Clinical Remission Using Personalized Low-Dose Intravenous Infusions of N-acetylcysteine with Minimal Toxicities for Interstitial Cystitis/Bladder Pain Syndrome
Abstract
1. Introduction
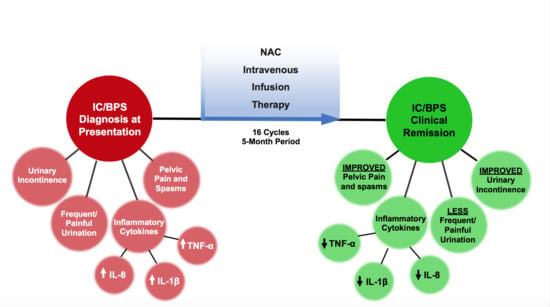
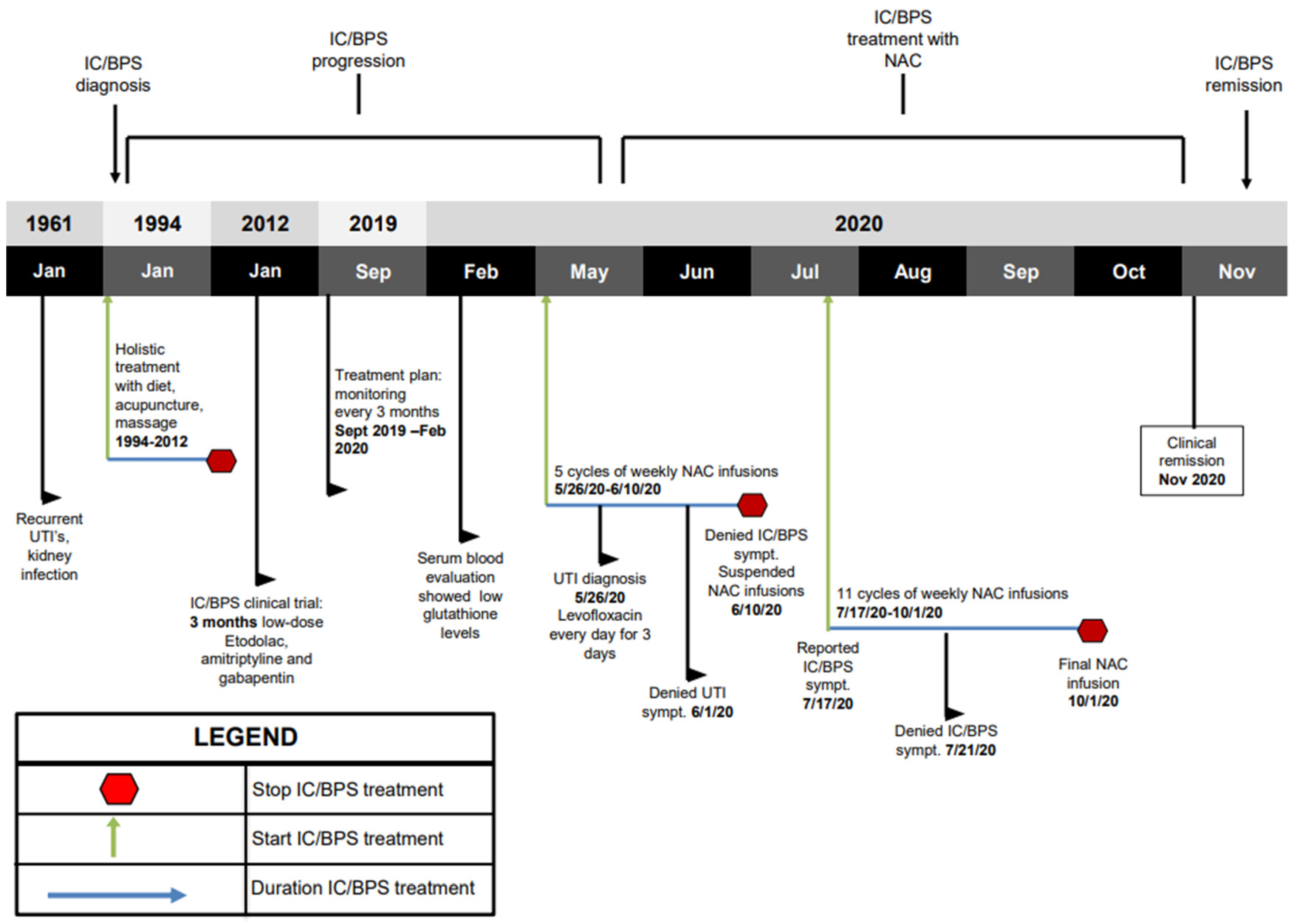
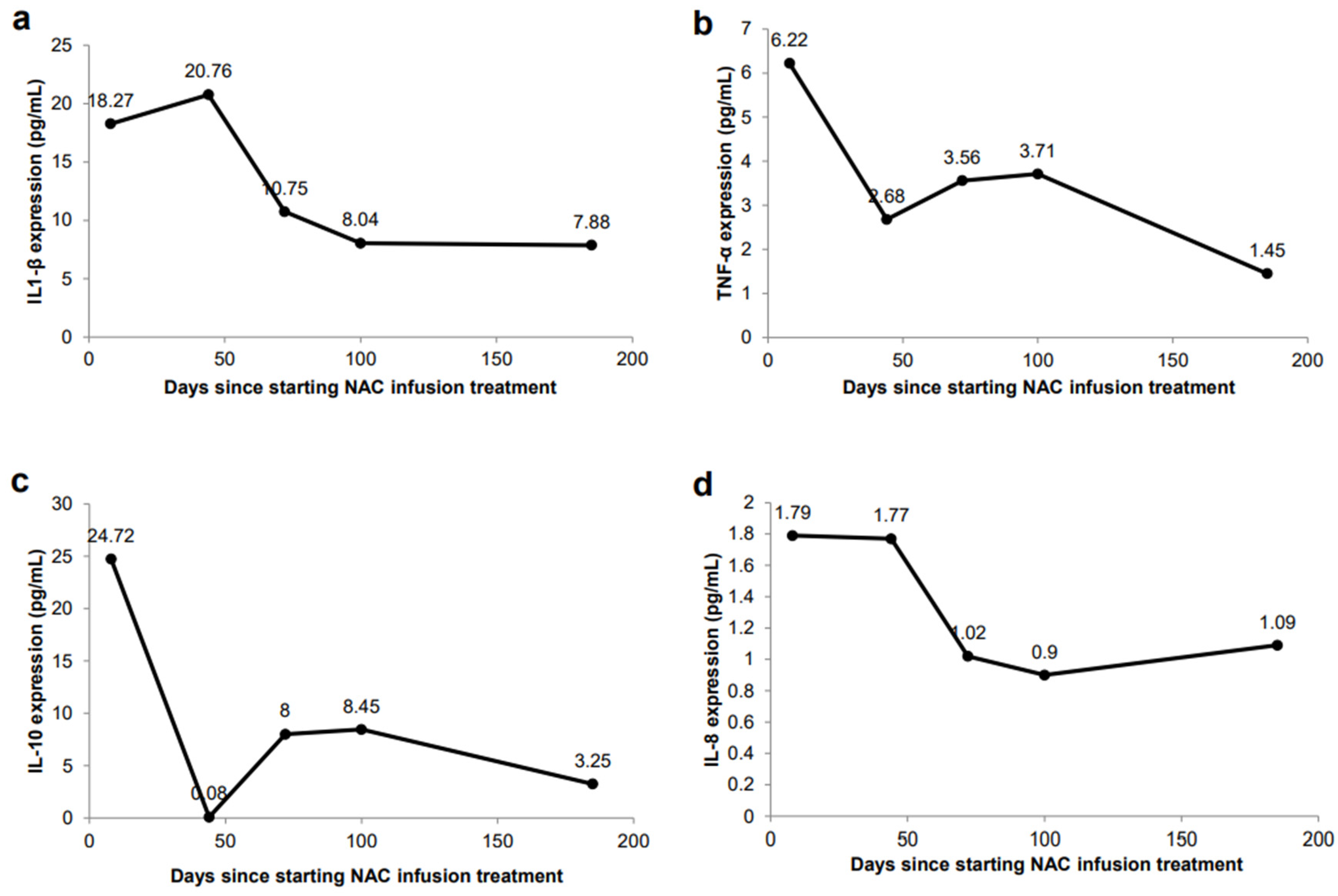
2. Results
Case Report
3. Discussion
4. Conclusions
5. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wesselmann, U. Interstitial cystitis: A chronic visceral pain syndrome. Urology 2001, 57 (Suppl. 1), 32–39. [Google Scholar] [CrossRef]
- Dasgupta, J.; Tincello, D.G. Interstitial cystitis/bladder pain syndrome: An update. Maturitas 2009, 64, 212–217. [Google Scholar] [CrossRef]
- Hanno, P.M.; Burks, D.A.; Clemens, J.Q.; Dmochowski, R.R.; Erickson, D.; Fitzgerald, M.P.; Forrest, J.B.; Gordon, B.; Gray, M.; Mayer, R.D.; et al. Interstitial cystitis guidelines panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J. Urol. 2011, 185, 2162–2170. [Google Scholar] [CrossRef]
- Hanno, P.M.; Erickson, D.; Moldwin, R.; Faraday, M.M.; American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol. 2015, 193, 1545–1553. [Google Scholar] [CrossRef]
- Rovner, E.; Propert, K.J.; Brensinger, C.; Wein, A.J.; Foy, M.; Kirkemo, A.; Landis, J.R.; Kusek, J.W.; Nyberg, L.M. Treatments used in women with interstitial cystitis: The Interstitial Cystitis Data Base (ICDB) study experience. Urology 2000, 56, 940–945. [Google Scholar] [CrossRef]
- Cox, A.; Golda, N.; Nadeau, G.; Curtis Nickel, J.; Carr, L.; Corcos, J.; Teichman, J. CUA guideline: Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Can. Urol. Assoc. J. 2016, 10, E136–E155. [Google Scholar] [CrossRef] [PubMed]
- Hanno, P.M.; Sant, G.R. Clinical highlights of the National Institute of Diabetes and Digestive and Kidney Diseases/Interstitial Cystitis Association scientific conference on interstitial cystitis. Urology 2001, 57 (Suppl. 1), 2–6. [Google Scholar] [CrossRef]
