Abstract
Better preoperative characterization of patients with pancreatic ductal adenocarcinoma (PDAC) would aid in treatment optimization. Extracellular vesicles (EV) are promising, largely unexplored biomarkers in PDAC. This study aimed to evaluate if plasma EV characteristics are associated with PDAC clinical characteristics and overall survival (OS). The prospective cohort included 34 PDAC patients undergoing surgery with curative intent. Patient data and plasma samples were collected preoperatively, intraoperatively and one month postoperatively. Small plasma EV (sEV) concentration and size were determined by nanoparticle-tracking analysis. A Mann–Whitney test, Spearman’s rho and Cox regression were used in statistical analysis. Preoperatively, patients with poorly differentiated tumors had significantly larger plasma sEVs when compared to patients with well/moderately differentiated tumors (mean diameter 176.9 vs. 149.2 nm, p = 0.021), the sEV size even enabling discrimination of the two groups (AUC = 0.742, 95% CI = 0.560–0.923). Plasma sEV characteristics were also a predictor of OS in multivariable analysis. Patients with a more than 33.8% increase in sEV concentration after one month had 7.2 months shorter median OS (p = 0.002), while patients with a more than 28.0% decrease in sEV size had 9.2 months shorter median OS (p = 0.045). Plasma sEV concentration and size correlate with tumor differentiation and may predict OS in PDAC patients. In the future, plasma sEV characteristics could contribute to improved patient stratification for optimized treatment.
1. Introduction
The overall 5 year survival of less than 10% makes the pancreatic ductal adenocarcinoma (PDAC) one of the deadliest cancers known, with the incidence rising in the developed world [1,2,3]. Due to late onset of symptoms, 80% of patients are diagnosed with advanced, unresectable stage of disease [1,3,4]. Current management strategies for resectable disease employ upfront radical surgery followed by adjuvant treatment, while for borderline resectable PDAC, neoadjuvant therapy followed by resection is proposed [4,5,6]. To improve survival, important advances in neoadjuvant and adjuvant regimens were achieved recently [4,5,7], with huge efforts dedicated to identifying specific biomarkers that would enable earlier diagnosis and more optimal treatment of PDAC [8]. While biomarkers for early detection are still lacking [9], preoperative identification of patients with advanced disease or poor prognosis, despite tumor resectability, could aid in treatment optimization. Surgery could be avoided or postponed in these patients and systemic treatment immediately applied, possibly resulting in improved survival or at least quality of life [10,11]. Among preoperatively obtainable characteristics, tumor differentiation [12,13] and serum carbohydrate antigen 19-9 (CA 19-9) levels are associated with survival and tumor resectability [10,14]. As tissue histological grading from preoperative fine needle aspiration/biopsy is invasive and unreliable and serum CA 19-9 has its own limitations [8,15], non-invasive liquid biopsy reflecting tumor heterogeneity could importantly contribute to improved patient stratification for optimized therapy [16].
Liquid biopsy is a test performed on biofluid samples, most commonly blood, in order to diagnose and monitor various diseases, among which several cancers have been in the spotlight. Only 1% of the literature on liquid biopsy in cancer focuses on PDAC [17]; still, meta-analysis of this supports the use of liquid biopsy as surrogate for tissue biopsy [18]. By obtaining tumor-derived material from peripheral blood of PDAC patients, genetic alterations (e.g., KRAS) reflecting tumor heterogeneity were identified in circulating tumor DNA (ctDNA), while analysis of circulating tumor cells (CTCs) and ctDNA showed potential for monitoring treatment outcome and disease progression (reviewed in [16,18,19]). Still, studies on the use of CTCs and ctDNA as PDAC biomarkers are not conclusive; therefore, novel approaches based on ctDNA methylation profiling or fragmentation patterns were proposed [20,21]. DNA methylation can help determine the tissue origin of ctDNA, as it is highly tissue specific but consistent among different individuals and cancer patients [20,21]. Another promising and still largely unexplored liquid biopsy biomarkers in PDAC are extracellular vesicles (EVs).
EVs are a heterogeneous population of membrane bound particles, which are shed from all cell types and accumulate in all body fluids, including blood and pancreatic juice [22,23,24]. According to their size and site of formation, EVs are subdivided into exosomes, microvesicles and apoptotic bodies. In PDAC, EVs are implicated in the pathogenesis, local progression, metastasis, immune evasion and intercellular communication [24]. EVs molecular composition and biophysical properties mirror the (patho)physiological state of the cell of origin and thus they have great potential for human diagnostics and therapeutic applications [22,23]. Importantly, the multiple distinct biological materials contained within the EV can enable improved sensitivity and specificity of combined EV biomarkers [25,26]. EV DNA [27], miRNA [28] and protein [29,30] cargo were shown to correlate with disease stage and survival in PDAC patients, and EVs were also studied as therapeutic targets or agents [24]. EV concentration itself could also be used as a biomarker for PDAC, since several cancers are characterized by a remarkable increase in total plasma levels of EVs [22], but this has not been specifically studied to date.
