Preferences for Updates on General Research Results: A Survey of Participants in Genomic Research from Two Institutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment Criteria and Survey Distribution
2.2. Measures
2.2.1. Social and Demographic Characteristics
2.2.2. Preferences for Research Updates
2.2.3. Opinions about Research Focus and Budgeting
2.3. Analytical Strategy
3. Results
3.1. Social and Demographic Characteristics
3.2. Ranking of Preferences for Research Updates
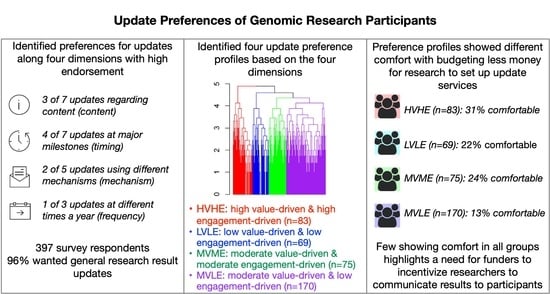
3.3. Cluster Analyses to Identify Preference Profiles
- cluster 1 (n = 75), moderate value-driven and moderate engagement-driven, MVME
- cluster 2 (n = 170), moderate value-driven and low engagement-driven, MVLE
- cluster 3 (n = 69), low value-driven and low engagement-driven, LVLE
- cluster 4 (n = 83), high value-driven and high engagement-driven, HVHE
3.4. Opinions about Research Focus and Budgeting among Preference Profiles
3.5. Characteristics of Preference Profiles
4. Discussion
4.1. Limitations
4.2. Implications for Stakeholders
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnsson, L.; Helgesson, G.; Rafnar, T.; Halldorsdottir, I.; Chia, K.-S.; Eriksson, S.; Hansson, M.G. Hypothetical and factual willingness to participate in biobank research. Eur. J. Hum. Genet. 2010, 18, 1261–1264. [Google Scholar] [CrossRef]
- Boden-Albala, B.; Carman, H.; Southwick, L.; Parikh, N.S.; Roberts, E.; Waddy, S.; Edwards, R. Examining Barriers and Practices to Recruitment and Retention in Stroke Clinical Trials. Stroke 2015, 46, 2232–2237. [Google Scholar] [CrossRef]
- Caenazzo, L.; Tozzo, P. The Future of Biobanking: What Is Next? BioTech 2020, 9, 23. [Google Scholar] [CrossRef]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Sinitsyna, A.; Izotov, A.; Kaysheva, A. Biobanks—A Platform for Scientific and Biomedical Research. Diagnostics 2020, 10, 485. [Google Scholar] [CrossRef]
