Gut-Brain Axis Cross-Talk and Limbic Disorders as Biological Basis of Secondary TMAU
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. DNA Extraction and Sequencing
2.3. Statistical Analysis
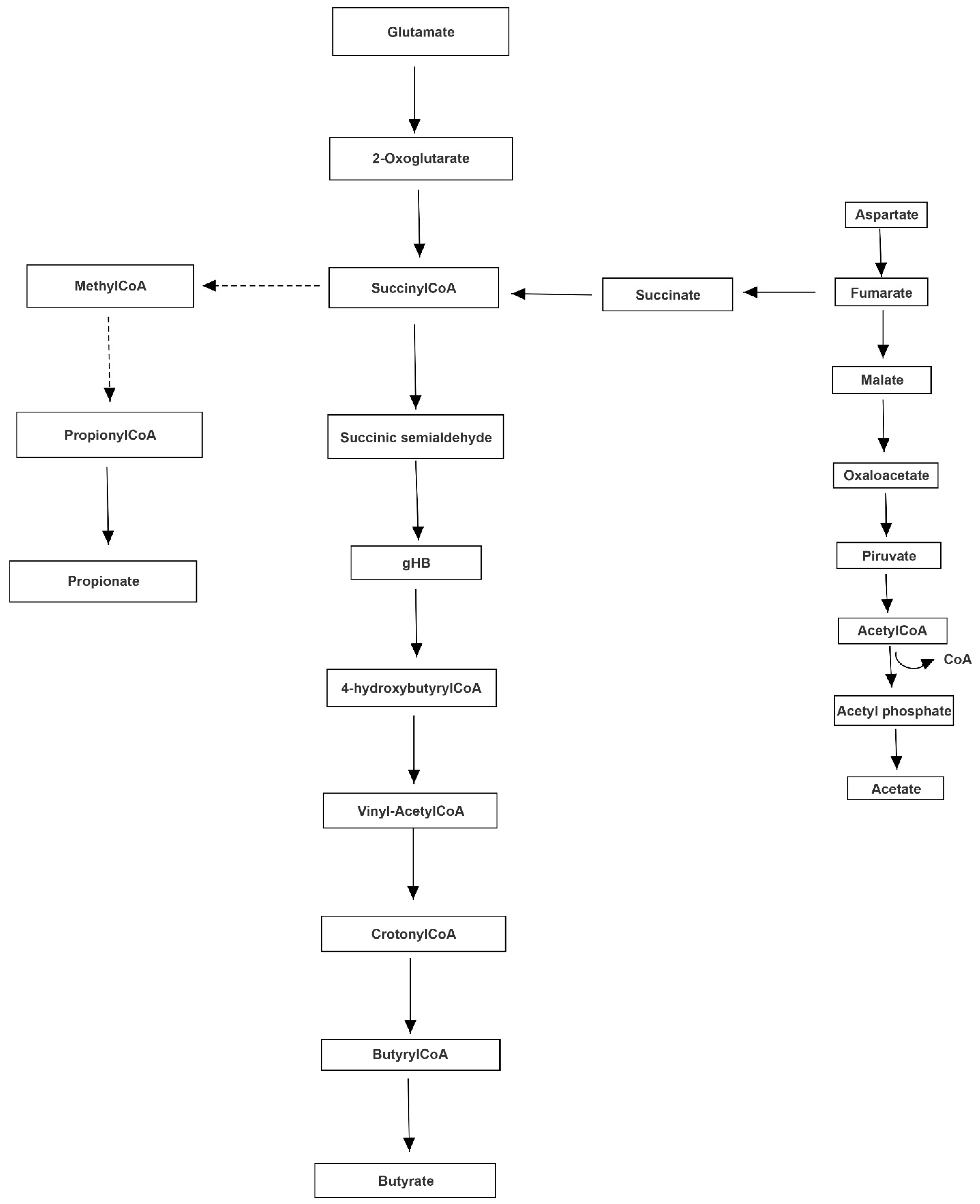
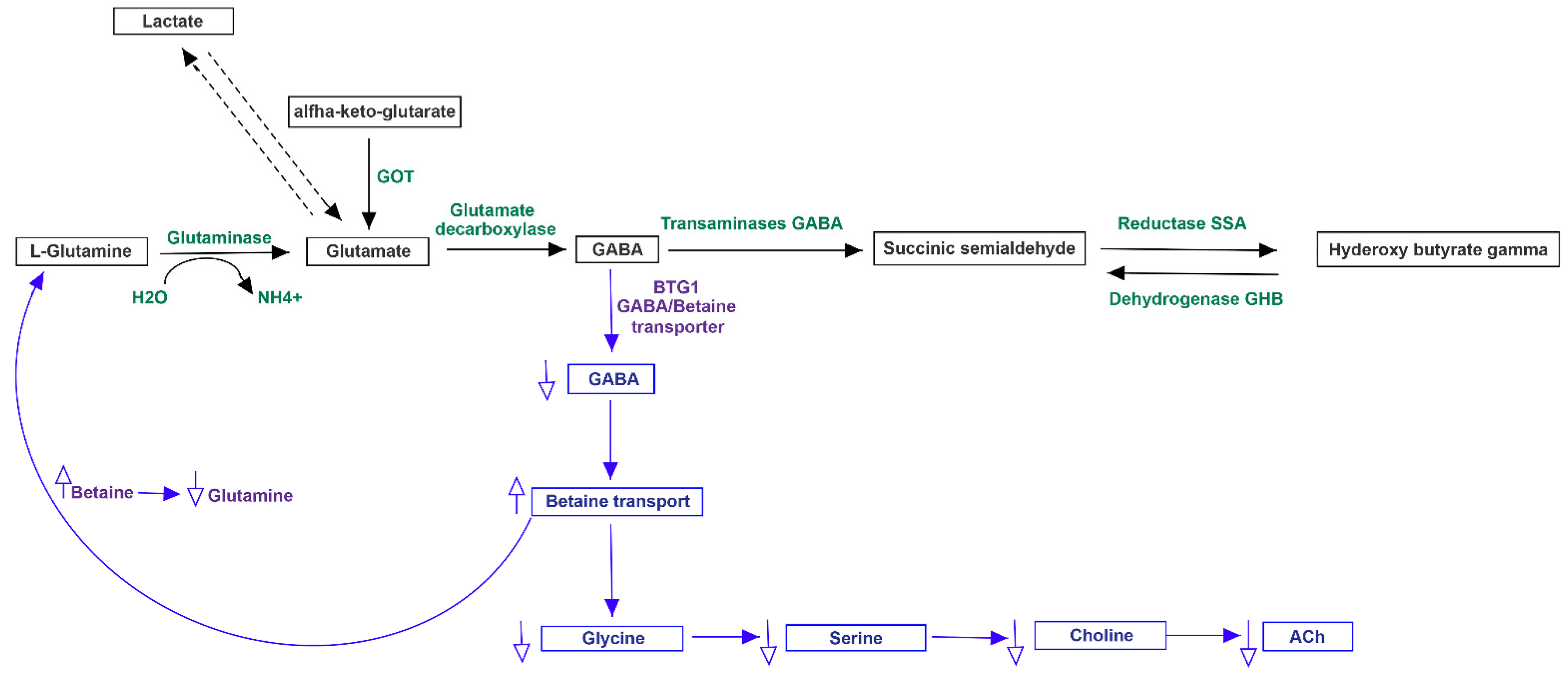
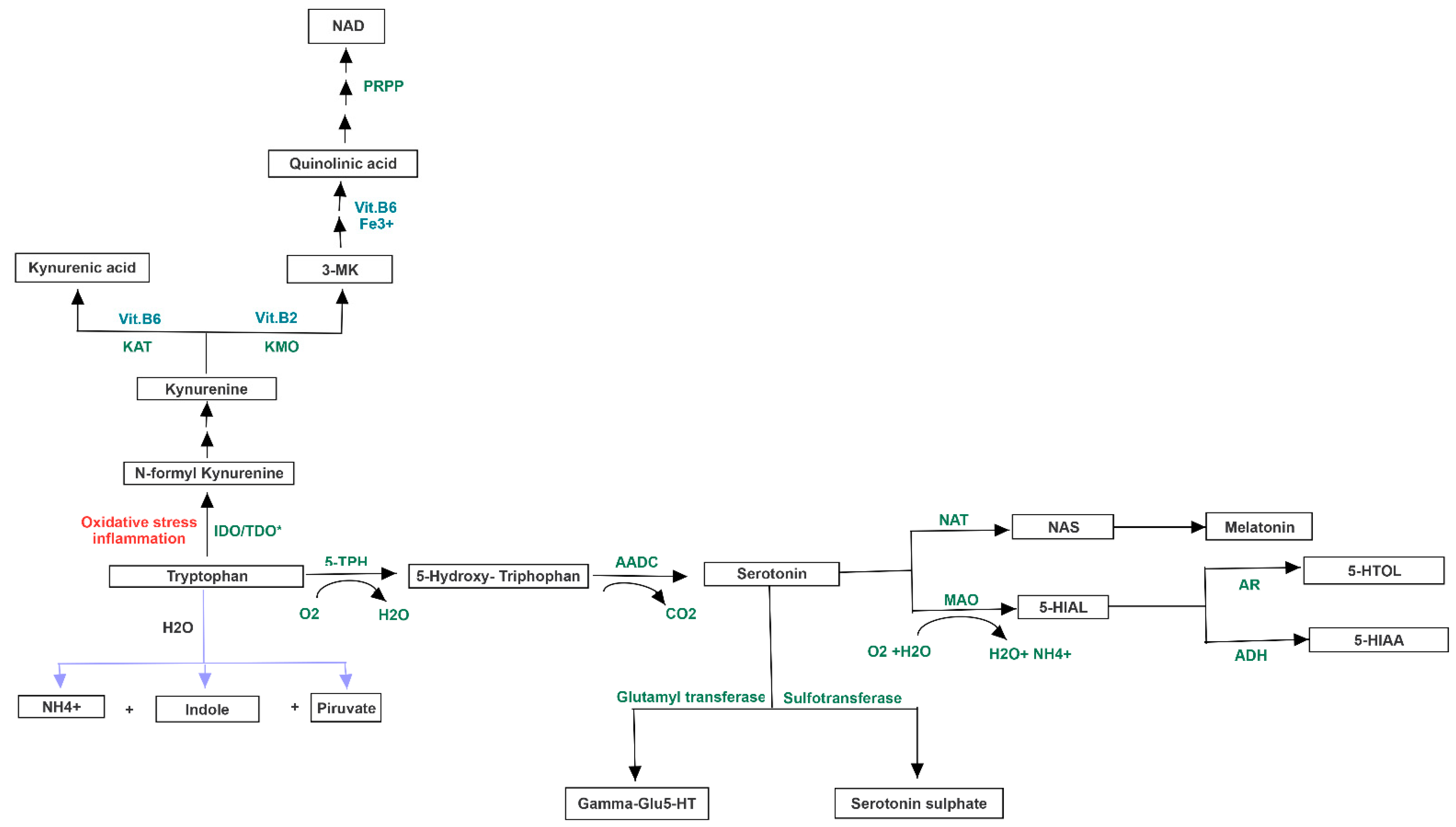
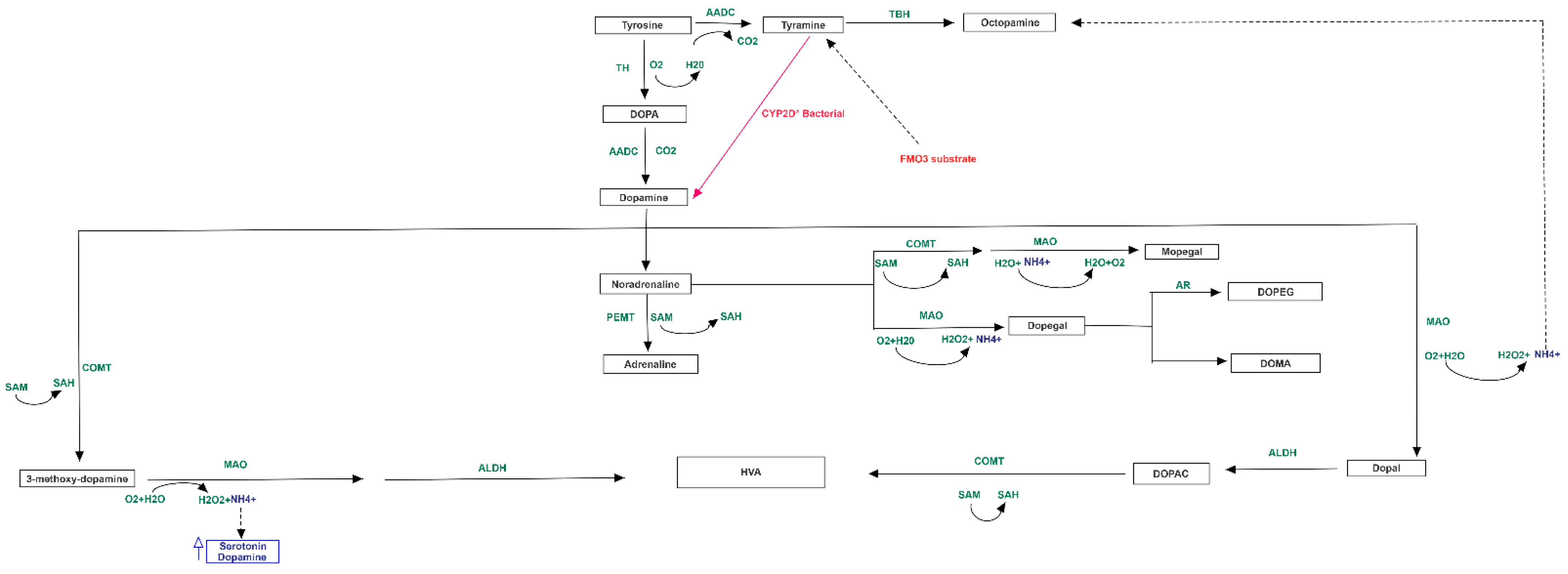
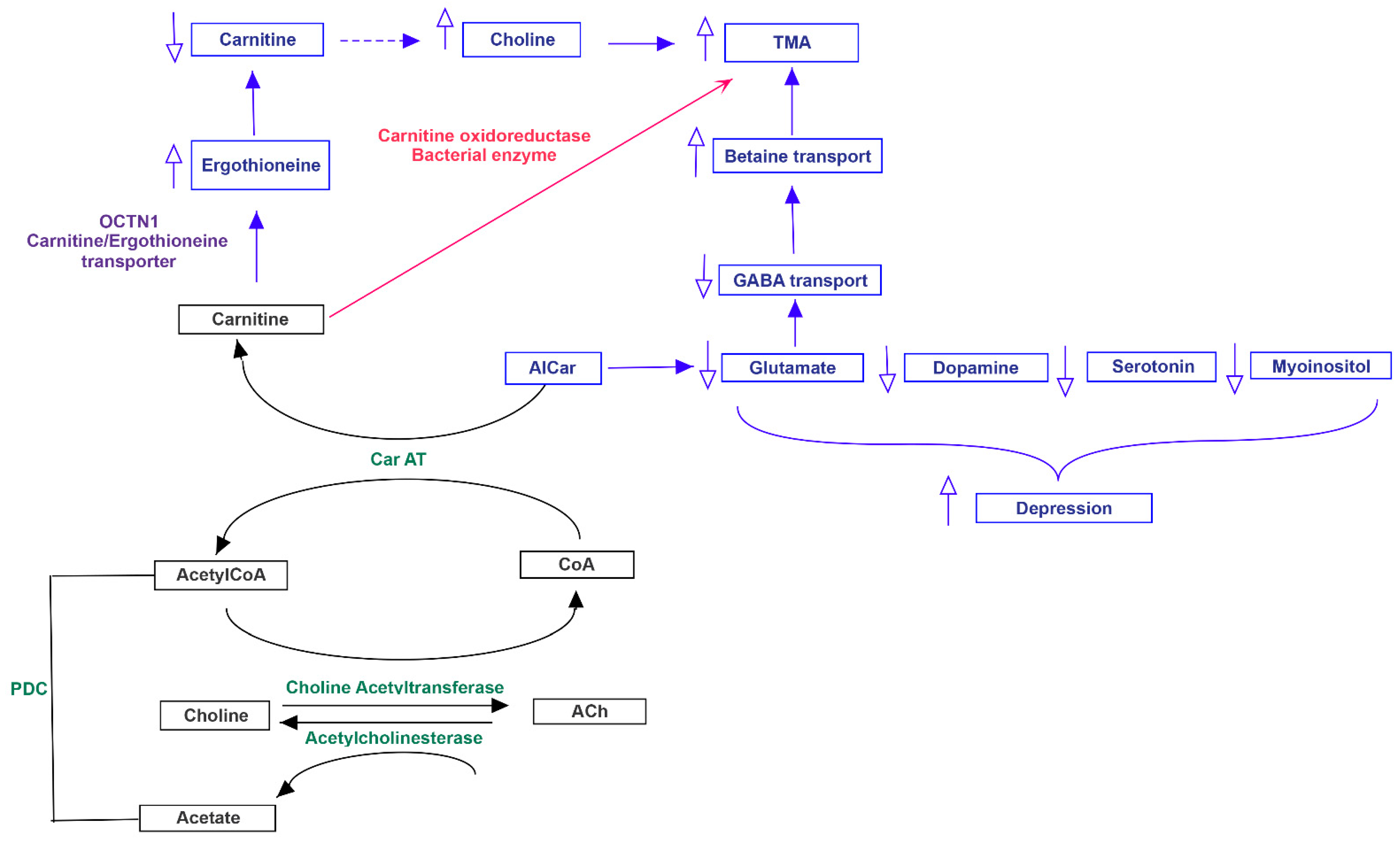
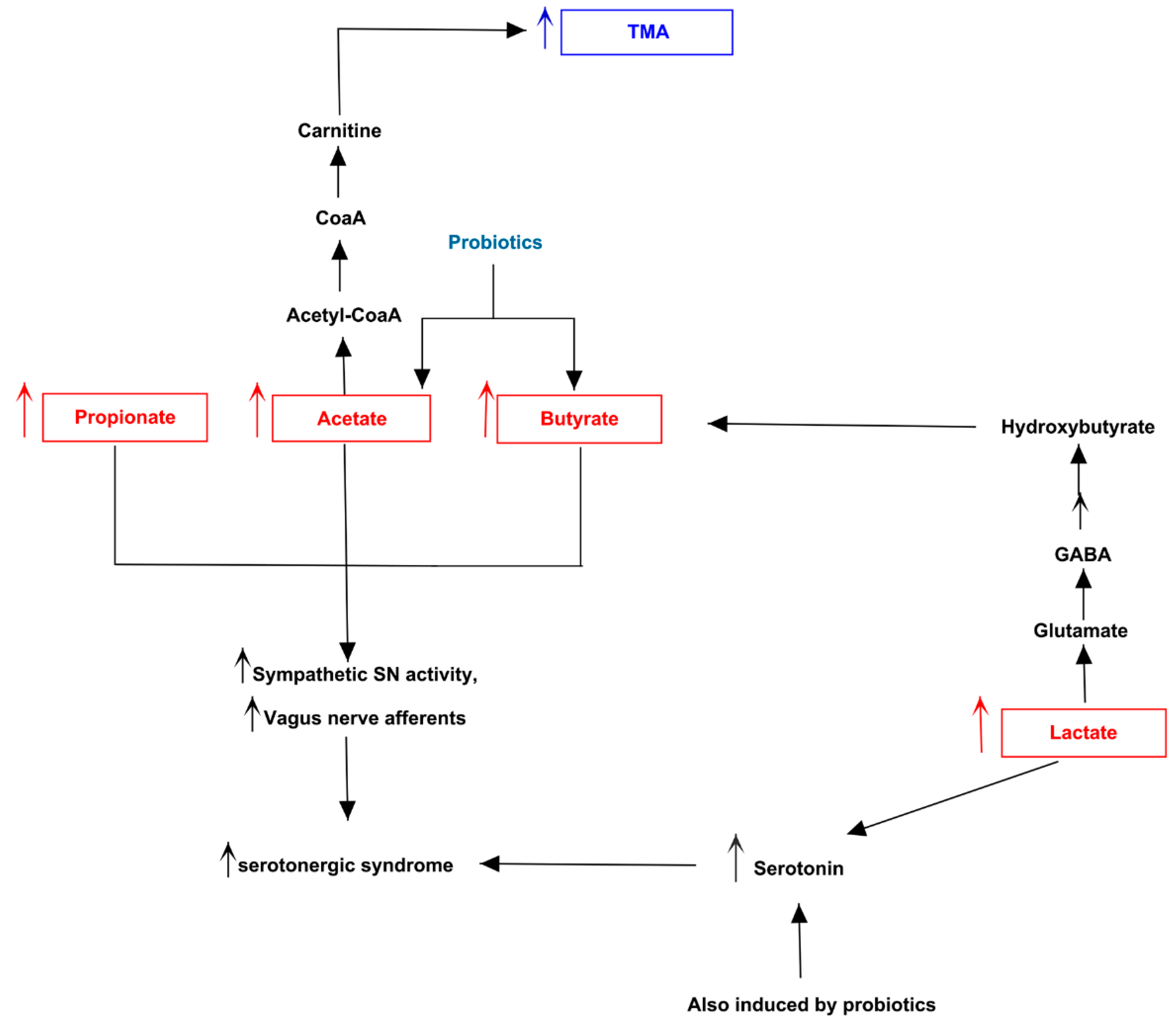
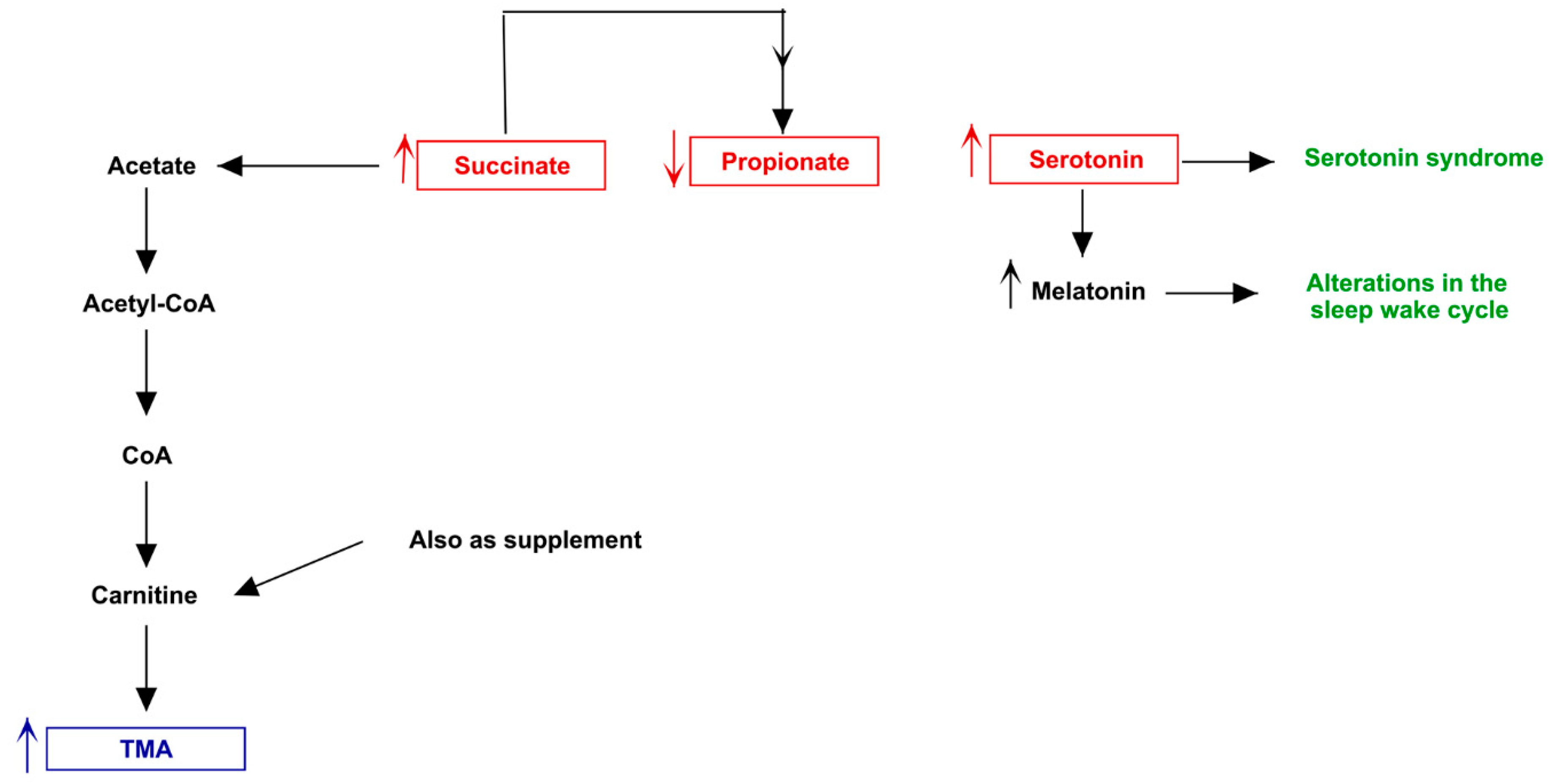
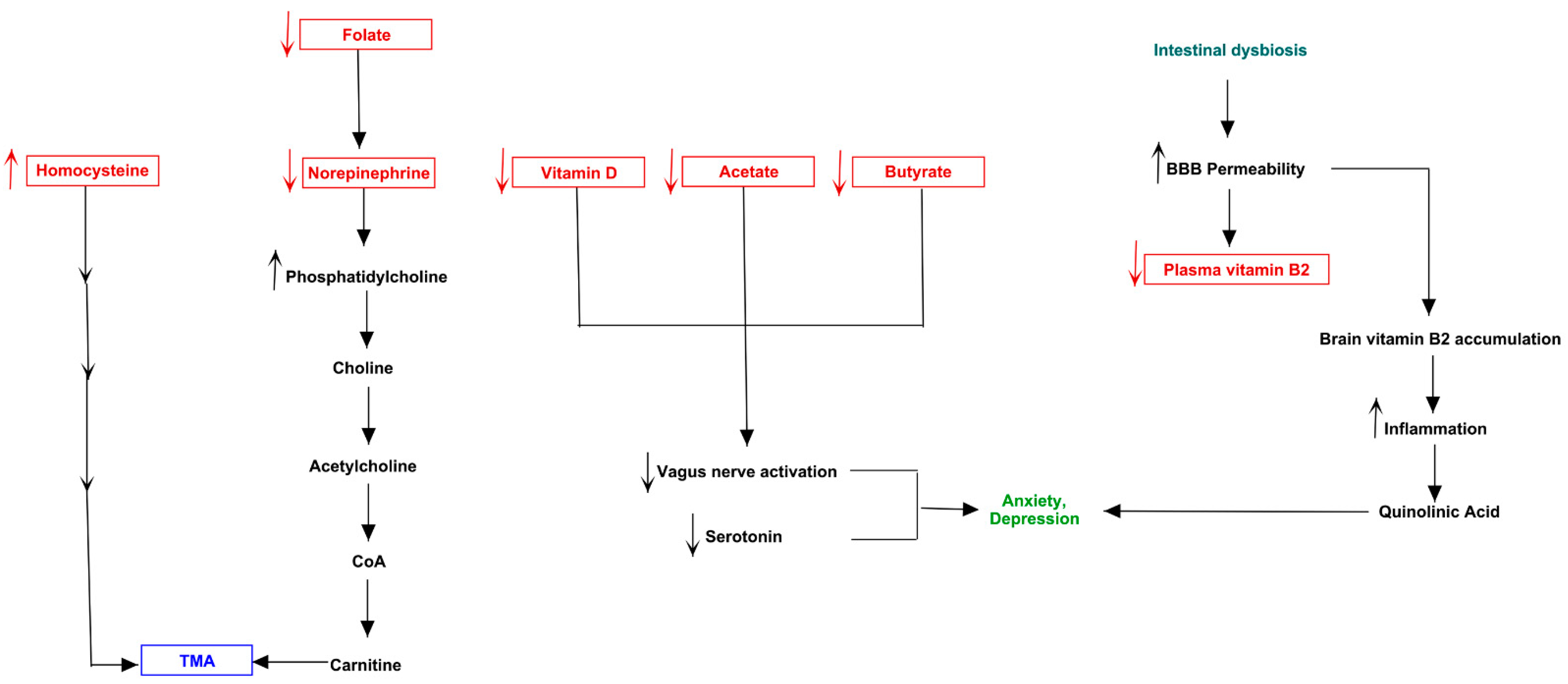
2.4. Neurotransmission Pathway Analysis of Gut-Brain Axis
3. Results
3.1. Microbiota of Neuro-Disordered TMAU Patients Revealed Huge Differences in Composition and Relative Abundances If Compared with “Brain-Healthy” TMAU Affected Individuals
3.2. Altered Bacterial Families of Neuro-Disordered TMAU Patients’ Microbiomes Produce Neurotransmitters and/or a Wide Range of Metabolites Involved in Their Biochemical Pathways
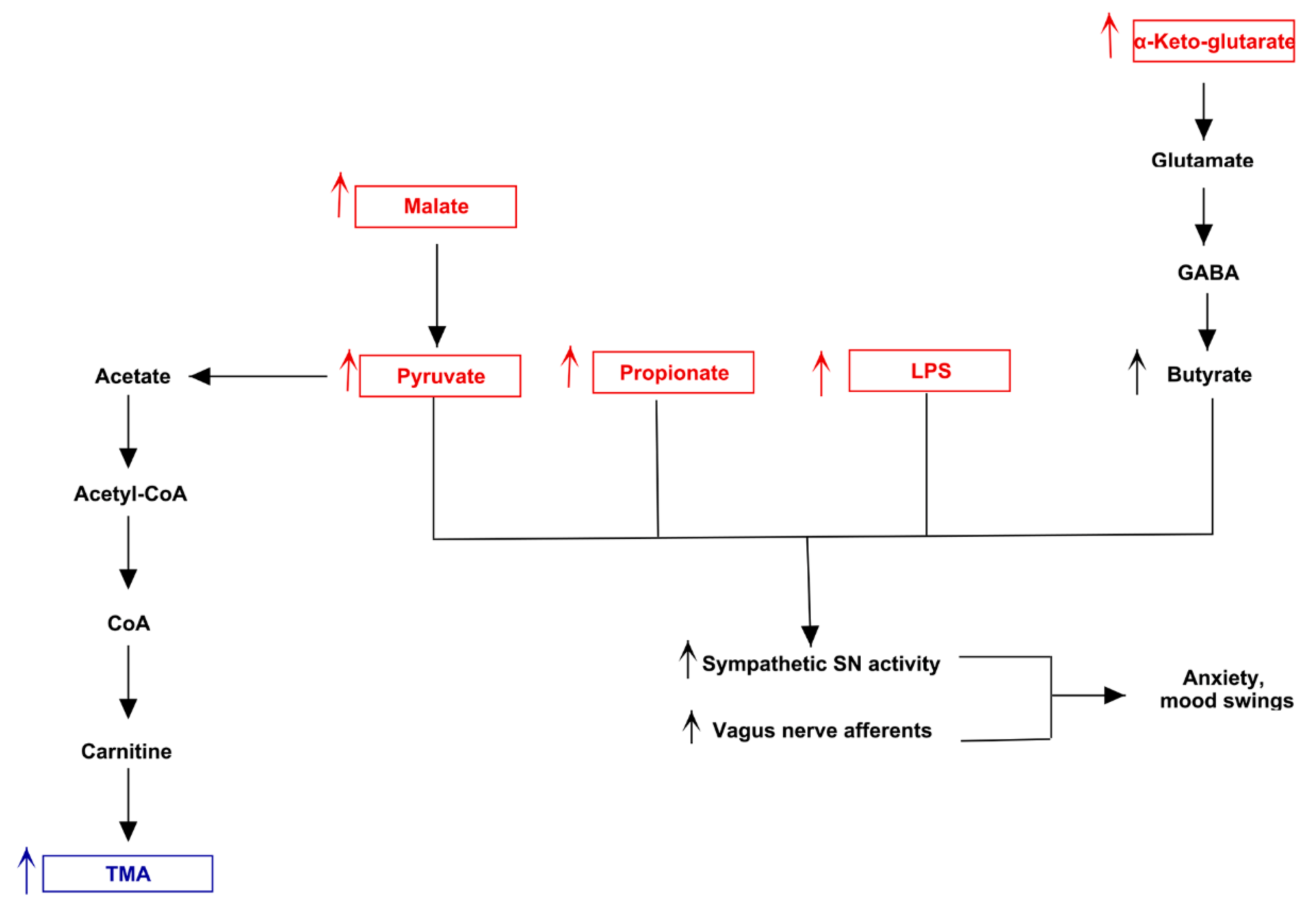
3.3. Pathway Analysis of Differential Abundances of Bacterial Families Suggested a Possible Biochemical Link between Microbiota Produced Metabolites, TMA Biosynthesis and Mood/Behavioral Disorders
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schmidt, A.C.; Leroux, J.C. Treatments of trimethylaminuria: Where we are and where we might be heading. Drug Discov. Today 2020, 25, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Messenger, J.; Clark, S.; Massick, S.; Bechtel, M. A Review of Trimethylaminuria: (Fish Odor Syndrome). J. Clin. Aesthetic Dermatol. 2013, 6, 45–48. [Google Scholar]
- Fraser-Andrews, E.A.; Manning, N.J.; Ashton, G.H.; Eldridge, P.; McGrath, J.; Menage, H.D.P. Fish odour syndrome with features of both primary and secondary trimethylaminuria. Clin. Exp. Dermatol. 2003, 28, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Varriale, B.; D’Angelo, R.; Amato, A.; Sidoti, A. Regulation of flavin-containing mono-oxygenase (Fmo3) gene expression by steroids in mice and humans. Horm. Mol. Biol. Clin. Investig. 2014, 20, 99–109. [Google Scholar] [CrossRef]