Our study aimed to evaluate if small plasma EV (sEV) concentration and size are associated with PDAC clinical characteristics and patients’ overall survival (OS) in a prospective cohort of PDAC patients undergoing surgery with curative intent. We showed that patients who underwent tumor resection did not differ significantly from patients with solely surgical exploration in studied clinical and EV characteristics. Importantly, however, patients with poorly differentiated tumors had significantly larger plasma sEVs when compared to patients with well/moderately differentiated tumors. Furthermore, plasma sEV concentration and size were significant predictors of OS after adjustment for clinical variables.
2. Materials and Methods
2.1. Study Design and Data Collection
Patients with definite or suspected diagnosis of PDAC were eligible for inclusion in this prospective cohort study, and they all underwent surgery with curative intent from 1 January to 30 September 2018, at the Department of Abdominal Surgery, University Medical Centre Ljubljana, Ljubljana, Slovenia. Depending on the intraoperative assessment of the extent of the disease, patients underwent either surgical resection or exploration without resection. If diagnosis of PDAC was not confirmed by histopathological examination of the resected tissue or intraoperative biopsy obtained at exploration, patients were excluded from the study. Patients who received neoadjuvant treatment were not eligible for study enrollment. The study was conducted in accordance with the Declaration of Helsinki and approved by the Republic of Slovenia National Medical Ethics Committee (Study No. 0120-155/2016-2, KME 106/03/16). Written informed consent was obtained from all subjects prior to their enrollment.
Patient data were collected before, during and one month after surgery. Patients’ vital status was determined on 24 May 2019. Data included patient demographics, American Society of Anesthesiologists (ASA) score, smoking status, alcohol consumption, body mass index (BMI), tumor size on preoperative computed tomography scan and adjuvant chemotherapy if applicable. Laboratory report included white blood cell (WBC) count, C-reactive protein (CRP), CA 19-9 and carcinoembryonic antigen (CEA). Pathology report included surgical resection status (R0, R1 and R2), tumor differentiation (well, moderate or poor) and tumor TNM classification. Any missing patient data due to follow-up non-attendance (poor health, disease progression and death) are clearly indicated.
Blood samples for EV isolation were collected immediately before surgery and again one month after surgery in K2-EDTA collection tubes (6 mL). Samples were processed within 4 h by centrifugation at 2500× g for 10 min at 4 °C and plasma aliquots stored at –80 °C. Any sample exclusion due to visually positive hemolysis is clearly indicated.
2.2. Small EV Isolation from Blood Plasma
One milliliter of plasma was thawed on ice and centrifuged at 10,000× g, for 20 min at 4 °C. Next, supernatant was diluted to 9 mL with phosphate-buffered saline (PBS) and pipetted over 2 mL of 20% sucrose in 13 mL tubes. After centrifugation at 100,000× g, for 2 h 15 min at 4 °C (MLA-55 in Optima MAX-XP, Beckman Coulter), supernatant was aspirated, the pellet suspended in 60 μL of PBS and aliquots stored at −20 °C until analysis. The described procedure enables isolation of sEVs and exclusion of most lipoproteins from plasma, as determined for 10 healthy volunteers by electron microscopy, nanoparticle-tracking analysis, asymmetrical flow field-flow fractionation connected to detectors and miRNA expression analysis [31,32].
2.3. Quantification of sEV Concentration and Size
Small EV concentration and size were determined by nanoparticle-tracking (NTA) analysis using the NanoSight NS300 instrument (488 nm laser) connected to an automated sample assistant (both Malvern Panalytical). Samples were diluted 200 and 400 times in PBS and recorded five times at camera level 14. Raw data were analyzed by the NanoSight NTA 3.3 program at the following settings: detection threshold 5, water viscosity, temperature 25 °C, automatic settings for minimum expected particle size and blur, and minimum track length 10. Output data were expressed as sEV concentration, that is, the number of particles per 1 mL plasma, and sEV size, that is, the mean, modal and median hydrodynamic diameter in nm. Coefficients of variation for sEV concentration and size measurements were 5% and 2–6%, respectively. Relative change was defined as the difference of sEV concentration or size values one month after and before surgery, divided by its value before surgery.
