Betel Nut Chewing Was Associated with Obstructive Lung Disease in a Large Taiwanese Population Study
Abstract
1. Introduction
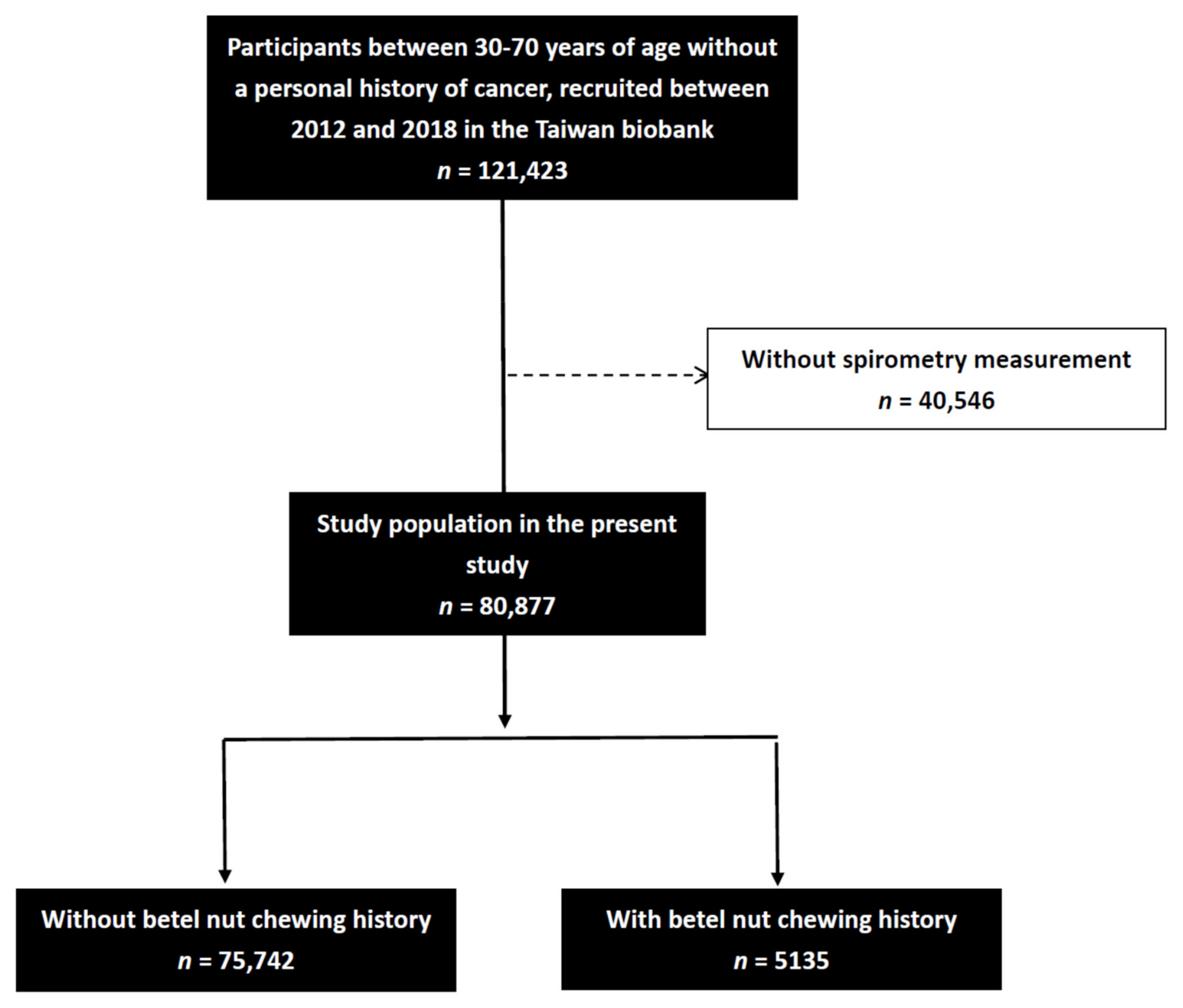
2. Methods
2.1. Ethics Statement
2.2. The TWB
2.3. Study Data
2.4. Assessment of Betel Nut Chewing
- ‘How many years have you chewed betel nut?’