- Cruciani, G.; Valeri, A.; Goracci, L.; Pellegrino, R.M.; Buonerba, F.; Baroni, M. Flavin Monooxygenase Metabolism: Why Medicinal Chemists Should Matter. J. Med. Chem. 2014, 57, 6183–6196. [Google Scholar] [CrossRef]
- Shephard, E.A.; Treacy, E.P.; Phillips, I.R. Clinical utility gene card for: Trimethylaminuria. Eur. J. Hum. Genet. 2012, 20, 4–5. [Google Scholar] [CrossRef][Green Version]
- Scimone, C.; Donato, L.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. First case of Currarino syndrome and trimethylaminuria: Two rare diseases for a complex clinical presentation. J. Dig. Dis. 2016, 17, 628–632. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Mackay, R.J.; McEntyre, C.J.; Henderson, C.; Lever, M.; George, P.M. Trimethylaminuria: Causes and Diagnosis of a Socially Distressing Condition. Clin. Biochem. Rev. 2011, 32, 33–43. [Google Scholar]
- Gu, M.; Mei, X.-L.; Zhao, Y.N. Sepsis and Cerebral Dysfunction: BBB Damage, Neuroinflammation, Oxidative Stress, Apoptosis and Autophagy as Key Mediators and the Potential Therapeutic Approaches. Neurotox. Res. 2020. [Google Scholar] [CrossRef]
- Abushik, P.A.; Karelina, T.V.; Sibarov, D.A.; Stepanenko, J.D.; Giniatullin, R.; Antonov, S.M. Homocysteine-induced membrane currents, calcium responses and changes of mitochondrial potential in rat cortical neurons. Zh. Evol. Biokhim Fiziol. 2015, 51, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.R.; Ferreira, A.G.; Da Cunha, A.A.; Tagliari, B.; Mussulini, B.H.; Wofchuk, S.; Wyse, A.T. Homocysteine alters glutamate uptake and Na+,K+-ATPase activity and oxidative status in rats hippocampus: Protection by vitamin C. Metab. Brain Dis. 2011, 26, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Reas, D.L.; Pedersen, G.; Karterud, S.; Ro, O. Self-harm and suicidal behavior in borderline personality disorder with and without bulimia nervosa. J. Consult. Clin. Psychol. 