2.4. Statistical Analysis
All analyses were performed using IBM SPSS Statistics, version 21.0 (IBM Corporation, Armonk, NY, USA). Continuous and categorical variables were described using the median with interquartile (25–75%) range and frequencies, respectively. A nonparametric Mann–Whitney test and Fisher’s exact test were used to compare the distribution of continuous variables and categorical variables among different patient groups, respectively. Spearman’s rho correlation coefficient (ρ) was used to assess correlations between continuous variables. In survival analysis, Cox regression was used to calculate hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs). Clinical variables used for adjustment in multivariable survival analysis were selected among all reported clinical variables using stepwise forward conditional selection. Kaplan–Meier analysis was used to calculate median survival and follow-up times. OS was defined as the time from surgery to death from any cause. A receiver operating characteristic (ROC) curve was used to determine the area under the curve (AUC) and cutoff with the highest sum of specificity and sensitivity. All statistical tests were two sided with the level of significance set to 0.05.
3. Results
3.1. Patient Characteristics
Characteristics of 34 included patients are presented in Table 1. Curative resection was achieved in 11 patients (four R0 ≤ 1 mm, 22.2%; seven R0 > 1 mm, 38.9%), resection margins were microscopically positive in four patients (R1; 22.2%) and two had macroscopic residual tumor (R2; 11.1%). For one patient, resection margins were not described. Seven patients without resection had stage III and nine stage IV disease; among patients who underwent resection, two had stage IIA, 13 stage IIB, one stage III and two stage IV disease. No significant difference was observed in the clinical characteristics between patients with or without tumor resection, with the exception of distant metastases, which were less likely to be present in patients with tumor resection (p = 0.009).

Table 1.
Baseline patients’ characteristics.
3.2. Patients’ Plasma sEV Characteristics
Plasma sEV concentration and size were determined immediately before (n = 34) and one month after surgery (n = 27, 79.4%) (Table 2). No statistically significant difference in sEV concentration was found between patients with and without resection. On the other hand, larger sEVs were detected before surgery in patients undergoing resection compared to patients without resection (modal diameter 144.0 vs. 132.2 nm, p = 0.039). One month after surgery, sEVs were still larger in patients undergoing resection, but the difference was no longer statistically significant (modal diameter 136.5 vs. 124.8 nm, p = 0.286).

Table 2.
Patients’ small plasma extracellular vesicle (EV) characteristics.
Since mostly no statistically significant differences in patients’ clinical and sEV characteristics with regard to tumor resection were found, all further analyses were performed on the entire study cohort. Higher sEV concentration correlated with smaller sEVs (ρ = −0.363, p = 0.035; ρ = −0.387, p = 0.024; ρ = −0.366, p = 0.034 for mean, modal and median diameter, respectively). Additionally, relative increase in sEV concentration at one month after surgery was associated with a relative decrease in sEV size (ρ = −0.570, p = 0.002; ρ = −0.573, p = 0.002; ρ = −0.568, p = 0.002 for mean, modal and median diameter, respectively). Relative change in EV characteristics was defined as the difference of sEV concentration or size values one month after and before surgery, divided by its value before surgery.
3.3. Association between Patients’ Clinical and Plasma sEV Characteristics
Association between patients’ clinical and plasma sEV characteristics are presented in Table S1. Increased inflammatory parameters, such as CRP and WBC count, tended to be associated with smaller sEVs (modal diameter), but the association did not reach statistical significance for WBC count (ρ = −0.376, p = 0.031 for CRP levels; ρ = −0.342, p = 0.051 for WBC count). Patients with higher ASA score had larger sEVs (mean diameter, p = 0.038), while other clinical characteristics were not significantly associated with sEV characteristics. Preoperatively evaluated tumor size or presence of distant metastases were thus not associated with sEV concentration or size (see Table S1).
Small EV concentration and size in regard to tumor differentiation are presented in Table 3 and Figure 1a. Importantly, before surgery, sEVs were significantly larger in poorly differentiated tumors when compared to well/moderately differentiated tumors (mean diameter 176.9 vs. 149.2 nm, p = 0.021 and median diameter 159.9 vs. 149.2 nm, p = 0.023). Lower sEV concentration tended to be associated with decreasing tumor differentiation (p = 0.984) (Figure 1a), the only patient with well differentiated tumor having the highest sEV concentration. At one month after surgery, a trend towards a higher (more positive) relative change in sEV concentration and lower (more negative) relative change in sEV size was observed for decreasing tumor differentiation (Figure 1b).

Table 3.
Association between plasma small EV characteristics and tumor differentiation.
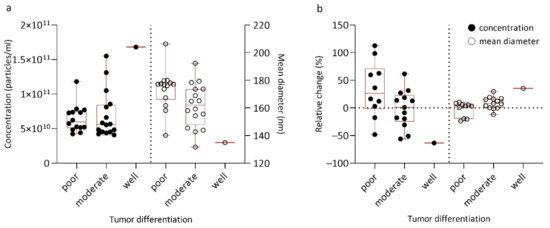
Figure 1.
