Comparison of Shoulder Ultrasonographic Assessments between Polymyalgia Rheumatica and Frozen Shoulder in Patients with Bilateral Shoulder Pain: A Comparative Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. US Protocol
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
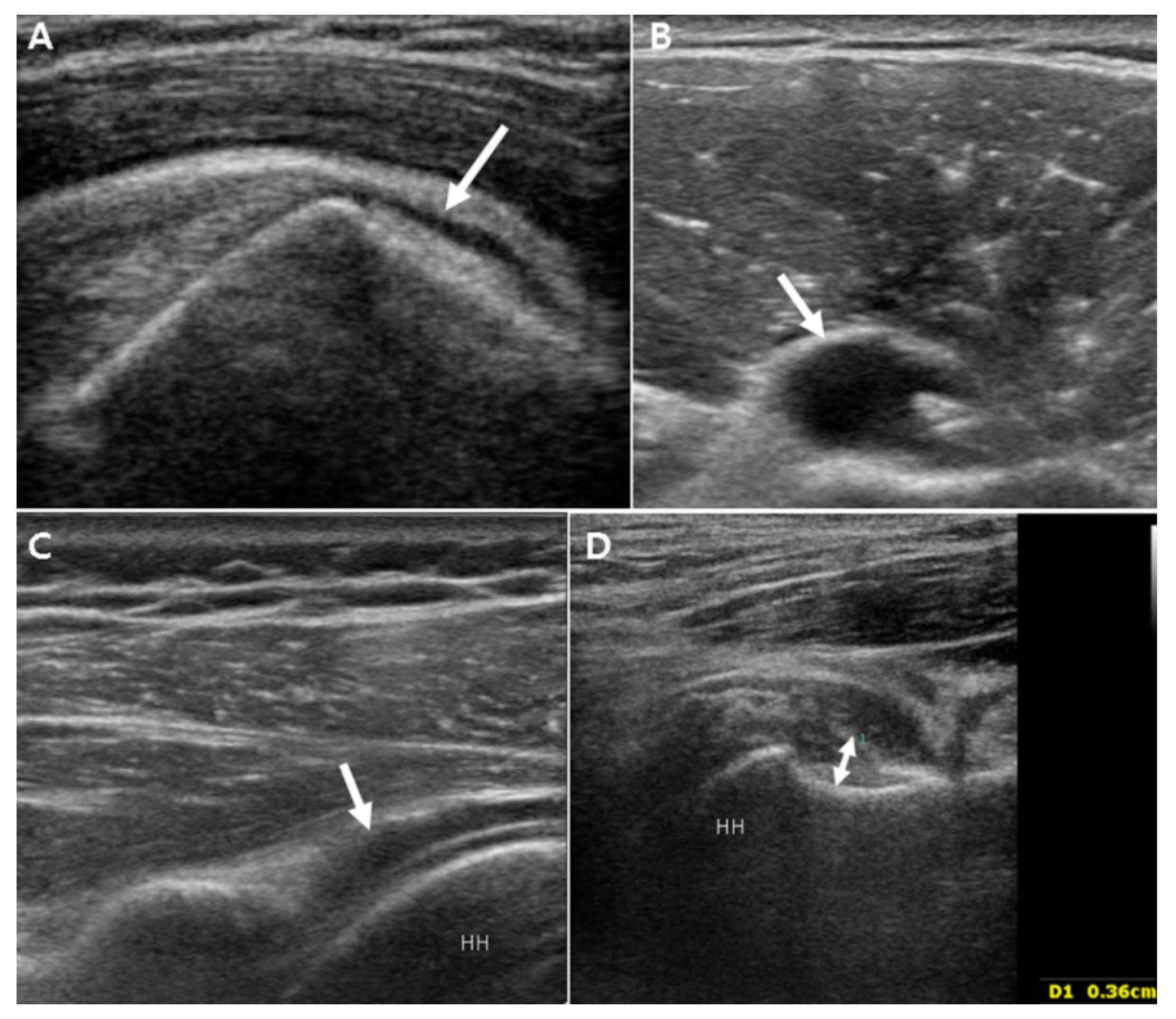
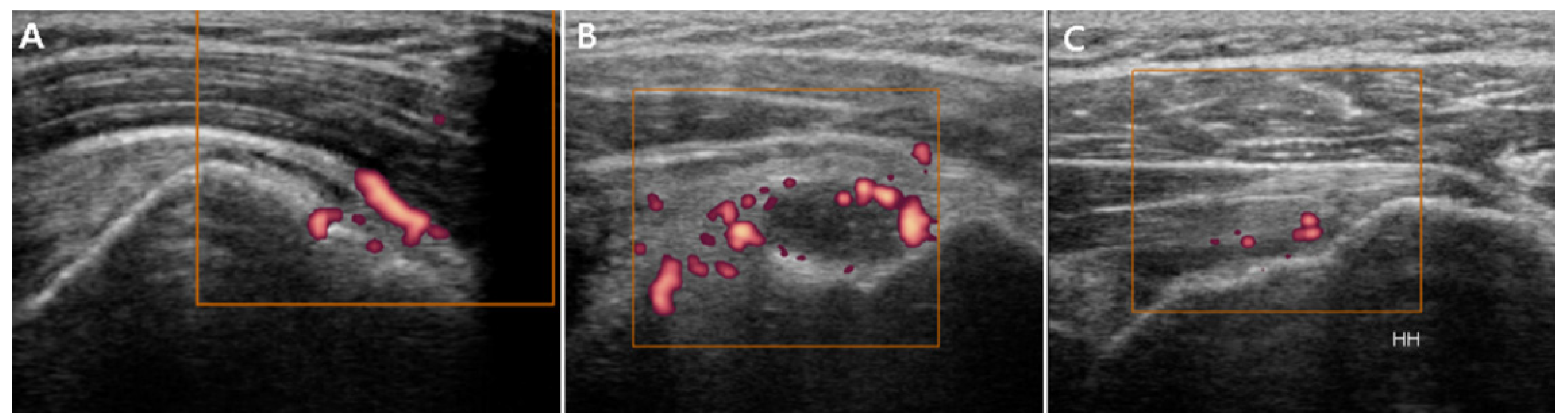
3.2. Ultrasound Findings
3.2.1. Unilateral Shoulder US Findings in PMR Patients and Bilateral FS Patients
3.2.2. Comparison of Bilaterality of Abnormal Shoulder US Findings in PMR Patients and Bilateral FS Patients
3.2.3. Power Doppler Findings
3.2.4. Utility of US Evidence of Bilateral Inferior GH Synovitis without Bilateral SASD Bursitis for the Differential Diagnosis between PMR and Bilateral FS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neviaser, A.S.; Hannafin, J.A. Adhesive capsulitis: A review of current treatment. Am. J. Sports Med. 2010, 38, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Neviaser, A.S.; Neviaser, R.J. Adhesive capsulitis of the shoulder. J. Am. Acad. Orthop. Surg. 2011, 19, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Brue, S.; Valentin, A.; Forssblad, M.; Werner, S.; Mikkelsen, C.; Cerulli, G. Idiopathic adhesive capsulitis of the shoulder: A review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2007, 15, 1048–1054. [Google Scholar] [CrossRef]
- Itoi, E.; Arce, G.; Bain, G.I.; Diercks, R.L.; Guttmann, D.; Imhoff, A.B.; Mazzocca, A.D.; Sugaya, H.; Yoo, Y.S. Shoulder Stiffness: Current Concepts and Concerns. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2016, 32, 1402–1414. [Google Scholar] [CrossRef]
- Ando, A.; Sugaya, H.; Hagiwara, Y.; Takahashi, N.; Watanabe, T.; Kanazawa, K.; Itoi, E. Identification of prognostic factors for the nonoperative treatment of stiff shoulder. Int. Orthop. 2013, 37, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Dejaco, C.; Matteson, E.L.; Dasgupta, B. Polymyalgia Rheumatica and Giant Cell Arteritis: A Systematic Review. JAMA 2016, 315, 2442–2458. [Google Scholar] [CrossRef]
- Maestri Brittain, J.; Gormsen, L.C.; von Benzon, E.; Andersen, K.F. Concomitant Polymyalgia Rheumatica and Large-Vessel Vasculitis Visualized on (18)F-FDG PET/CT. Diagnostics 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Matteson, E.L.; Dejaco, C. Polymyalgia Rheumatica. Ann. Intern. Med. 2017, 166, Itc65–Itc80. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Cimmino, M.A.; Kremers, H.M.; Schmidt, W.A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S.; et al. 2012 Provisional classification criteria for polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012, 64, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Do, J.G.; Park, J.; Sung, D.H. Characteristics of Korean Patients with Polymyalgia Rheumatica: A Single Locomotive Pain Clinic Cohort Study. J. Korean Med. Sci. 2018, 33, e241. [Google Scholar] [CrossRef]