2015, 83, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Allaband, C.; McDonald, D.; Vazquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Golob, J.L.; Margolis, E.; Hoffman, N.G.; Fredricks, D.N. Evaluating the accuracy of amplicon-based microbiome computational pipelines on simulated human gut microbial communities. BMC Bioinform. 2017, 18, 283. [Google Scholar] [CrossRef]
- Leong, L.E.X.; Taylor, S.L.; Shivasami, A.; Goldwater, P.N.; Rogers, G.B. Intestinal Microbiota Composition in Sudden Infant Death Syndrome and Age-Matched Controls. J. Pediatr. 2017, 191, 63–68. [Google Scholar] [CrossRef]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Niu, J.; Xu, L.; Qian, Y.; Sun, Z.; Yu, D.; Huang, J.; Zhou, X.; Wang, Y.; Zhang, T.; Ren, R.; et al. Evolution of the Gut Microbiome in Early Childhood: A Cross-Sectional Study of Chinese Children. Front. Microbiol. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- McNeill, T.W.; Sinkora, G.; Leavitt, F. Psychologic Classification of Low-Back Pain Patients: A Prognostic Tool. Spine 1986, 11, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Le Boulch, M.; Dehais, P.; Combes, S.; Pascal, G. The MACADAM database: A MetAboliC pAthways DAtabase for Microbial taxonomic groups for mining potential metabolic capacities of archaeal and bacterial taxonomic groups. Database 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Murphy, M.M.; Gueant, J.L. B vitamins and one carbon metabolism micronutrients in health and disease. Biochime 2020, 173, 1–2. [Google Scholar] [CrossRef]