Box plot representing small EV concentration and size in regard to tumor differentiation at different timepoints: (a) before surgery; (b) one month after surgery expressed as relative change. Relative change in EV characteristics was defined as the difference of small plasma EV (sEV) concentration or size values one month after and before surgery, divided by its value before surgery.
Using ROC curve analysis, we determined cutoff values for sEV characteristics to discriminate between poorly and well/moderately differentiated tumors (see Table S2). At the cutoff value of 173.55 nm for mean diameter before surgery, sensitivity for predicting poor differentiation was 0.765 and specificity 0.714, with an AUC of 0.742 (95% CI = 0.560–0.923, p = 0.022). Similarly, at the cutoff value of 158.85 nm for the median diameter before surgery, sensitivity for predicting poor differentiation was 0.824 and specificity 0.643, with an AUC of 0.736 (95% CI = 0.534–0.917, p = 0.025).
3.4. Patients’ Clinical and Plasma sEV Characteristics and Overall Survival
The median OS of study patients was 9.6 (5.2–15.9) months, with a follow-up time of 12.5 (11.3–14.3) months. At the time of vital status data collection, 14 (41.2%) patients were still alive. In univariable analysis, higher age and CA 19-9 before surgery were associated with shorter OS (HR = 1.08, 95% CI = 1.03–1.14, p = 0.004 and HR = 1.00, 95% CI = 1.00–1.00, p = 0.007, respectively), while adjuvant chemotherapy improved OS (HR = 0.20, 95% CI = 0.08–0.54, p = 0.001). If tumor resection was performed, patients had slightly longer OS compared to patients with exploration only, but the difference did not reach statistical significance (HR = 0.45, 95% CI = 0.18–1.11, p = 0.082). In a multivariable regression model, adjuvant chemotherapy and CRP before surgery were the only significant predictors of OS (HR = 0.11, 95% CI = 0.04–0.37, p < 0.001 and HR = 1.04, 95% CI = 1.01–1.06, p = 0.002, respectively).
Small EV concentration or size before surgery were not associated with OS. However, when adjusted for CRP levels before surgery and for adjuvant chemotherapy, shorter OS was observed in patients with higher (more positive) relative change in sEV concentration (HR = 1.25, 95% CI = 1.05–1.50, p = 0.015) and lower (more negative) relative change in sEV size (modal diameter; HR = 0.74, 95% CI = 0.57–0.95, p = 0.019) (Table 4). Patients were next stratified according to the cutoff values for sEV characteristics (see Table S2), and the association with OS was evaluated. If sEV concentration increased by more than 33.8%, patients had shorter OS (8.7 (3.4–8.7) months compared to 15.9 (7.7–15.9) months). Even though the association with OS was not significant in univariable analysis (HR = 2.67, 95% CI = 0.84–8.45, p = 0.095), relative change in sEV concentration was a significant predictor of OS after adjustment for clinical variables (HR = 10.21, 95% CI = 2.33–44.67, p = 0.002) (Figure 2a). If sEV size (modal diameter) decreased by more than 28.0%, patients had shorter OS (6.7 (2.1–7.7) months compared to 15.9 (8.2–15.9) months) both in univariable and multivariable analysis (HR = 0.18, 95% CI = 0.05–0.67, p = 0.010 and HR = 0.24, 95% CI = 0.06–0.97, p = 0.045, respectively) (Figure 2b).

Table 4.
Association between small EV characteristics and overall survival.
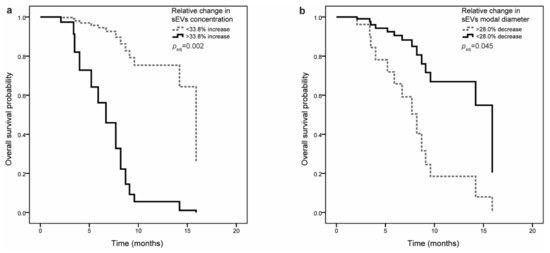
Figure 2.
Multivariable Cox regression analysis of overall survival in pancreatic ductal adenocarcinoma (PDAC) patients (n = 34): (a) Association of relative change in small EV concentration with overall survival. If sEV concentration increased by more than 33.8%, patients had shorter overall survival. (b) Association of relative change in small EV in modal diameter with overall survival. If sEV modal diameter decreased by more than 28.0%, patients had shorter overall survival.