- ‘How often do you chew betel nut?’ (frequency)
- 1–3 days/month (score = 1);
- 1, 2 days/week (score = 2);
- 3–5 days/week (score = 3);
- Every day (score = 4).
- ‘How many quid a day?’ (daily amount)
- <10 quids (score = 1);
- 10–20 quids (score = 2);
- 21–30 quids (score = 3);
- ≥31 quids (score = 4).
- Cumulative dose = years of chewing betel nut*frequency score*daily score.
2.5. Spirometry Measurements
2.6. Statistical Analysis
3. Results
3.1. Comparisons of Clinical Characteristics between the Normal Lung Function and Obstructive Lung Function Groups
3.2. Correlations between Chewing Betel Nut and FEV1/FVC < 70% in All Participants
3.3. Comparisons of Betel Nut Chewing Characteristics among the Participants Who Chewed Betel Nut According to FEV1/FVC ≥ 70% or <70%
3.4. Correlations between Betel Nut Chewing Characteristics with FEV1/FVC < 70% in the Participants Who Chewed Betel Nut
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labaki, W.W.; Han, M.K. Chronic respiratory diseases: A global view. Lancet Respir. Med. 2020, 8, 531–533. [Google Scholar] [CrossRef]
- MacNee, W. Pathology, pathogenesis, and pathophysiology. BMJ 2006, 332, 1202–1204. [Google Scholar] [CrossRef]
- Decramer, M.; Janssens, W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir. Med. 2013, 1, 73–83. [Google Scholar] [CrossRef]
- Volgin, A.D.; Bashirzade, A.; Amstislavskaya, T.G.; Yakovlev, O.A.; Demin, K.A.; Ho, Y.J.; Wang, D.; Shevyrin, V.A.; Yan, D.; Tang, Z.; et al. DARK Classics in Chemical Neuroscience: Arecoline. ACS Chem. Neurosci. 2019, 10, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.-S. Effects of betel chewing on the central and autonomic nervous systems. J. Biomed. Sci. 2001, 8, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Dhingra, C.; Prasad, S.; Menon, I. Betel nut chewing and its deleterious effects on oral cavity. J. Cancer Res. Ther. 2014, 10, 499–505. [Google Scholar] [PubMed]
- World Health Organization; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer. Betel-Quid and Areca-Nut Chewing and Some Areca-Nut Derived Nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 85; International Agency for Research on Cancer: Lyon, France, 2004. [Google Scholar]
- Chen, Y.Y.; Fang, W.H.; Wang, C.C.; Kao, T.W.; Chang, Y.W.; Yang, H.F.; Wu, C.-J.; Sun, Y.-S.; Chen, W.-L. Detrimental association between betel nut chewing and colorectal polyps in adult populations. PLoS ONE 2018, 13, e0206383. [Google Scholar]
- Khan, M.S.; Bawany, F.I.; Ahmed, M.U.; Hussain, M.; Khan, A.; Lashari, M.N. Betel nut usage is a major risk factor for coronary artery disease. Glob. J. Health Sci. 2013, 6, 189–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, Y.C.; Huang, T.J.; Yeh, M.H.; Lin, M.S.; Chen, M.Y. Lung function impairment and cardiometabolic risks among rural adults: Implication for an aging society. BMC Public Health 2021, 21, 960. [Google Scholar] [CrossRef]
- Wang, T.N.; Huang, M.S.; Lin, M.C.; Duh, T.H.; Lee, C.H.; Wang, C.C.; Chen, P.-H.; Chiang, S.-L.; Sheu, C.-C.; Chen, V.C.-H.; et al. Betel chewing and arecoline affects eotaxin-1, asthma and lung function. PLoS ONE 2014, 9, e91889. [Google Scholar] [CrossRef]