- Kutmon, M.; Van Iersel, M.P.; Bohler, A.; Kelder, T.; Nunes, N.; Pico, A.R.; Evelo, C.T. PathVisio 3: An Extendable Pathway Analysis Toolbox. PLoS Comput. Biol. 2015, 11, e1004085. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.S.; Xia, X.T.; Wu, Y.F.; Zhao, L.; Xiang, H.; Du, G.H.; Zhang, X.; Qin, X.M. Discovery, screening and evaluation of a plasma biomarker panel for subjects with psychological suboptimal health state using (1)H-NMR-based metabolomics profiles. Sci. Rep. 2016, 6, 33820. [Google Scholar] [CrossRef] [PubMed]
- Nagalski, A.; Kozinski, K.; Wisniewska, M.B. Metabolic pathways in the periphery and brain: Contribution to mental disorders? Int. J. Biochem. Cell Biol. 2016, 80, 19–30. [Google Scholar] [CrossRef] [PubMed]
- McNiven, V.; Mamane, S.; Zai, G.; So, J. The Nose Knows… or Does it? Olfactory Reference Syndrome in Patients Presenting for Assessment of Unusual Body Odor. J. Nerv. Ment. Dis. 2019, 207, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.R.; Shephard, E.A. Flavin-containing monooxygenase 3 (FMO3): Genetic variants and their consequences for drug metabolism and disease. Xenobiotica 2020, 50, 19–33. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Newington, J.T.; Harris, R.A.; Cumming, R.C. Reevaluating Metabolism in Alzheimer’s Disease from the Perspective of the Astrocyte-Neuron Lactate Shuttle Model. J. Neurodegener. Dis. 2013, 2013, 234572. [Google Scholar] [CrossRef]
- Jin, X.T.; Galvan, A.; Wichmann, T.; Smith, Y. Localization and Function of GABA Transporters GAT-1 and GAT-3 in the Basal Ganglia. Front. Syst. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Ahs, F.; Engman, J.; Jonasson, M.; Alaie, I.; Bjorkstrand, J.; Frans, O.; Faria, V.; Linnman, C.; Appel, L.; et al. Serotonin Synthesis and Reuptake in Social Anxiety Disorder: A Positron Emission Tomography Study. JAMA Psychiatry 2015, 72, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Mischoulon, D. Folate in depression: Efficacy, safety, differences in formulations, and clinical issues. J. Clin. Psychiatry 2009, 70 (Suppl. 5), 12–17. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Johanson, C.E. Vitamin transport and homeostasis in mammalian brain: Focus on Vitamins B and E. J. Neurochem. 