4. Discussion
This study is to our knowledge the first to correlate sEV concentration and size to tumor differentiation and OS in PDAC patients undergoing surgery, and only a few similar studies can be found in other cancers [33,34,35,36]. Patients who underwent tumor resection did not differ significantly from patients with solely surgical exploration in studied clinical characteristics, sEV characteristics and OS. Importantly, however, patients with poorly differentiated tumors had significantly larger plasma sEVs before operation when compared to patients with well/moderately differentiated tumors, the sEV size even enabling discrimination of the two groups. Furthermore, plasma sEV characteristics were a significant predictor of OS after adjustment for clinical variables, with shorter OS observed in patients with higher relative change in sEV concentration and lower relative change in sEV size in one month after surgery.
As shown here and by others, certain PDAC patients undergoing resection have OS similar to those with advanced disease [15], with tumor differentiation being an important prognostic factor of resectability and OS [11,12,15]. Poorly differentiated tumors are associated with worse outcome, but the parameter is routinely obtained intraoperatively by tumor biopsy or resection, while the preoperative endoscopic ultrasound-guided fine-needle biopsy lacks accuracy [15]. Consequently, patients can be exposed to surgical overtreatment with associated complications and suboptimal PDAC management, as those with poorly differentiated PDAC are most likely to benefit from neoadjuvant therapy or immediate systemic treatment initiation [7]. Serum CA 19-9, an alternative preoperative parameter correlating with resectability and OS [10,14], similarly lacks in specificity (elevated in various cancers and benign diseases) and sensitivity (Lewis antigen-negative individuals) [8].
Importantly, our study demonstrated that preoperative plasma sEV size is associated with tumor differentiation. Previously, larger sEVs were associated with metastatic compared to non-metastatic PDAC [27]. We additionally showed that sEVs with a mean diameter >173.55 nm or a median diameter >158.85 nm could discriminate patients with poorly differentiated tumors from those with well/moderately differentiated tumors, yet with modest AUC and limited sensitivity and specificity (0.765 and 0.714, 0.824 and 0.643, respectively). To improve the clinical utility of sEV size, further larger studies combining EV characteristics and other molecular biomarkers, such as EV cargo or CA 19-9, are needed, as composite biomarkers are more likely to have a better predictive ability [25,26]. Alternatively, classification based on sEV size could be used complementary to endoscopic ultrasound-guided fine-needle biopsy findings to improve preoperative assessment of tumor histological grade and thus aid in the personalized treatment of PDAC. Plasma EVs better represent tumor heterogeneity and real-time state of the disease [16,25]. EV concentration and protein levels were similarly associated with tumor differentiation in colorectal and glioma cancer, respectively [33,34,35].
In our study cohort, previously recognized parameters associated with OS in PDAC were identified, such as age, CA 19-9, CRP and adjuvant chemotherapy [11,13,14], but we additionally showed that changes in sEV concentration and size are significant predictors of OS after adjustment for clinical variables. Higher relative change in sEV concentration and lower relative change in sEV size in one month after surgery were associated with shorter OS. Patients with a more than 33.8% increase in sEV concentration had 7.2 months shorter median OS than patients below this cutoff value, while patients with a more than 28.0% decrease in sEV size had 9.2 months shorter median OS than patients above this cutoff value. Similarly, higher plasma sEV [27] or serum glypican-1-positive exosome [30] levels predicted worse OS in localized and metastatic PDAC, while a greater decrease in serum glypican-1-enriched exosomes was proposed to improve OS in all stages of PDAC [29]. Supporting the relevance of high EV levels in predicting OS in PDAC, plasma EVs with >5% exosome KRAS mutant allele fraction, high miR-4525, miR-451a, miR-222, miR-21 or circ-PDE8A expression were all associated with worse OS in previous studies [37,38,39,40].
EVs have been shown to be an important prognostic factor in various cancers [23,41], with high EV concentration or small EV size shown to be predictive of less time to relapse and/or worse OS in colorectal, prostate, esophageal and lung cancers [34,36,42,43,44]. High plasma EV concentration in cancer can to some extent be associated with tumor burden [28,45,46]; however, inflammation and response to systemic treatment could also contribute [47,48]. The observed increase in plasma EVs might be connected to physiological factors, such as hypoxia, autophagy or stress, which are often altered in tumors [49]. In our study, there was no significant impact of tumor size or presence of metastases on EV characteristics. One month after surgery, a higher relative change in sEV concentration was observed in patients who did not undergo resection compared to those with resected tumors (14.7% vs. 3.1%, respectively), which might indicate a connection of EV concentration to tumor burden, but the difference was not significant. This might be due to the longer time interval after the surgery in our study, as blood samples were collected after one month, while in other studies, which showed a correlation of EV concentration to tumor burden, they were collected up to one week after surgery [28,45,46].
A limitation of our study was a small sample size and relatively short observational period. As only one patient had a well-differentiated tumor, we could not evaluate the association with sEV characteristics for this subgroup. However, we investigated sEV concentration and size in a well characterized population of PDAC patients treated according to the same protocol and in the same institution. Our results should be validated in an independent larger cohort in the future and association of plasma EV characteristics with tumor burden examined in more detail.