- Critchley, C.R.; Nicol, D.; Otlowski, M.F.; Stranger, M.J. Predicting intention to biobank: A national survey. Eur. J. Public Health 2010, 22, 139–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mester, J.L.; Mercer, M.; Goldenberg, A.; Moore, R.A.; Eng, C.; Sharp, R.R. Communicating with Biobank Participants: Preferences for Receiving and Providing Updates to Researchers. Cancer Epidemiol. Biomark. Prev. 2015, 24, 708–712. [Google Scholar] [CrossRef]
- O’Daniel, J.; Haga, S. Public Perspectives on Returning Genetics and Genomics Research Results. Public Health Genom. 2011, 14, 346–355. [Google Scholar] [CrossRef]
- Mathews, D.J.H.; Jamal, L. Revisiting Respect for Persons in Genomic Research. Genes 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Overby, C.L.; Maloney, K.A.; Alestock, T.D.; Chavez, J.; Berman, D.M.; Sharaf, R.M.; Fitzgerald, T.; Kim, E.-Y.; Palmer, K.; Shuldiner, A.R.; et al. Prioritizing Approaches to Engage Community Members and Build Trust in Biobanks: A Survey of Attitudes and Opinions of Adults within Outpatient Practices at the University of Maryland. J. Pers. Med. 2015, 5, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Long, C.R.; Stewart, M.K.; McElfish, P.A. Health research participants are not receiving research results: A collaborative solution is needed. Trials 2017, 18, 449. [Google Scholar] [CrossRef]
- Knoppers, B.M.; Deschênes, M.; Zawati, M.H.; Tassé, A.M. Population studies: Return of research results and incidental findings Policy Statement. Eur. J. Hum. Genet. 2012, 21, 245–247. [Google Scholar] [CrossRef]
- Shalowitz, D.I.; Miller, F.G. Disclosing Individual Results of Clinical Research: Implications of respect for participants. JAMA 2005, 294, 737–740. [Google Scholar] [CrossRef]
- Jarvik, G.P.; Amendola, L.M.; Berg, J.S.; Brothers, K.; Clayton, E.W.; Chung, W.; Evans, B.J.; Evans, J.P.; Fullerton, S.M.; Gallego, C.J.; et al. Return of Genomic Results to Research Participants: The Floor, the Ceiling, and the Choices in between. Am. J. Hum. Genet. 2014, 94, 818–826. [Google Scholar] [CrossRef]