2007, 103, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Baldani, J.I.; Rouws, L.; Cruz, L.M.; Olivares, F.L.; Schmid, M.; Hartmann, A. The Family Oxalobacteraceae. In The Prokaryotes: Alphaproteobacteria and Betaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 919–974. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]



| ID | AGE | SEX | TMAU AGE of ONSET | DIET | ANTIBIOTIC MASSIVE USE | PROBIOTIC/FOOD SUPPLEMENTS | BEHAVIOR DISORDER | KIND OF BEHAVIOR DISORDER | OTHER |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | M | 17 | Chocolate, Eggs, Peas | NO | NO | YES | Anxiety, Fear, Suicidal instincts, Mood alteration | / |
| 2 | 40 | F | 14 | Fish, Vegetables | NO | NO | YES | Excessive emotionality, Anxiety | / |
| 3 | 54 | F | 6 | Dairy products, Meat, Fish | NO | L-carnitine, bromelain | YES | Migraine, Sleep disorders, Mood alteration, Sense of marginalization, Difficulties in social relations | / |
| 4 | 45 | F | 7 | Chocolate, Legumes, Eggs, Fish | YES | NO | YES | Chronic and rapid mental fatigue, Frequent headaches, Dizziness, Anxiety, Depression | Low levels of Folate, Plasmatic Vitamin B2 and D, Cu2+, Zn2+; High levels of PTH, homocysteine, Ca2+ |
| 5 | 44 | M | 34 | Coffee, Tea, White Meat, Vegetables, Fish | YES | L. acidophilus, Bifidobacterium lactis, L. rhamnosus, Streptococcus thermophilus and L. Paracasei | YES | Obsessive-compulsive disorder, Sense of marginalization | / |
| 6 | 36 | F | 9 | Vegetables, Coffee, Eggs | YES | Zinc, selenium, folic acid, iron, inulin, magnesium, L. Helveticus, B. longum spp.longum, Vitamin B6, Vitamin B1 and Vitamin D | YES | Mood alteration, Sense of marginalization, Suicidal instincts | / |
| 7 | 25 | F | 4 | Fish, Eggs, Chocolate, Legumes | NO | NO | YES | Depression, Obsessive-compulsive disorder, Sense of persecution | / |