- Whitmore, K.E. Complementary and alternative therapies as treatment approaches for interstitial cystitis. Rev. Urol. 2002, 4 (Suppl. 1), S28–S35. [Google Scholar]
- Greiman, A.; Cox, L. Pharmacotherapy for interstitial cystitis/bladder pain syndrome. Curr. Bladder Dysfunct. Rep. 2019, 14, 365–376. [Google Scholar] [CrossRef]
- Thakur, S.A.; Nyska, A.; White, K.L., Jr.; Smith, M.J.; Auttachoat, W.; Germolec, D.R. Immunomodulatory activity of orphan drug Elmiron® in female B6C3F1/N mice. Food Chem. Toxicol. 2014, 68, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.-A.; Ahn, S.H.; Oh, T.H.; Lee, J.W.; Han, D.Y.; Jeong, H.J. Effect of low-dose triple therapy using gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug for overactive bladder symptoms in patients with bladder pain syndrome. Int. Neurourol. J. 2013, 17, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Han, D.Y.; Jeong, H.J. Bladder pain syndrome treated with triple therapy with gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug. Int. Neurourol. J. 2010, 14, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, D.; Liu, G.; Chen, Y.; Cai, Z. Effect of low-dose amitriptyline treatment for interstitial cystitis/bladder pain syndrome. Clin. Res. Urol. 2018, 1, 1–5. [Google Scholar]
- Quintero, G.C. Review about gabapentin misuse, interactions, contraindications and side effects. J. Exp. Pharmacol. 2017, 9, 13–21. [Google Scholar] [CrossRef]
- Thour, A.; Marwaha, R. Amitriptyline. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kamble, S.V.; Motlekar, S.A.; D’souza, L.L.; Kudrigikar, V.N.; Rao, S.E. Low doses of amitriptyline, pregabalin, and gabapentin are preferred for management of neuropathic pain in India: Is there a need for revisiting dosing recommendations? Korean J. Pain 2017, 30, 183–191. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A review on various uses of N-acetyl cysteine. Cell J. 2017, 19, 11–17. [Google Scholar]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Peng, C.-H.; Liu, H.-T.; Kuo, H.-C. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS ONE 2013, 8, e76779. [Google Scholar] [CrossRef]