- McEwen, J.E.; Boyer, J.T.; Sun, K.Y. Evolving approaches to the ethical management of genomic data. Trends Genet. 2013, 29, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Beskow, L.M.; Burke, W. Offering Individual Genetic Research Results: Context Matters. Sci. Transl. Med. 2010, 2, 38cm20. [Google Scholar] [CrossRef]
- Khodyakov, D.; Mendoza-Graf, A.; Berry, S.; Nebeker, C.; Bromley, E. Return of Value in the New Era of Biomedical Research—One Size Will Not Fit All. AJOB Empir. Bioeth. 2019, 10, 265–275. [Google Scholar] [CrossRef]
- Downey, A.S.; Busta, E.R.; Mancher, M.; Botkin, J.R. (Eds.) Returning Individual Research Results to Participants: Guidance for a New Research Paradigm; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Beskow, L.M.; Burke, W.; Fullerton, S.M.; Sharp, R.R. Offering aggregate results to participants in genomic research: Opportunities and challenges. Genet. Med. 2012, 14, 490–496. [Google Scholar] [CrossRef]
- Cook, S.; Mayers, S.; Goggins, K.; Schlundt, D.; Bonnet, K.; Williams, N.; Alcendor, D.; Barkin, S. Assessing research participant preferences for receiving study results. J. Clin. Transl. Sci. 2019, 4, 243–249. [Google Scholar] [CrossRef]
- Wong, C.A.; Hernandez, A.F.; Califf, R.M. Return of Research Results to Study Participants. JAMA 2018, 320, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Shalowitz, D.I.; Miller, F.G. Communicating the Results of Clinical Research to Participants: Attitudes, Practices, and Future Directions. PLoS Med. 2008, 5, e91. [Google Scholar] [CrossRef] [PubMed]
- Augustine, E.F.; Dorsey, E.R.; Hauser, R.A.; Elm, J.J.; Tilley, B.C.; Kieburtz, K.K. Communicating with participants during the conduct of multi-center clinical trials. Clin. Trials 2016, 13, 592–596. [Google Scholar] [CrossRef]
- Elzinga, K.E.; Khan, O.F.; Tang, A.R.; Fernandez, C.V.; Elzinga, C.L.; Heng, D.Y.; Vickers, M.M.; Truong, T.H.; Tang, P.A. Adult patient perspectives on clinical trial result reporting: A survey of cancer patients. Clin. Trials 2016, 13, 574–581. [Google Scholar] [CrossRef]