| 1c | 47 | F | 8 | Gluten-free foods, Vegetables, Coffee | NO | NO | NO | NO | / |
| 2c | 26 | M | 10 | Fish, Chocolate, Red meat, Coffee, Alcohol | NO | Bifidobacterium lactis, L. acidophilus, L. plantarum, L. paracasei; Streptococcus salivarius subsp. thermophilus, Bifidobacterium brevis, Lactobacillus delbrueckii subsp. bulgaricus, Enterococcus faecium. | NO | NO | / |
| 3c | 20 | M | 16 | Gluten-free foods, Vegetables, Meat | NO | L. acidophilus, Bifidobacterium lactis, L. rhamnosus, Streptococcus thermophilus and L. Paracasei | NO | NO | / |
| 4c | 72 | F | 2 | Gluten-free and Lactose-free foods, Fish | NO | NO | NO | NO | High ROS and Arachidonic Acid |
| 5c | 35 | M | 35 | Red meat, Legumes, vegetables, Salmon | NO | NO | NO | NO | Use of alcohol |
| ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1c | 2c | 3c | 4c | 5c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterobacteriaceae [0.1–1.1] | 0.85 | 1.08 | 0.45 | 0.1 | 0.74 | 0.15 | 0.15 | 0.02 | 0.01 | 0.1 | 2.8 | 0.05 |
| Oxalobacteraceae [0.0–0.0] | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 | 0 |
| Enterococcaceae [0.0–0.0] | 0.02 | 0 | 0.02 | 0 | 0 | 0.68 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erysipelotrichaceae [0.1–2.9] | 2.8 | 0.4 | 0.78 | 0.1 | 0.38 | 3.9 | 3.3 | 0.15 | 0.21 | 0.1 | 3.8 | 2.62 |
| Rikenellaceae [0.2–5.3] | 0.48 | 5.22 | 1.25 | 0.2 | 2.2 | 6.95 | 0.2 | 0.2 | 0.2 | 0.2 | 6.78 | 0.48 |
| Veilloneaceae [0.8–7.7] | 6.35 | 3.15 | 1.58 | 0.8 | 2.8 | 5.35 | 3.35 | 0.8 | 0.8 | 0.8 | 0.48 | 1.85 |
| Roseburia [0.0–0.9] | 0 | 0.15 | 0.25 | 0.85 | 0 | 0.04 | 1.03 | 3.09 | 4.4 | 0 | 1 | 1.53 |
| Streptococcaceae [0.1–1.8] | 0.28 | 0.22 | 3.48 | 0.01 | 0.15 | 2.62 | 0.15 | 0.1 | 0.1 | 0.03 | 0.32 | 0.08 |
| Clostridiaceae [0.1–1.4] | 0.28 | 1.45 | 1.25 | 287.8 | 134.1 | 0.28 | 1.6 | 0.1 | 0.1 | 0..23 | 0.32 | 0.18 |
| Lachnospiraceae [12.8–37.26] | 20.52 | 9.98 | 24.78 | 1.86 | 15.8 | 3.78 | 23.22 | 72.24 | 44.65 | 0.04 | 18.58 | 23.25 |