In conclusion, plasma sEV concentration and size correlate with tumor differentiation and may predict OS in PDAC patients undergoing surgery with curative intent. Further longitudinal studies on larger study cohorts are needed to evaluate sEVs as composite or complementary biomarkers for preoperative assessment of tumor grade and as prognostic biomarkers for OS, in order to improve patient stratification and treatment optimization. Our study thus complements other innovative approaches in cancer liquid biopsy, such as ctDNA methylation profile and fragmentation [20,21].
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4426/11/2/77/s1, Table S1: Association between patients’ clinical and plasma small EV characteristics; Table S2: ROC curve analysis to assess the ability of small EV characteristics to discriminate between poorly and well/moderately differentiated tumors.
Author Contributions
Conceptualization, D.B., V.D., A.T. and M.L.; methodology, K.G., T.L., N.M. and M.L.; validation, K.G., T.L. and N.M.; formal analysis, K.G. and T.L.; investigation, D.B., H.Z., M.P. and A.T.; resources, V.D., A.T. and M.L.; writing—Original draft preparation, D.B., K.G., H.Z., A.T., V.D. and M.L.; writing—Review and editing, D.B., K.G., H.Z., A.T., V.D. and M.L.; visualization, D.B., K.G., H.Z., T.L. and M.L.; supervision, A.T., V.D. and M.L.; project administration, D.B. and M.P.; funding acquisition, A.T., M.P., V.D. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Javna Agencija za Raziskovalno Dejavnost RS (Eng. Slovenian Research Agency) (ARRS), research projects P1-170 and J3-9255, and by the University Medical Centre Ljubljana research project No. 20150180.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Republic of Slovenia National Medical Ethics Committee (Study No. 0120-155/2016-2, KME 106/03/16; date of approval: 15 March 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors are grateful to Tanja Blagus and Savica Soldat from the Institute of Biochemistry, Faculty of Medicine, University of Ljubljana, for their help with collection and processing of blood samples, and to all the nurses and administrators at the Department of Abdominal Surgery, University Medical Centre Ljubljana, Slovenia, for their help with patient enrollment and patient care.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2014. Ann. Oncol. 2014, 25, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic Adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Sa Cuhna, A.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Brousse, P. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Ulrich, A.; Büchler, M.W. Borderline resectable pancreatic cancer. Cancer Lett. 2016, 375, 231–237. [Google Scholar] [CrossRef]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef]
- Nurmi, A.; Mustonen, H.; Parviainen, H.; Peltola, K.; Haglund, C.; Seppänen, H. Neoadjuvant therapy offers longer survival than upfront surgery for poorly differentiated and higher stage pancreatic cancer. Acta Oncol. (Madr). 2018, 57, 799–806. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Van Veldhuisen, E.; Vogel, J.A.; Klompmaker, S.; Busch, O.R.; van Laarhoven, H.W.M.; van Lienden, K.P.; Wilmink, J.W.; Marsman, H.A.; Besselink, M.G. Added value of CA19-9 response in predicting resectability of locally advanced pancreatic cancer following induction chemotherapy. HPB (Oxford) 2018, 20, 605–611. [Google Scholar] [CrossRef]
- Barugola, G.; Partelli, S.; Marcucci, S.; Sartori, N.; Capelli, P.; Bassi, C.; Pederzoli, P.; Falconi, M. Resectable pancreatic cancer: Who really benefits from resection? Ann. Surg. Oncol. 2009, 16, 3316–3322. [Google Scholar] [CrossRef] [PubMed]
