten, T.; Jaudszus, A. Successful Pregnancy of a Patient with Cystic Fibrosis Genotype F508del/F508del and Progressed Pulmonary Destruction on lumacaftor/ivacaftor. Klinische Pädiatrie 2019, 231, 271–273. [Google Scholar] [CrossRef]
- Nash, E.F.; Middleton, P.G.; Taylor-Cousar, J.L. Outcomes of pregnancy in women with cystic fibrosis (CF) taking CFTR modulators–an international survey. J. Cyst. Fibros. 2020, 19, 521–526. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services. Office on Women’s Health: Pregnancy Loss; U.S. Department of Health & Human Services: Washington, DC, USA, 2019. Available online: https://www.womenshealth.gov/pregnancy/youre-pregnant-now-what/pregnancy-loss (accessed on 19 April 2021).
- Trimble, A.T.; Donaldson, S.H. Ivacaftor withdrawal syndrome in cystic fibrosis patients with the G551D mutation. J. Cyst. Fibros. 2018, 17, e13–e16. [Google Scholar] [CrossRef]
- Carpino, E.A.; Fowler, R.E.; Uluer, A.Z.; Sawicki, G.S. Acute Clinical Outcomes Following Participation in Short-Term CFTR Modulator Trials in Adults with Cystic Fibrosis: A Retrospective Chart Review. Pediatr. Pulmonol. 2018, 53, 260–261. [Google Scholar]
- Godfrey, E.M.; Mody, S.; Schwartz, M.R.; Heltshe, S.L.; Taylor-Cousar, J.L.; Jain, R.; Sufian, S.; Josephy, T.; Aitken, M.L. Contraceptive use among women with cystic fibrosis: A pilot study linking reproductive health questions to the Cystic Fibrosis Foundation National Patient Registry. Contraception 2020, 101, 420–426. [Google Scholar] [CrossRef]
- Sun, X.; Yi, Y.; Yan, Z.; Rosen, B.H.; Liang, B.; Winter, M.C.; Evans, T.I.A.; Rotti, P.G.; Yang, Y.; Gray, J.S.; et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci. Transl. Med. 2019, 11, eaau7531. [Google Scholar] [CrossRef] [PubMed]
- Fortner, C.N.; Seguin, J.M.; Kay, D.M. Normal pancreatic function and false-negative CF newborn screen in a child born to a mother taking CFTR modulator therapy during pregnancy. J. Cyst. Fibros. 2021. [Google Scholar] [CrossRef]
- Middleton, P.G.; Gade, E.J.; Aguilera, C.; MacKillop, L.; Button, B.M.; Coleman, C.; Johnson, B.; Albrechtsen, C.; Edenborough, F.; Rigau, D.; et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur. Respir. J. 2019, 55, 1901208. [Google Scholar] [CrossRef] [PubMed]
- Edenborough, F.; Borgo, G.; Knoop, C.; Lannefors, L.; Mackenzie, W.; Madge, S.; Morton, A.; Oxley, H.; Touw, D.; Benham, M.; et al. Guidelines for the management of pregnancy in women with cystic fibrosis. J. Cyst. Fibros. 2008, 7 (Suppl. 1), S2–S32. [Google Scholar] [CrossRef]
- Cheng, E.Y.; Goss, C.H.; McKone, E.F.; Galic, V.; Debley, C.K.; Tonelli, M.R.; Aitken, M.L. Aggressive prenatal care results in successful fetal outcomes in CF women. J. Cyst. Fibros. 2006, 5, 85–91. [Google Scholar] [CrossRef]
- Litvin, M.; Yoon, J.C. Nutritional excess in cystic fibrosis: The skinny on obesity. J. Cyst. Fibros. 2020, 19, 3–5. [Google Scholar] [CrossRef] [PubMed]
| Impaired Fertility | Genotoxicity | Teratogenicity | Neonatal Cataracts | Presence in Breast Milk | |
|---|---|---|---|---|---|
| Ivacaftor | Yes at toxic human doses | None | At maternally toxic doses: ↓ fetal body weight; no impact on survival or organogenesis | Cataracts observed at all doses administered to juvenile rats | Yes * |
| Lumacaftor | No | None | No | When using combination therapy (i.e., LUM/IVA), see IVA | Yes * |
| Tezacaftor | No | None | At maternally toxic doses: ↓ fetal body weight, early development delay in pinna detachment/eye opening; no impact on survival or organogenesis | When using combination therapy (i.e., TEZ/IVA), see IVA | Yes |
| Elexacaftor | Yes at toxic human doses | None | At maternally toxic doses: ↓ fetal body weight; no impact on survival or organogenesis | When using combination therapy (i.e., ELX/TEZ/IVA), see IVA | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, R.; Taylor-Cousar, J.L. Fertility, Pregnancy and Lactation Considerations for Women with CF in the CFTR Modulator Era. J. Pers. Med. 2021, 11, 418. https://doi.org/10.3390/jpm11050418
Jain R, Taylor-Cousar JL. Fertility, Pregnancy and Lactation Considerations for Women with CF in the CFTR Modulator Era. Journal of Personalized Medicine. 2021; 11(5):418. https://doi.org/10.3390/jpm11050418
Chicago/Turabian StyleJain, Raksha, and Jennifer L. Taylor-Cousar. 2021. "Fertility, Pregnancy and Lactation Considerations for Women with CF in the CFTR Modulator Era" Journal of Personalized Medicine 11, no. 5: 418. https://doi.org/10.3390/jpm11050418
APA StyleJain, R., & Taylor-Cousar, J. L. (2021). Fertility, Pregnancy and Lactation Considerations for Women with CF in the CFTR Modulator Era. Journal of Personalized Medicine, 11(5), 418. https://doi.org/10.3390/jpm11050418