- Long, C.R.; Stewart, M.K.; Cunningham, T.V.; Warmack, T.S.; McElfish, P.A. Health research participants’ preferences for receiving research results. Clin. Trials 2016, 13, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Purvis, R.S.; Abraham, T.H.; Long, C.R.; Stewart, M.K.; Warmack, T.S.; McElfish, P.A. Qualitative study of participants’ perceptions and preferences regarding research dissemination. AJOB Empir. Bioeth. 2017, 8, 69–74. [Google Scholar] [CrossRef]
- Partridge, A.H.; Winer, E.P. Informing clinical trial participants about study results. JAMA 2002, 288, 363–365. [Google Scholar] [CrossRef]
- Scherr, C.L.; Aufox, S.; Ross, A.A.; Ramesh, S.; Wicklund, C.A.; Smith, M. What People Want to Know About Their Genes: A Critical Review of the Literature on Large-Scale Genome Sequencing Studies. Healthcare 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Webb, F.J.; Khubchandani, J.; Striley, C.W.; Cottler, L.B. Black–White Differences in Willingness to Participate and Perceptions About Health Research: Results from the Population-Based HealthStreet Study. J. Immigr. Minor. Health 2019, 21, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]
- Wendler, D.; Kington, R.; Madans, J.; Van Wye, G.; Christ-Schmidt, H.; Pratt, L.A.; Brawley, O.W.; Gross, C.P.; Emanuel, E. Are Racial and Ethnic Minorities Less Willing to Participate in Health Research? PLoS Med. 2005, 3, e19. [Google Scholar] [CrossRef] [PubMed]
- Mullarkey, M.C.; Dobias, M.; Maron, A.; Bearman, S.K. A systematic review of randomized trials for engaging socially disadvantaged groups in health research: A distillation approach. PsyArXiv 2019. [Google Scholar] [CrossRef]
- All of Us Research Program. All of Us Research Program Protocol; National Institute of Health: Bethesda, MD, USA, 2020. Available online: https://allofus.nih.gov/about/all-us-research-program-protocol (accessed on 18 July 2020).
- Kohane, I.S.; Taylor, P.L. Multidimensional Results Reporting to Participants in Genomic Studies: Getting It Right. Sci. Transl. Med. 2010, 2, 37cm19. [Google Scholar] [CrossRef]