- Park, H.B.; Gwark, J.Y.; Jung, J.; Jeong, S.T. Association Between High-Sensitivity C-Reactive Protein and Idiopathic Adhesive Capsulitis. J. Bone Jt. Surg. Am. 2020, 102, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Hirose, Y.; Takeda, E.; Ikusaka, M. Polymyalgia Rheumatica with Normal Inflammatory Markers. Am. J. Med. 2018, 131, e301–e302. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, A.; Fede, C.; De Caro, R.; Stecco, C.; Özçakar, L. Ultrasound imaging of a scar on the knee: Sonopalpation for fascia and subcutaneous tissues. Eur. J. Transl. Myol. 2020, 30, 8909. [Google Scholar] [CrossRef]
- Quin, K.; Madhoun, H.M. Ultrasound as a Biomarker in Rheumatic Diseases. Diagnostics 2020, 10, 933. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Albano, D.; Allen, G.; Bazzocchi, A.; Bignotti, B.; Chianca, V.; Facal de Castro, F.; Drakonaki, E.E.; Gallardo, E.; Gielen, J.; et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur. Radiol. 2018, 28, 5338–5351. [Google Scholar] [CrossRef]
- Vahed, L.K.; Arianpur, A.; Gharedaghi, M.; Rezaei, H. Ultrasound as a diagnostic tool in the investigation of patients with carpal tunnel syndrome. Eur. J. Transl. Myol. 2018, 28, 7380. [Google Scholar] [CrossRef] [PubMed]
- Homsi, C.; Bordalo-Rodrigues, M.; da Silva, J.J.; Stump, X.M. Ultrasound in adhesive capsulitis of the shoulder: Is assessment of the coracohumeral ligament a valuable diagnostic tool? Skelet. Radiol. 2006, 35, 673–678. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, C.H.; Sung, D.H. Ultrasound measurements of axillary recess capsule thickness in unilateral frozen shoulder: Study of correlation with MRI measurements. Skelet. Radiol. 2018, 47, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Sykes, C.; Saifuddin, A.; Connell, D. Adhesive capsulitis: Sonographic changes in the rotator cuff interval with arthroscopic correlation. Skelet. Radiol. 2005, 34, 522–527. [Google Scholar] [CrossRef]
- Michelin, P.; Delarue, Y.; Duparc, F.; Dacher, J.N. Thickening of the inferior glenohumeral capsule: An ultrasound sign for shoulder capsular contracture. Eur. Radiol. 2013, 23, 2802–2806. [Google Scholar] [CrossRef]
- Walmsley, S.; Osmotherly, P.G.; Walker, C.J.; Rivett, D.A. Power Doppler ultrasonography in the early diagnosis of primary/idiopathic adhesive capsulitis: An exploratory study. J. Manip. Physiol. Ther. 2013, 36, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zappia, M.; Di Pietto, F.; Aliprandi, A.; Pozza, S.; De Petro, P.; Muda, A.; Sconfienza, L.M. Multi-modal imaging of adhesive capsulitis of the shoulder. Insights Imaging 2016, 7, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Iagnocco, A.; Finucci, A.; Ceccarelli, F.; Scirocco, C.; Rutigliano, I.M. Musculoskeletal ultrasound in the evaluation of Polymyalgia Rheumatica. Med. Ultrason. 2015, 17, 361–366. [Google Scholar] [CrossRef]