- Kondaiah, P.; Pant, I.; Khan, I. Molecular pathways regulated by areca nut in the etiopathogenesis of oral submucous fibrosis. Periodontology 2000 2019, 80, 213–224. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, J.H.; Chiang, C.W.K.; Hsiung, C.N.; Wu, P.E.; Chang, L.C.; Chu, H.-W.; Chang, J.; Song, I.-W.; Yang, S.-L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef]
- Fan, C.T.; Hung, T.H.; Yeh, C.K. Taiwan Regulation of Biobanks. J. Law Med. Ethics 2015, 43, 816–826. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Kiyingi, K.S. Betel-nut chewing may aggravate asthma. PNG Med. J. 1991, 34, 117–121. [Google Scholar]
- Taylor, R.F.; Al-Jarad, N.; John, L.M.; Conroy, D.M.; Barnes, N.C. Betel-nut chewing and asthma. Lancet 1992, 339, 1134–1136. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L.; Huang, M.S.; Chiang, S.L.; Ko, Y.C. Bronchial epithelium-derived IL-8 and RANTES increased bronchial smooth muscle cell migration and proliferation by Krüppel-like factor 5 in areca nut-mediated airway remodeling. Toxicol. Sci. 2011, 121, 177–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stich, H.F.; Anders, F. The involvement of reactive oxygen species in oral cancers of betel quid/tobacco chewers. Mutat. Res. 1989, 214, 47–61. [Google Scholar] [CrossRef]
- Thomas, S.J.; MacLennan, R. Slaked lime and betel nut cancer in Papua New Guinea. Lancet 1992, 340, 577–578. [Google Scholar] [CrossRef]
- Sazwi, N.N.; Nalina, T.; Abdul Rahim, Z.H. Antioxidant and cytoprotective activities of Piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complement. Altern. Med. 2013, 13, 351. [Google Scholar]
- Sari, E.F.; Prayogo, G.P.; Loo, Y.T.; Zhang, P.; McCullough, M.J.; Cirillo, N. Distinct phenolic, alkaloid and antioxidant profile in betel quids from four regions of Indonesia. Sci. Rep. 2020, 10, 16254. [Google Scholar] [CrossRef]
- Sarode, S.C.; Mahuli, A.; Sarode, G.S.; Mahuli, S. Why only areca nut chewing cannot cause oral submucous fibrosis? Med. Hypotheses 2013, 81, 47–49. [Google Scholar] [CrossRef]
- Pilette, C.; Colinet, B.; Kiss, R.; André, S.; Kaltner, H.; Gabius, H.J.; Delos, M.; Vaerman, J.P.; Decramer, M.; Sibille, Y. Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur. Respir. J. 2007, 29, 914–922. [Google Scholar] [CrossRef]
- Sapey, E.; Stockley, J.A.; Greenwood, H.; Ahmad, A.; Bayley, D.; Lord, J.M.; Insall, R.H.; Stockley, R.A. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 183, 1176–1186. [Google Scholar] [CrossRef]
- Thulborn, S.J.; Mistry, V.; Brightling, C.E.; Moffitt, K.L.; Ribeiro, D.; Bafadhel, M. Neutrophil elastase as a biomarker for bacterial infection in COPD. Respir. Res. 2019, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Dong, L.; Wu, Y.; Shen, H.; Chen, Z. Lipid metabolism in chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon Dis. 2019, 14, 1009–1018. [Google Scholar] [CrossRef]