- Hoell, C.; Wynn, J.; Rasmussen, L.V.; Marsolo, K.; Aufox, S.A.; Chung, W.K.; Connolly, J.J.; Freimuth, R.R.; Kochan, D.; Hakonarson, H.; et al. Participant choices for return of genomic results in the eMERGE Network. Genet. Med. 2020, 22, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.L.; Strong, K.A.; Dimmock, D.P. Choices of incidental findings of individuals undergoing genome wide sequencing, a single center’s experience. Clin. Genet. 2017, 91, 137–140. [Google Scholar] [CrossRef]
- Teare, H.J.; Morrison, M.; Whitley, E.A.; Kaye, J. Towards ‘Engagement 2.0′: Insights from a study of dynamic consent with biobank participants. Digit. Health 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Thiel, D.B.; Platt, J.; Platt, T.; King, S.B.; Fisher, N.; Shelton, R.; Kardia, S.L.R. Testing an Online, Dynamic Consent Portal for Large Population Biobank Research. Public Health Genom. 2015, 18, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Teare, H.J.A.; Hogg, J.; Kaye, J.; Luqmani, R.; Rush, E.; Turner, A.; Watts, L.; Williams, M.; Javaid, M.K. The RUDY study: Using digital technologies to enable a research partnership. Eur. J. Hum. Genet. 2017, 25, 816–822. [Google Scholar] [CrossRef]
- Aguilar-Quesada, R.; Aroca-Siendones, I.; de la Torre, L.; Panadero-Fajardo, S.; Rejón, J.; Sánchez-López, A.; Miranda, B. The Andalusian Registry of Donors for Biomedical Research: Five Years of History. BioTech 2021, 10, 6. [Google Scholar] [CrossRef]

| Variables | Categories | N (%, N = 397) |
|---|---|---|
| Gender | Male | 120 (30.2%) |
| Female | 268 (67.5%) | |
| Prefer not to say/missing | 9 (2.3%) | |
| Age | 18–29 years old | 9 (2.3%) |
| 30–44 years old | 90 (22.7%) | |
| 45–59 years old | 136 (34.3%) | |
| 60 years old or more | 154 (38.8%) | |
| Prefer not to say/missing | 8 (2.0%) | |
| Education (highest level) | Less than high school | 1 (0.3%) |
| High school graduate or GED | 26 (6.5%) | |
| Some college | 67 (16.9%) | |
| Bachelor’s degree | 121 (30.5%) | |
| Graduate or professional degree | 174 (43.8%) | |
| Prefer not to say/missing | 8 (2.0%) | |
| Ethnicity | Hispanic or Latino | 25 (6.3%) |