- Wasif, N.; Ko, C.Y.; Farrell, J.; Wainberg, Z.; Hines, O.J.; Reber, H.; Tomlinson, J.S. Impact of Tumor Grade on Prognosis in Pancreatic Cancer: Should We Include Grade in AJCC Staging? Ann. Surg. Oncol. 2010, 17, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Gemenetzis, G.; Blair, A.B.; Rivero-Soto, R.J.; Yu, J.; Javed, A.A.; Burkhart, R.A.; Rinkes, I.H.M.B.; Molenaar, I.Q.; Cameron, J.L.; et al. Defining and Predicting Early Recurrence in 957 Patients with Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, G.C.; Morganti, A.G.; Cellini, F.; Buwenge, M.; Casadei, R.; Farioli, A.; Alfieri, S.; Arcelli, A.; Bertini, F.; Calvo, F.A.; et al. Prognostic Impact of Presurgical CA19-9 Level in Pancreatic Adenocarcinoma: A Pooled Analysis. Transl. Oncol. 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Correale, L.; Ricci, R.; Abdulkader, I.; Monges, G.; Iglesias-Garcia, J.; Giovannini, M.; Attili, F.; Vitale, G.; Hassan, C.; et al. Interobserver agreement and accuracy of preoperative endoscopic ultrasound-guided biopsy for histological grading of pancreatic cancer. Endoscopy 2014, 47, 308–314. [Google Scholar] [CrossRef]
- Qi, Z.-H.; Xu, H.-X.; Zhang, S.-R.; Xu, J.-Z.; Li, S.; Gao, H.-L.; Jin, W.; Wang, W.-Q.; Wu, C.-T.; Ni, Q.-X.; et al. The Significance of Liquid Biopsy in Pancreatic Cancer. J. Cancer 2018, 9, 3417–3426. [Google Scholar] [CrossRef]
- Otandault, A.; Anker, P.; Al Amir Dache, Z.; Guillaumon, V.; Meddeb, R.; Pastor, B.; Pisareva, E.; Sanchez, C.; Tanos, R.; Tousch, G.; et al. Recent advances in circulating nucleic acids in oncology. Ann. Oncol. 2019, 30, 374–384. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Nottegar, A.; Cappelletti, V.; Daidone, M.G.; Smith, L.; Parris, C.; Brosens, L.A.A.; Caruso, M.G.; Cheng, L.; et al. cancers Liquid Biopsy as Surrogate for Tissue for Molecular Profiling in Pancreatic Cancer: A Meta-Analysis Towards Precision Medicine. Cancers (Basel) 2019, 11, 1152. [Google Scholar] [CrossRef]
- Rofi, E.; Vivaldi, C.; Del Re, M.; Arrigoni, E.; Crucitta, S.; Funel, N.; Fogli, S.; Vasile, E.; Musettini, G.; Fornaro, L.; et al. The emerging role of liquid biopsy in diagnosis, prognosis and treatment monitoring of pancreatic cancer. Pharmacogenomics 2019, 20, 49–68. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, P.; Chan, K.A.; Wong, J.; Cheng, Y.K.; Liang, R.H.; Chan, W.K.; Ma, E.S.; Chan, S.L.; Cheng, S.H.; et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc. Nat. Acad. Sci. USA 2015, 112, E5503–E5512. [Google Scholar] [CrossRef]
- Gai, W.; Sun, K. Epigenetic Biomarkers in Cell-Free DNA and Applications in Liquid Biopsy. Genes (Basel) 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Falcón-Pérez, J.M. Cell-derived extracellular vesicles as a platform to identify low-invasive disease biomarkers. Expert Rev. Mol. Diagn. 2015, 15, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Chen, Y.-C. Clinical significance of exosomes as potential biomarkers in cancer. World J. Clin. Cases 2019, 7, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, R.L.; Hines, O.J.; Eibl, G.; King, J.C. Emerging Evidence for the Clinical Relevance of Pancreatic Cancer Exosomes. Pancreas 2019, 48, 1–8. [Google Scholar] [CrossRef]
- Yee, N.S.; Zhang, S.; He, H.Z.; Zheng, S.Y. Extracellular vesicles as potential biomarkers for early detection and diagnosis of pancreatic cancer. Biomedicines 2020, 8, 1–20. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Kalluri, R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef]
- Allenson, K.; Castillo, J.; San Lucas, F.A.; Scelo, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef]
- Lai, X.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017, 393, 86–93. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Buscail, E.; Alix-Panabières, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdon, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers (Basel) 2019, 11, 1656. [Google Scholar] [CrossRef]
- Clayton, A.; Buschmann, D.; Byrd, J.B.; Carter, D.R.F.; Cheng, L.; Compton, C.; Daaboul, G.; Devitt, A.; Falcon-Perez, J.M.; Gardiner, C.; et al. Summary of the ISEV workshop on extracellular vesicles as disease biomarkers, held in Birmingham, UK, during December 2017. J. Extracell. Vesicles 2018, 7, 1473707. [Google Scholar] [CrossRef] [PubMed]
- Holcar, M.; Ferdin, J.; Sitar, S.; Tušek-Žnidarič, M.; Dolžan, V.; Plemenitaš, A.; Žagar, E.; Lenassi, M. Enrichment of plasma extracellular vesicles for reliable quantification of their size and concentration for biomarker discovery. Sci. Rep. 2020. [CrossRef]