- Gunay, S.; Sariaydin, M.; Acay, A. New Predictor of Atherosclerosis in Subjects With COPD: Atherogenic Indices. Respir. Care 2016, 61, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Han, F.; Gong, L.; Lv, Y.; Wan, Z.; Liu, H.; Zhang, D.; Jia, Y.; Yang, S.; Ren, L.; et al. Association between chronic obstructive pulmonary disease and serum lipid levels: A meta-analysis. Lipids Health Dis. 2018, 17, 263. [Google Scholar] [CrossRef] [PubMed]
- Kahnert, K.; Alter, P.; Welte, T.; Huber, R.M.; Behr, J.; Biertz, F.; Watz, H.; Bals, R.; Vogelmeier, C.F.; Jörres, R.A. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: A multi-dimensional approach. Respir. Res. 2018, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.R.; Monego, E.T.; Jardim, P.C.; Carvalho, M.M.; Sousa, A.L.; Oliveira, J.S.; Balestra Neto, O. Diet and medication in the treatment of hyperuricemia in hypertensive patients. Arq. Bras. Cardiol. 2001, 76, 463–472. [Google Scholar] [CrossRef]
- Wattanachayakul, P.; Rujirachun, P.; Charoenngam, N.; Ungprasert, P. Chronic obstructive pulmonary disease (COPD) is associated with a higher level of serum uric acid. A systematic review and meta-analysis. Adv. Respir. Med. 2020, 88, 215–222. [Google Scholar] [CrossRef]
- Nagao, H.; Nishizawa, H.; Tanaka, Y.; Fukata, T.; Mizushima, T.; Furuno, M.; Bamba, T.; Tsushima, Y.; Fujishima, Y.; Kita, S.; et al. Hypoxanthine Secretion from Human Adipose Tissue and its Increase in Hypoxia. Obesity 2018, 26, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.C. Purine release and inhibition of synaptic transmission during hypoxia and hypoglycemia in rat hippocampal slices. Neurosci. Lett. 1993, 157, 83–86. [Google Scholar] [CrossRef]
- Shaaban, O.G.; Abd El Razik, H.A.; A Shams El-Dine, S.E.; Ashour, F.A.; El-Tombary, A.A.; Afifi, O.S.; Abu-Serie, M.M. Purines and triazolo[4,3-e]purines containing pyrazole moiety as potential anticancer and antioxidant agents. Future Med. Chem. 2018, 10, 1449–1464. [Google Scholar] [CrossRef]
- Hoke, T.S.; Douglas, I.S.; Klein, C.L.; He, Z.; Fang, W.; Thurman, J.M.; Tao, Y.; Dursun, B.; Voelkel, N.F.; Edelstein, C.L.; et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J. Am. Soc. Nephrol. 2007, 18, 155–164. [Google Scholar] [CrossRef]
- Ambruso, S.L.; Gil, H.W.; Fox, B.; Park, B.; Altmann, C.; Bagchi, R.A.; Baker, P.R., II; Reisz, J.A.; Faubel, S. Lung metabolomics after ischemic acute kidney injury reveals increased oxidative stress, altered energy production, and ATP depletion. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L50–L64. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Slutsky, A.S.; Ronco, C. Lung-Kidney Cross-Talk in the Critically Ill Patient. Am. J. Respir. Crit. Care Med. 2016, 194, 402–414. [Google Scholar]
- Klein, C.L.; Hoke, T.S.; Fang, W.F.; Altmann, C.J.; Douglas, I.S.; Faubel, S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008, 74, 901–909. [Google Scholar] [CrossRef]
- Ahuja, N.; Andres-Hernando, A.; Altmann, C.; Bhargava, R.; Bacalja, J.; Webb, R.G.; He, Z.; Edelstein, C.L.; Faubel, S. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am. J. Physiol. Renal Physiol. 2012, 303, F864–F872. [Google Scholar] [CrossRef]