| Prevotellaceae [0.1–13.66] | 0.12 | 2.3 | 16.68 | 0.1 | 0.7 | 3.85 | 40.0 | 0.02 | 0.1 | 0.1 | 0.13 | 26.65 |
| Coriobacteriaceae [0.3–5.9] | 0.15 | 1.08 | 2.12 | 0.01 | 0.7 | 6.5 | 0.82 | 0.3 | 0.3 | 0.04 | 0.52 | 1.7 |
| Bacteroidaceae [3.2–35.36] | 55.62 | 17.5 | 9.98 | 3.2 | 9.2 | 25.38 | 1.4 | 3.2 | 3.2 | 3.2 | 14.58 | 9.45 |
| Ruminococcaceae [13.7–34.7] | 2.42 | 24.4 | 23.38 | 13.7 | 18.7 | 24.35 | 16.23 | 0.27 | 1.43 | 0.13 | 24.25 | 19.8 |
| Faecalibacterium [2.5–15.56] | 0 | 3.05 | 9.35 | 5.2 | 5.5 | 0.58 | 8.43 | 6.4 | 23.97 | 10.33 | 8.25 | 7.2 |
| Porphiromonodaceae [0.2–3.2] | 1.25 | 0.2 | 0.98 | 0.22 | 0.52 | 1.5 | 0.55 | 0.12 | 0.2 | 0.2 | 1.22 | 0.28 |
| Sutterellaceae [0.1–3.5] | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.01 | 0.61 | 0.1 | 0.1 | 0.1 |
| Bifidobacteriaceae [0.1–7.96] | 4.38 | 1.82 | 0.38 | 0.39 | 3.55 | 3.88 | 0.1 | 0.1 | 0.003 | 0.11 | 0.1 | 1.05 |
| BACTERIA/METABOLITES | Lactate | Dopamine | Norepinephrine | Acetate | Serotonin | Succinate | Butyrate | Glycolate | Propionate | Pyruvate | α-ketoglutarate | LPS | Malate | Tryptophan | GABA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterobacteriaceae | X | X | X | X | X | X | X | ||||||||
| Oxalobacteraceae | X | X | X | X | X | X | |||||||||
| Enterococcaceae | X | X | X | ||||||||||||
| Erysipelotrichaceae | X | X | |||||||||||||
| Bifidobacteriaceae | X | X | X | ||||||||||||
| Rikenellaceae | X | X | X | ||||||||||||
| Sutterellaceae | X | X | |||||||||||||
| Veilloneaceae | X | X | X | X | |||||||||||
| Roseburia | X | X | X | X | |||||||||||
| Ruminococcaceae | X | X | X | ||||||||||||
| Streptococcaceae | X | X | X | ||||||||||||
| Clostridiaceae | X | X | X | X | |||||||||||
| Lachnospiraceae | X | X | X | X | |||||||||||
| Prevotellaceae | X | X | X | ||||||||||||
| Coriobacteriaceae | X | X | |||||||||||||
| Bacteroidaceae | X | X | X | X | X | ||||||||||