- Ruta, S.; Rosa, J.; Navarta, D.A.; Saucedo, C.; Catoggio, L.J.; Monaco, R.G.; Soriano, E.R. Ultrasound assessment of new onset bilateral painful shoulder in patients with polymyalgia rheumatica and rheumatoid arthritis. Clin. Rheumatol. 2012, 31, 1383–1387. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, R.; Okamoto, A.; Seri, Y. Semiquantitative Evaluation of Extrasynovial Soft Tissue Inflammation in the Shoulders of Patients with Polymyalgia Rheumatica and Elderly-Onset Rheumatoid Arthritis by Power Doppler Ultrasound. Biomed Res. Int. 2017, 2017, 4272560. [Google Scholar] [CrossRef]
- Hannafin, J.A.; Chiaia, T.A. Adhesive capsulitis. A treatment approach. Clin. Orthop. Relat. Res. 2000, 372, 95–109. [Google Scholar] [CrossRef]
- Shah, A.; Amin, M.; Srinivasan, S.; Botchu, R. Improving efficiency and decreasing scanning time of sonographic examination of the shoulder by using a poster illustrating proper shoulder positioning to the patient. J. Clin. Ultrasound. 2015, 43, 417–420. [Google Scholar] [CrossRef]
- Kim, K.T.; Lee, D.G.; Lee, S.; Kim, H. Ultrasonographic Measurement of the Thickness of Axillary Recess Capsule in Healthy Volunteers. Ann. Rehabil. Med. 2016, 40, 502–508. [Google Scholar] [CrossRef]
- Jimenez-Palop, M.; Naredo, E.; Humbrado, L.; Medina, J.; Uson, J.; Francisco, F.; Garcia-Yebenes, M.J.; Garrido, J. Ultrasonographic monitoring of response to therapy in polymyalgia rheumatica. Ann. Rheum. Dis. 2010, 69, 879–882. [Google Scholar] [CrossRef]
- Falsetti, P.; Acciai, C.; Volpe, A.; Lenzi, L. Ultrasonography in early assessment of elderly patients with polymyalgic symptoms: A role in predicting diagnostic outcome? Scand. J. Rheumatol. 2011, 40, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Frediani, B.; Falsetti, P.; Storri, L.; Bisogno, S.; Baldi, F.; Campanella, V.; Acciai, C.; Filippou, G.; Chellini, F.; Cosentino, R.; et al. Evidence for synovitis in active polymyalgia rheumatica: Sonographic study in a large series of patients. J. Rheumatol. 2002, 29, 123–130. [Google Scholar]
- Lange, U.; Piegsa, M.; Teichmann, J.; Neeck, G. Ultrasonography of the glenohumeral joints--a helpful instrument in differentiation in elderly onset rheumatoid arthritis and polymyalgia rheumatica. Rheumatol. Int. 2000, 19, 185–189. [Google Scholar] [CrossRef]