- Huang, K.; Fang, C.; Yi, K.; Liu, X.; Qi, H.; Tan, Y.; Zhou, J.; Li, Y.; Liu, M.; Zhang, Y.; et al. The role of PTRF/Cavin1 as a biomarker in both glioma and serum exosomes. Theranostics 2018, 8, 1540–1557. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Garcia, V.; Rodriguez, M.; Compte, M.; Cisneros, E.; Veguillas, P.; Garcia, J.M.; Dominguez, G.; Campos-Martin, Y.; Cuevas, J.; et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes. Chromosomes Cancer 2012, 51, 409–418. [Google Scholar] [CrossRef]
- Muller, L.; Muller-Haegele, S.; Mitsuhashi, M.; Gooding, W.; Okada, H.; Whiteside, T.L. Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology 2015, 4, e1008347. [Google Scholar] [CrossRef]
- Navarro, A.; Molins, L.; Marrades, R.M.; Moises, J.; Viñolas, N.; Morales, S.; Canals, J.; Castellano, J.J.; Ramírez, J.; Monzo, M. Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery. Cancers (Basel) 2019, 21, 11. [Google Scholar] [CrossRef]
- Takahasi, K.; Iinuma, H.; Wada, K.; Minezaki, S.; Kawamura, S.; Kainuma, M.; Ikeda, Y.; Shibuya, M.; Miura, F.; Sano, K. Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. J. Hepato Biliary Pancreat. Sci. 2018, 25, 155–161. [Google Scholar] [CrossRef]
- Kawamura, S.; Iinuma, H.; Wada, K.; Takahashi, K.; Minezaki, S.; Kainuma, M.; Shibuya, M.; Miura, F.; Sano, K. Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21 in portal vein blood is a high-sensitive liquid biomarker for the selection of high-risk pancreatic ductal adenocarcinoma patients. J. Hepato Biliary Pancreat. Sci. 2019, 26, 63–72. [Google Scholar] [CrossRef]
- Li, Z.; Yanfang, W.; Li, J.; Jiang, P.; Peng, T.; Chen, K.; Zhao, X.; Zhang, Y.; Zhen, P.; Zhu, J.; et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018, 432, 237–250. [Google Scholar] [CrossRef]
- Li, Z.; Tao, Y.; Wang, X.; Jiang, P.; Li, J.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Zhen, P.; et al. Tumor-Secreted Exosomal miR-222 Promotes Tumor Progression via Regulating P27 Expression and Re-Localization in Pancreatic Cancer. Cell. Physiol. Biochem. 2018, 51, 610–629. [Google Scholar] [CrossRef]
- Oehme, F.; Krahl, S.; Gyorffy, B.; Muessle, B.; Rao, V.; Greif, H.; Ziegler, N.; Lin, K.; Thepkaysone, M.-L.; Polster, H.; et al. Low level of exosomal long non-coding RNA HOTTIP is a prognostic biomarker in colorectal cancer. RNA Biol. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Helley, D.; Banu, E.; Bouziane, A.; Banu, A.; Scotte, F.; Fischer, A.-M.; Oudard, S. Platelet Microparticles: A Potential Predictive Factor of Survival in Hormone-Refractory Prostate Cancer Patients Treated with Docetaxel-Based Chemotherapy. Eur. Urol. 2009, 56, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kano, M.; Akutsu, Y.; Hanari, N.; Hoshino, I.; Murakami, K.; Usui, A.; Suito, H.; Takahashi, M.; Otsuka, R.; et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol. Rep. 2016, 36, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xiang, Y.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Wu, N.; Wu, L.; Yu, Z.; Bai, L.; et al. Plasma exosome levels in non-small-cell lung cancer: Correlation with clinicopathological features and prognostic implications. Cancer Biomark. 2018, 22, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Osti, D.; Del Bene, M.; Rappa, G.; Santos, M.; Matafora, V.; Richichi, C.; Faletti, S.; Beznoussenko, G.V.; Mironov, A.; Bachi, A.; et al. Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients. Clin. Cancer Res. 2019, 5, 266–276. [Google Scholar] [CrossRef]
- Rodríguez Zorrilla, S.; Pérez-Sayans, M.; Fais, S.; Logozzi, M.; Torreira, M.G.; García García, A. A Pilot Clinical Study on the Prognostic Relevance of Plasmatic Exosomes Levels in Oral Squamous Cell Carcinoma Patients. Cancers (Basel) 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.D.; Wong, W.; Lee, M.M.; Cho, W.C.; Yee, B.K.; Kwan, Y.W.; Tai, W.C. Exosomes in Inflammation and Inflammatory Disease. Proteomics 2019, 19, 1800149. [Google Scholar] [CrossRef]
- Stevic, I.; Buescher, G.; Ricklefs, F.L. Monitoring Therapy Efficiency in Cancer through Extracellular Vesicles. Cells 2020, 9, 130. [Google Scholar] [CrossRef]
- Vasconcelos, M.H.; Caires, H.R.; Ābols, A.; Xavier, C.P.R.; Linē, A. Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist. Updates 2019, 47, 100647. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).