- Liu, H.-T.; Kuo, H.-C. Biomarkers for patients with interstitial cystitis/bladder pain syndrome. Urol. Sci. 2015, 26, 225–229. [Google Scholar] [CrossRef]
- Mohamaden, W.I. Alterations of pro-inflammatory cytokines and tissue protein expressions in cats with interstitial cystitis. Pak. Vet. J. 2019, 39, 151–156. [Google Scholar] [CrossRef]
- Carr, L.K.; Corcos, J.; Nickel, J.C.; Teichman, J. Diagnosis of interstitial cystitis June 2007. Can. Urol. Assoc. J. 2009, 3, 81–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kushner, L.; Moldwin, R.M. Efficiency of questionnaires used to screen for interstitial cystitis. J. Urol. 2006, 176, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.L.; Dell, J.; Stanford, E.J.; Bullen, M.; Kahn, B.S.; Waxell, T.; Koziol, J.A. Increased prevalence of interstitial cystitis: Previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology 2002, 60, 573–578. [Google Scholar]
- Ryu, C.-M.; Shin, J.H.; Yu, H.Y.; Ju, H.; Kim, S.; Lim, J.; Heo, J.; Lee, S.; Shin, D.-M.; Choo, M.-S. N-acetylcysteine prevents bladder tissue fibrosis in a lipopolysaccharide-induced cystitis rat model. Sci. Rep. 2019, 9, 8134. [Google Scholar] [CrossRef]
- Shin, J.H.; Ryu, C.-M.; Ju, H.; Yu, H.Y.; Song, S.; Shin, D.-M.; Choo, M.-S. Synergistic effects of N-acetylcysteine and mesenchymal stem cell in a lipopolysaccharide-induced interstitial cystitis rat model. Cells 2019, 9, 86. [Google Scholar] [CrossRef]
- Sprong, R.C.; Winkelhuyzen-Janssen, A.M.; Aarsman, C.J.; van Oirschot, J.F.; van der Bruggen, T.; van Asbeck, B.S. Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1283–1293. [Google Scholar] [CrossRef]