- Macchioni, P.; Catanoso, M.G.; Pipitone, N.; Boiardi, L.; Salvarani, C. Longitudinal examination with shoulder ultrasound of patients with polymyalgia rheumatica. Rheumatology 2009, 48, 1566–1569. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, R.; Hidaka, Y.; Seri, Y. Proliferative Synovitis of the Shoulder Bursae is a Key Feature for Discriminating Elderly Onset Rheumatoid Arthritis Mimicking Polymyalgia Rheumatica From Polymyalgia Rheumatica. Clin. Med. Insights. Arthritis Musculoskelet. Disord. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Salvarani, C.; Olivieri, I.; Niccoli, L.; Padula, A.; Macchioni, L.; Boiardi, L.; Ciancio, G.; Mastrorosato, M.; Rubini, F.; et al. Shoulder ultrasonography in the diagnosis of polymyalgia rheumatica: A case-control study. J. Rheumatol. 2001, 28, 1049–1055. [Google Scholar] [PubMed]
- Bulgen, D.Y.; Binder, A.; Hazleman, B.L.; Park, J.R. Immunological studies in frozen shoulder. J. Rheumatol. 1982, 9, 893–898. [Google Scholar] [CrossRef] [PubMed]
- de Sa, D.; Phillips, M.; Catapano, M.; Simunovic, N.; Belzile, E.L.; Karlsson, J.; Ayeni, O.R. Adhesive capsulitis of the hip: A review addressing diagnosis, treatment and outcomes. J. Hip Preserv. Surg. 2016, 3, 43–55. [Google Scholar] [CrossRef][Green Version]
- Miller, A.R.; Arnot, D.; Wake, M. A healthy patient with bilateral frozen hips preceding bilateral frozen shoulders: A cautionary tale. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Rodriguez-Valverde, V.; Blanco, R.; Fernandez-Sueiro, J.L.; Armona, J.; Figueroa, M.; Martinez-Taboada, V.M. Polymyalgia rheumatica without significantly increased erythrocyte sedimentation rate. A more benign syndrome. Arch. Intern. Med. 1997, 157, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.; Hazleman, B.L.; Parr, G.; Roberts, S. A controlled study of oral prednisolone in frozen shoulder. Br. J. Rheumatol. 1986, 25, 288–292. [Google Scholar] [CrossRef]
- Wakura, D.; Kotani, T.; Takeuchi, T.; Komori, T.; Yoshida, S.; Makino, S.; Hanafusa, T. Differentiation between Polymyalgia Rheumatica (PMR) and Elderly-Onset Rheumatoid Arthritis Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Is Enthesitis a New Pathological Lesion in PMR? PLoS ONE 2016, 11, e0158509. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R. Evaluation of the Canadian Rheumatology Association Choosing Wisely recommendation concerning anti-nuclear antibody (ANA) testing. Clin. Rheumatol. 2015, 34, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Catanoso, M.G.; Macchioni, P.; Boiardi, L.; Pipitone, N.; Salvarani, C. Treatment of refractory polymyalgia rheumatica with etanercept: An open pilot study. Arthritis Rheum. 2007, 57, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
| PMR (n = 19) | Bilateral FS (n = 19) | p | |