| Non-Hispanic | 362 (91.2%) | |
| Prefer not to say/missing | 10 (2.5%) | |
| English (first language) | Yes | 368 (92.7%) |
| No | 21 (5.3%) | |
| Prefer not to say/missing | 8 (2.0%) | |
| Race | ||
| White Caucasian | 336 (84.6%) | |
| Black African American | 27 (6.8%) | |
| Asian or Asian American | 5 (1.3%) | |
| Multiracial | 19(4.8%) | |
| Other/missing | 10 (2.5%) | |
| Primary healthcare institution | ||
| Johns Hopkins University Columbia University | 313(78.8%) 84(21.2%) | |
| Remember donating sample | ||
| Yes | 327(82.4%) | |
| No | 38(9.6%) | |
| Unsure | 32(8.1%) | |
| Want to be updated about research | Yes | 382(96.2%) |
| No/Unsure | 15(3.8%) |
| Research Updates Preferences | Clusters | Rank of Statements | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | p | 1 | 2 | 3 | 4 | |
| N = 75 | N = 170 | N = 69 | N = 83 | ||||||
| Update Content | |||||||||
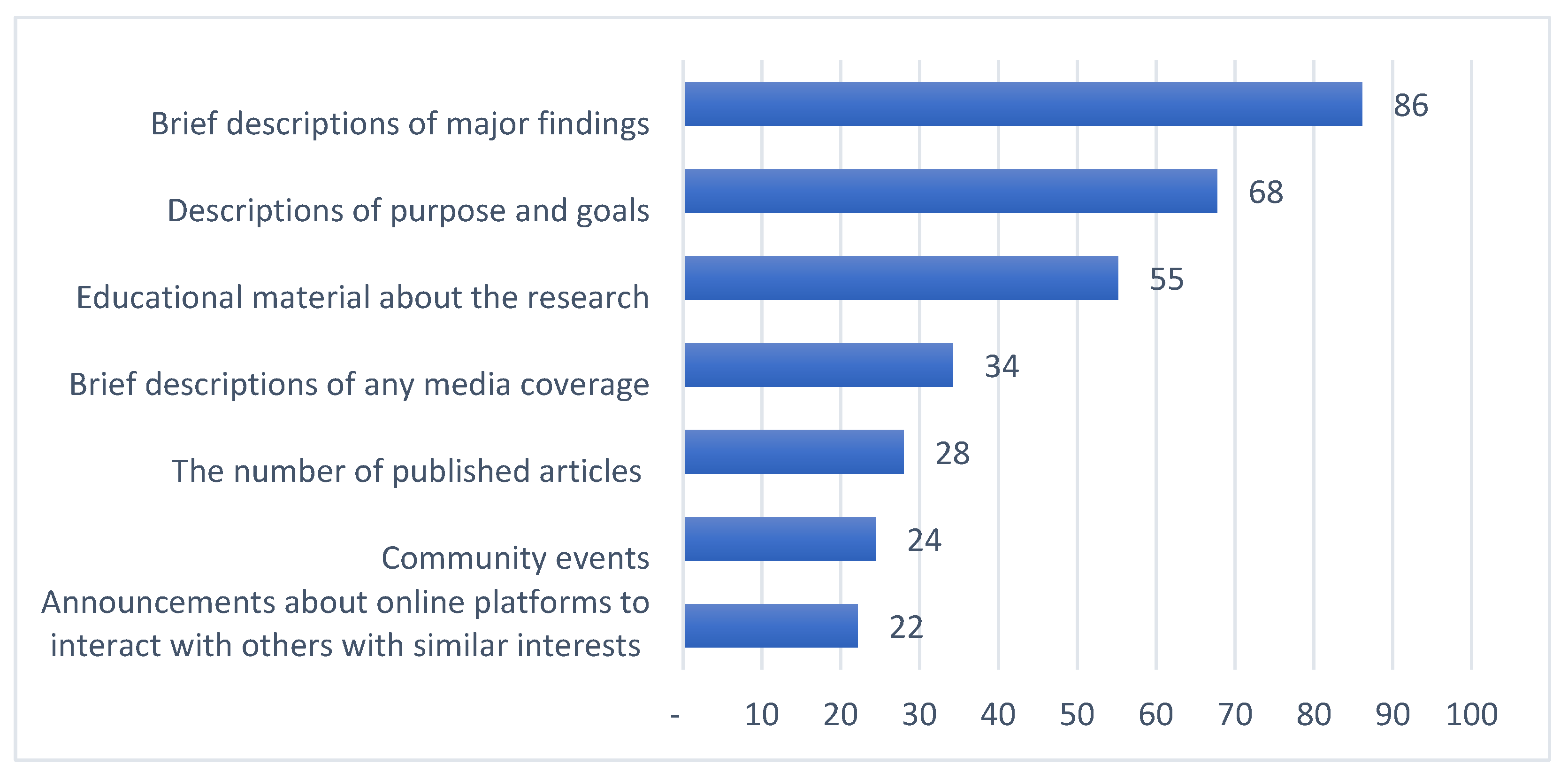
| Brief descriptions of major findings | 74 (98.7%) | 163 (95.9%) | 28 (40.6%) | 77 (92.8%) | <0.001 | 1 | 1 | 1 | 1 |
| Descriptions of purpose and goals | 66 (88.0%) | 132 (77.6%) | 4 (5.8%) | 67 (80.7%) | <0.001 | 2 | 2 | 3 | 3 |
| Educational material about the research | 54 (72.0%) | 84 (49.4%) | 14 (20.3%) | 67 (80.7%) | <0.001 | 3 | 3 | 2 | 3 |
| The number of published articles | 31 (41.3%) | 38 (22.4%) | 1 (1.4%) | 41 (49.4%) | <0.001 | 4 | 4 | 7 | 7 |
| Brief descriptions of any media coverage | 31 (41.3%) | 32 (18.8%) | 2 (2.9%) | 71 (85.5%) | <0.001 | 4 | 5 | 4 | 2 |
| Community events | 13 (17.3%) | 17 (10.0%) | 2 (2.9%) | 65 (78.3%) | <0.001 | 7 | 6 | 4 | 5 |
| Announcements about online platforms to interact with others with similar interests | 18 (24.0%) | 15 (8.8%) | 2 (2.9%) | 53 (63.9%) | <0.001 | 6 | 7 | 4 | 6 |
| Update Timing | |||||||||
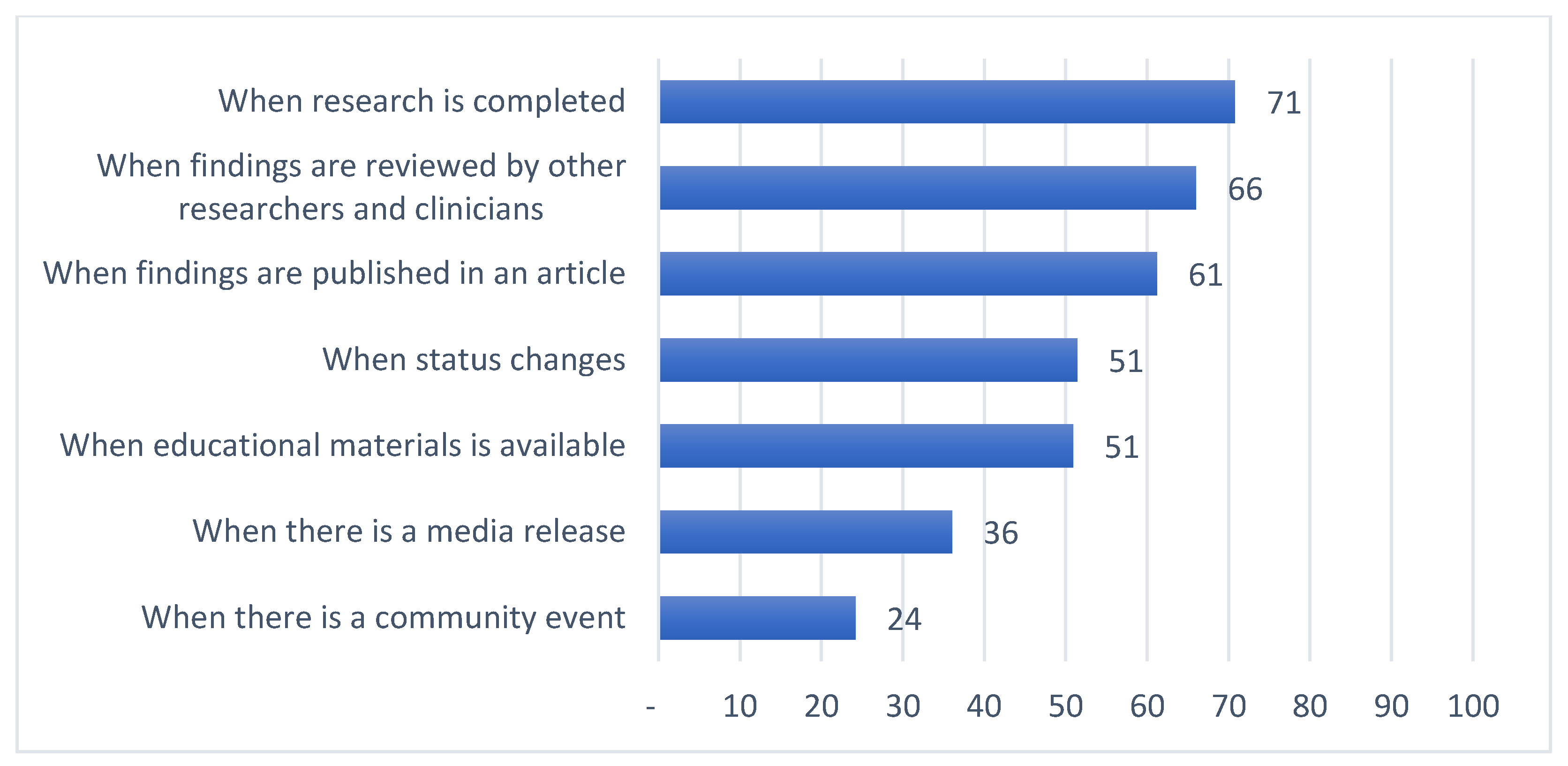
| When findings are reviewed by other researchers and clinicians | 67 (89.3%) | 119 (70.0%) | 12 (17.4%) | 64 (77.1%) | <0.001 | 1 | 1 | 3 | 7 |
| When research is completed | 67 (89.3%) | 115 (67.6%) | 27 (39.1%) | 72 (86.7%) | <0.001 | 1 | 2 | 1 | 4 |