- Szkudlarek, U.; Zdziechowski, A.; Witkowski, K.; Kasielski, M.; Luczyńska, M.; Luczyński, R.; Sarniak, A.; Nowak, D. Effect of inhaled N-acetylcysteine on hydrogen peroxide exhalation in healthy subjects. Pulm. Pharmacol. Ther. 2004, 17, 155–162. [Google Scholar] [CrossRef]
- Sun, Z.; Fu, Q.; Cao, L.; Jin, W.; Cheng, L.; Li, Z. Intravenous N-acetylcysteine for prevention of contrast-induced nephropathy: A meta-analysis of randomized, controlled trials. PLoS ONE 2013, 8, e55124. [Google Scholar]
- Ibrahim, H.; Perl, A.; Smith, D.; Lewis, T.; Kon, Z.; Goldenberg, R.; Yarta, K.; Staniloae, C.; Williams, M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin. Immunol. 2020, 219, 108544. [Google Scholar] [CrossRef]
- Yang, W.; Searl, T.J.; Yaggie, R.; Schaeffer, A.J.; Klumpp, D.J. A MAPP network study: Overexpression of tumor necrosis factor-α in mouse urothelium mimics interstitial cystitis. Am. J. Physiol. Renal Physiol. 2018, 315, F36–F44. [Google Scholar] [CrossRef]
- Mühl, H. Pro-inflammatory signaling by IL-10 and IL-22: Bad habit stirred up by interferons? Front. Immunol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Döcke, W.-D.; Asadullah, K.; Belbe, G.; Ebeling, M.; Höflich, C.; Friedrich, M.; Sterry, W.; Volk, H.-D. Comprehensive biomarker monitoring in cytokine therapy: Heterogeneous, time-dependent, and persisting immune effects of interleukin-10 application in psoriasis. J. Leukoc. Biol. 2009, 85, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.J.; Fekade, D.; Remick, D.G.; Grint, P.; Wherry, J.; Griffin, G.E. Recombinant human interleukin-10 fails to alter proinflammatory cytokine production or physiologic changes Associated with the Jarisch-Herxheimer reaction. J. Infect. Dis. 2000, 181, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Duell, B.L.; Carey, A.J.; Tan, C.K.; Cui, X.; Webb, R.I.; Totsika, M.; Schembri, M.A.; Derrington, P.; Irving-Rodgers, H.; Brooks, A.J.; et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J. Immunol. 