| Faecalibacteriaceae | X | ||||||||||||||
| Porphiromonodaceae | X | X | X |
| ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1c | 2c | 3c | 4c | 5c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| Lactate | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | ↓ | ↑ | ||||
| Succinate | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ||||||
| Dopamine | ↓ | ↑ | ↑ | |||||||||
| Norepinephrine | ↓ | ↓ | ↑ | ↑ | ||||||||
| Serotonin | ↑ | ↓ | ↑ | ↓ | ↓ | ↑ | ||||||
| α-ketoglutarate | ↑ | ↑ | ||||||||||
| Malate | ↑ | ↑ | ||||||||||
| Pyruvate | ↑ | ↑ | ||||||||||
| LPS | ↑ | ↑ | ↓ | ↑ | ||||||||
| Propionate | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | |||||
| Butyrate | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Tryptophan | ↓ | ↓ | ||||||||||
| GABA | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donato, L.; Alibrandi, S.; Scimone, C.; Castagnetti, A.; Rao, G.; Sidoti, A.; D’Angelo, R. Gut-Brain Axis Cross-Talk and Limbic Disorders as Biological Basis of Secondary TMAU. J. Pers. Med. 2021, 11, 87. https://doi.org/10.3390/jpm11020087
Donato L, Alibrandi S, Scimone C, Castagnetti A, Rao G, Sidoti A, D’Angelo R. Gut-Brain Axis Cross-Talk and Limbic Disorders as Biological Basis of Secondary TMAU. Journal of Personalized Medicine. 2021; 11(2):87. https://doi.org/10.3390/jpm11020087
Chicago/Turabian StyleDonato, Luigi, Simona Alibrandi, Concetta Scimone, Andrea Castagnetti, Giacomo Rao, Antonina Sidoti, and Rosalia D’Angelo. 2021. "Gut-Brain Axis Cross-Talk and Limbic Disorders as Biological Basis of Secondary TMAU" Journal of Personalized Medicine 11, no. 2: 87. https://doi.org/10.3390/jpm11020087
APA StyleDonato, L., Alibrandi, S., Scimone, C., Castagnetti, A., Rao, G., Sidoti, A., & D’Angelo, R. (2021). Gut-Brain Axis Cross-Talk and Limbic Disorders as Biological Basis of Secondary TMAU. Journal of Personalized Medicine, 11(2), 87. https://doi.org/10.3390/jpm11020087