| When findings are published in an article | 61 (81.3%) | 98 (57.6%) | 11 (15.9%) | 73 (88.0%) | <0.001 | 2 | 3 | 4 | 2 |
| When educational materials are available | 50 (66.7%) | 74 (43.5%) | 7 (10.1%) | 71 (85.5%) | <0.001 | 4 | 4 | 5 | 6 |
| When status changes | 53 (70.7%) | 65 (38.2%) | 14 (20.3%) | 72 (86.7%) | <0.001 | 3 | 5 | 2 | 4 |
| When there is a media release | 25 (33.3%) | 37 (21.8%) | 5 (7.2%) | 76 (91.6%) | <0.001 | 5 | 6 | 6 | 1 |
| When there is a community event | 10 (13.3%) | 12 (7.1%) | 1 (1.4%) | 73 (88.0%) | <0.001 | 6 | 7 | 7 | 2 |
| Update Mechanism | |||||||||
| An email | 61 (81.3%) | 131 (77.1%) | 27 (39.1%) | 71 (85.5%) | <0.001 | 1 | 1 | 1 | 1 |
| An electronic newsletter by email | 48 (64.0%) | 115 (67.6%) | 20 (29.0%) | 64 (77.1%) | <0.001 | 2 | 2 | 2 | 2 |
| A newsletter by mail | 28 (37.3%) | 37 (21.8%) | 11 (15.9%) | 35 (42.2%) | <0.001 | 3 | 3 | 4 | 3 |
| A text message | 20 (26.7%) | 22 (12.9%) | 12 (17.4%) | 28 (33.7%) | 0.001 | 4 | 4 | 3 | 4 |
| A call prerecorded message | 5 (6.7%) | 3 (1.8%) | 1 (1.4%) | 10 (12.0%) | 0.002 | 5 | 5 | 5 | 5 |
| Update Frequency | |||||||||
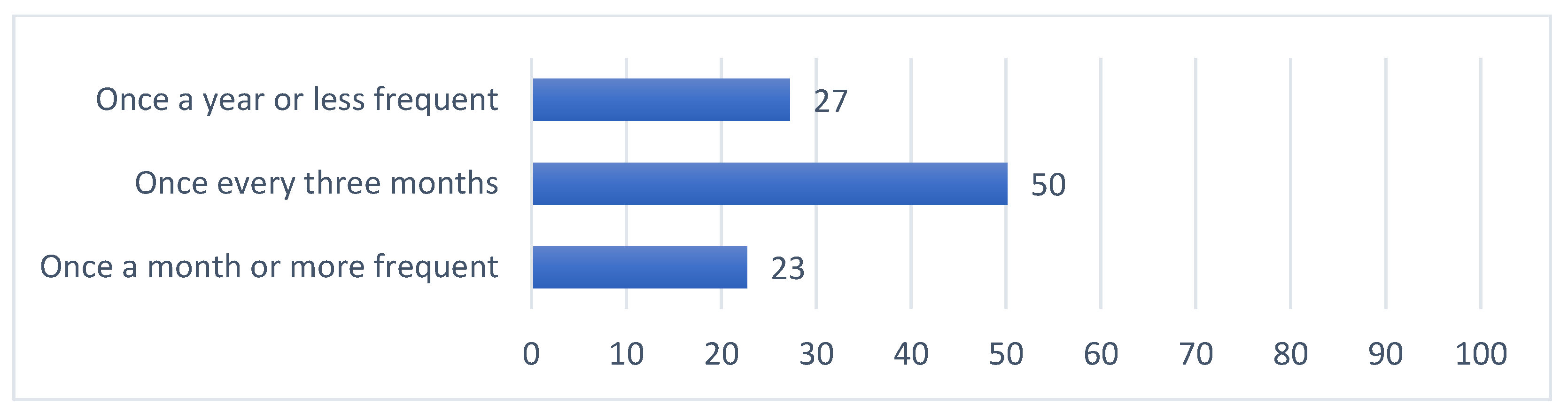
| Once every three months | 19 (25.3%) | 97 (57.1%) | 30 (43.5%) | 53 (63.9%) | 2 | 1 | 1 | 1 | |
| Once a year or less frequent | 0 (0%) | 72 (42.4%) | 27 (39.1%) | 9 (10.8%) | <0.001 | 3 | 2 | 2 | 3 |
| Once a month or more frequent | 56 (74.7%) | 1 (0.6%) | 12 (17.4%) | 21 (25.3%) | 1 | 3 | 3 | 2 | |
| Clusters | |||||
|---|---|---|---|---|---|
| Opinions | 1 | 2 | 3 | 4 | p Value |
| N = 75 | N = 170 | N = 69 | N = 83 | ||
| Comfortable with less money for research | 18 (24.0%) | 23 (13.5%) | 15 (21.7%) | 26 (31.3%) | 0.01 |
| Interest in research focus | 63 (84.0%) | 142 (83.5%) | 52 (75.4%) | 76 (91.6%) | 0.06 |
| Characteristics | Clusters | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | p Value | ||
| 75 | 170 | 69 | 83 | |||
| Gender | Male | 16 (21.3%) | 55 (32.4%) | 23 (33.3%) | 26 (31.3%) | |
| Female | 57 (76.0%) | 111 (65.3%) | 43 (62.3%) | 57 (68.7%) | 0.27 | |