2012, 188, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Drage, L.K.L.; Robson, W.; Mowbray, C.; Ali, A.; Perry, J.D.; Walton, K.E.; Harding, C.; Pickard, R.; Hall, J.; Aldridge, P.D. Elevated urine IL-10 concentrations associate with Escherichia coli persistence in older patients susceptible to recurrent urinary tract infections. Immun. Ageing 2019, 16, 16. [Google Scholar] [CrossRef]
- Sairanen, J.; Hotakainen, K.; Tammela, T.L.J.; Stenman, U.-H.; Ruutu, M. Urinary epidermal growth factor and interleukin-6 levels in patients with painful bladder syndrome/interstitial cystitis treated with cyclosporine or pentosan polysulfate sodium. Urology 2008, 71, 630–633. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharaj, D.; Srinivasan, G.; Makepeace, S.; Hickey, C.J.; Gouvea, J. Clinical Remission Using Personalized Low-Dose Intravenous Infusions of N-acetylcysteine with Minimal Toxicities for Interstitial Cystitis/Bladder Pain Syndrome. J. Pers. Med. 2021, 11, 342. https://doi.org/10.3390/jpm11050342
Maharaj D, Srinivasan G, Makepeace S, Hickey CJ, Gouvea J. Clinical Remission Using Personalized Low-Dose Intravenous Infusions of N-acetylcysteine with Minimal Toxicities for Interstitial Cystitis/Bladder Pain Syndrome. Journal of Personalized Medicine. 2021; 11(5):342. https://doi.org/10.3390/jpm11050342
Chicago/Turabian StyleMaharaj, Dipnarine, Gayathri Srinivasan, Sarah Makepeace, Christopher J. Hickey, and Jacqueline Gouvea. 2021. "Clinical Remission Using Personalized Low-Dose Intravenous Infusions of N-acetylcysteine with Minimal Toxicities for Interstitial Cystitis/Bladder Pain Syndrome" Journal of Personalized Medicine 11, no. 5: 342. https://doi.org/10.3390/jpm11050342
APA StyleMaharaj, D., Srinivasan, G., Makepeace, S., Hickey, C. J., & Gouvea, J. (2021). Clinical Remission Using Personalized Low-Dose Intravenous Infusions of N-acetylcysteine with Minimal Toxicities for Interstitial Cystitis/Bladder Pain Syndrome. Journal of Personalized Medicine, 11(5), 342. https://doi.org/10.3390/jpm11050342