|---|---|---|---|
| Age, years | 67.7 ± 11.7 | 59.6 ± 8.6 | 0.025 |
| Sex, male:female | 8:11 | 11:8 | 0.330 |
| Disease duration, months | 4.5 ± 1.4 | 5.4 ± 1.8 | 0.234 |
| Diabetes, n | 2 | 7 | 0.003 |
| Abnormal ESR, n | 18 | 10 | 0.004 |
| Abnormal CRP, n | 19 | 7 | <0.001 |
| ANA positive, n | 7 | 1 | 0.017 |
| RF positive, n | 1 | 0 | 0.311 |
| Anti-CCP antibody, n | 0 | 0 | 1 |
| PMR (n = 38) | Bilateral FS (n = 38) | p | |
|---|---|---|---|
| SASD bursitis, n (%) | 25 (67%) | 11 (29%) | 0.001 |
| LHB tenosynovitis, n (%) | 29 (76%) | 23 (61%) | 0.108 |
| Posterior GH synovitis, n (%) | 12 (32%) | 9 (24%) | 0.304 |
| Inferior GH synovitis, n (%) | 18 (47%) | 34 (89%) | <0.001 |
| Axillary pouch thickness, mm | 3.3 ± 0.7 | 4.2 ± 0.7 | <0.001 |
| PMR (n = 19) | Bilateral FS (n = 19) | p | |
|---|---|---|---|
| SASD bursitis, n (%) | 13 (68%) | 1 (5%) | <0.001 |
| LHB tenosynovitis, n (%) | 14 (74%) | 8 (42%) | 0.049 |
| Posterior GH synovitis, n (%) | 5 (26%) | 3 (16%) | 0.426 |
| Inferior GH synovitis, n (%) | 9 (47%) | 14 (74%) | 0.044 |
| PMR (n = 38) | Bilateral FS (n = 38) | Fisher’s Exact Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | p value |
| LHB | 4 | 14 | 13 | 7 | 12 | 5 | 19 | 2 | 0.007 |
| SASD | 7 | 11 | 18 | 2 | 24 | 8 | 6 | 0 | <0.001 |
| Posterior GH | 22 | 14 | 2 | 0 | 33 | 5 | 0 | 0 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.-W.; Cho, J.-H.; Cho, C.-H.; Sung, D.-H.; Kim, D.-H. Comparison of Shoulder Ultrasonographic Assessments between Polymyalgia Rheumatica and Frozen Shoulder in Patients with Bilateral Shoulder Pain: A Comparative Retrospective Study. J. Pers. Med. 2021, 11, 372. https://doi.org/10.3390/jpm11050372
Park E-W, Cho J-H, Cho C-H, Sung D-H, Kim D-H. Comparison of Shoulder Ultrasonographic Assessments between Polymyalgia Rheumatica and Frozen Shoulder in Patients with Bilateral Shoulder Pain: A Comparative Retrospective Study. Journal of Personalized Medicine. 2021; 11(5):372. https://doi.org/10.3390/jpm11050372
Chicago/Turabian StylePark, Eun-Woo, Jang-Hyuk Cho, Chul-Hyun Cho, Duk-Hyun Sung, and Du-Hwan Kim. 2021. "Comparison of Shoulder Ultrasonographic Assessments between Polymyalgia Rheumatica and Frozen Shoulder in Patients with Bilateral Shoulder Pain: A Comparative Retrospective Study" Journal of Personalized Medicine 11, no. 5: 372. https://doi.org/10.3390/jpm11050372
APA StylePark, E.-W., Cho, J.-H., Cho, C.-H., Sung, D.-H., & Kim, D.-H. (2021). Comparison of Shoulder Ultrasonographic Assessments between Polymyalgia Rheumatica and Frozen Shoulder in Patients with Bilateral Shoulder Pain: A Comparative Retrospective Study. Journal of Personalized Medicine, 11(5), 372. https://doi.org/10.3390/jpm11050372