| Age | 18–59 | 42 (56.0%) | 107 (62.9%) | 38 (55.1%) | 48 (57.8%) | |
| 60+ | 31 (41.3%) | 59 (34.7%) | 29 (42.0%) | 35 (42.2%) | 0.59 | |
| Education | Bachelor’s degree or less | 42 (56.0%) | 82 (48.2%) | 42 (60.9%) | 49 (59.0%) | |
| Graduate or professional degree | 31 (41.3%) | 84 (49.4%) | 25 (36.2%) | 34 (41.0%) | 0.29 | |
| Health care institute | Columbia University | 20 (26.7%) | 27 (15.9%) | 21 (30.4%) | 16 (19.3%) | |
| Johns Hopkins University | 55 (73.3%) | 143 (84.1%) | 48 (69.6%) | 67 (80.7%) | 0.047 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, C.O.; Manov, N.F.; Crew, K.D.; Weng, C.; Connolly, J.J.; Chute, C.G.; Ford, D.E.; Lehmann, H.; Rahm, A.K.; Kullo, I.J.; et al. Preferences for Updates on General Research Results: A Survey of Participants in Genomic Research from Two Institutions. J. Pers. Med. 2021, 11, 399. https://doi.org/10.3390/jpm11050399
Taylor CO, Manov NF, Crew KD, Weng C, Connolly JJ, Chute CG, Ford DE, Lehmann H, Rahm AK, Kullo IJ, et al. Preferences for Updates on General Research Results: A Survey of Participants in Genomic Research from Two Institutions. Journal of Personalized Medicine. 2021; 11(5):399. https://doi.org/10.3390/jpm11050399
Chicago/Turabian StyleTaylor, Casey Overby, Natalie Flaks Manov, Katherine D. Crew, Chunhua Weng, John J. Connolly, Christopher G. Chute, Daniel E. Ford, Harold Lehmann, Alanna Kulchak Rahm, Iftikhar J. Kullo, and et al. 2021. "Preferences for Updates on General Research Results: A Survey of Participants in Genomic Research from Two Institutions" Journal of Personalized Medicine 11, no. 5: 399. https://doi.org/10.3390/jpm11050399
APA StyleTaylor, C. O., Manov, N. F., Crew, K. D., Weng, C., Connolly, J. J., Chute, C. G., Ford, D. E., Lehmann, H., Rahm, A. K., Kullo, I. J., Caraballo, P. J., Holm, I. A., & Mathews, D. (2021). Preferences for Updates on General Research Results: A Survey of Participants in Genomic Research from Two Institutions. Journal of Personalized Medicine, 11(5), 399. https://doi.org/10.3390/jpm11050399