- Lie, M.L.; White, L.E.; Santora, R.J.; Park, J.M.; Rabb, H.; Hassoun, H.T. Lung T lymphocyte trafficking and activation during ischemic acute kidney injury. J. Immunol. 2012, 189, 2843–2851. [Google Scholar] [CrossRef]
- Miranda Machado, P.A.; Baños Álvarez, I.; Gaitán Duarte, H.G. Association between anemia and chronic obstructive pulmonary disease exacerbations in Cartagena Colombia: A prospective cohort study. Medwave 2019, 19, e7602. [Google Scholar] [CrossRef]
- Ergan, B.; Ergün, R. Impact of anemia on short-term survival in severe COPD exacerbations: A cohort study. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 1775–1783. [Google Scholar] [CrossRef]
- Boutou, A.K.; Hopkinson, N.S.; Polkey, M.I. Anaemia in chronic obstructive pulmonary disease: An insight into its prevalence and pathophysiology. Clin. Sci. 2015, 128, 283–295. [Google Scholar] [CrossRef]
- John, M.; Hoernig, S.; Doehner, W.; Okonko, D.D.; Witt, C.; Anker, S.D. Anemia and inflammation in COPD. Chest 2005, 127, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | FEV1/FVC ≥ 70% (n = 65,199) | FEV1/FVC < 70% (n = 15,678) | p-Value |
|---|---|---|---|
| Age (year) | 49.7 ± 10.9 | 50.6 ± 11.0 | <0.001 |
| Male gender (%) | 37.7 | 35.4 | <0.001 |
| Smoking history (%) | 28.4 | 27.8 | 0.167 |
| DM (%) | 5.0 | 5.1 | 0.601 |
| Hypertension (%) | 12.0 | 12.5 | 0.090 |
| Asthma (%) | 4.2 | 3.6 | 0.001 |
| Emphysema or bronchitis (%) | 1.2 | 1.5 | 0.011 |
| Betel nut chewing history (%) | 6.3 | 6.7 | 0.076 |
| Regular exercise habit (%) | 40.4 | 43.8 | <0.001 |
| BMI (kg/m2) | 24.3 ± 3.8 | 23.9 ± 3.6 | <0.001 |
| Laboratory parameters | |||
| White blood cell (*103/uL) | 5.8 ± 1.6 | 5.9 ± 1.6 | <0.001 |
| Hemoglobin (g/dL) | 13.8 ± 1.6 | 13.7 ± 1.6 | <0.001 |
| Fasting glucose (mg/dL) | 95.9 ± 20.1 | 95.5 ± 20.3 | 0.061 |
| Triglyceride (mg/dL) | 117.1 ± 93.8 | 112.2 ± 83.6 | <0.001 |
| Total cholesterol (mg/dL) | 195.8 ± 35.9 | 195.1 ± 35.0 | 0.034 |
| HDL-C (mg/dL) | 54.5 ± 13.5 | 54.4 ± 13.3 | 0.511 |
| LDL-C (mg/dL) | 121.1 ± 31.8 | 120.9 ± 31.4 | 0.542 |
| eGFR (mL/min/1.73 m2) | 35.3 ± 46.8 | 33.3 ± 46.5 | <0.001 |
| Uric acid (mg/dL) | 5.4 ± 1.4 | 5.4 ± 1.4 | 0.584 |
| Lung function | |||
| FVC (L) | 2.83 ± 0.80 | 2.75 ± 0.84 | <0.001 |
| FEV1 (L) | 2.48 ± 0.71 | 1.40 ± 0.57 | <0.001 |
| FEV1/FVC (%) | 87.8 ± 7.1 | 50.9 ± 13.7 | <0.001 |
| Variables | Multivariable (FEV1/FVC < 70%) | |
|---|---|---|
| Odds Ratio (95% CI) | p-Value | |
| Betel nut chewing history | 1.159 (1.072–1.254) | <0.001 |
| Age (per 1 year) | 1.007 (1.006–1.009) | <0.001 |
| Male (vs. female) | 0.645 (0.550–0.755) | <0.001 |
| Smoking history | 1.017 (0.970–1.067) | 0.482 |
| DM | 0.982 (0.896–1.077) | 0.700 |
| Hypertension | 1.042 (0.984–1.104) | 0.163 |
| Asthma | 0.857 (0.781–0.941) | 0.001 |
| Emphysema or bronchitis | 1.190 (1.025–1.383) | 0.022 |
| Regular exercise habit | 1.107 (1.066–1.150) | <0.001 |
| BMI (per 1 kg/m2) | 0.961 (0.955–0.966) | <0.001 |
| Laboratory parameters | ||
| White blood cell (per 1 × 103/uL) | 1.080 (1.068–1.092) | <0.001 |
| Hemoglobin (per 1 g/dL) | 0.967 (0.953–0.981) | <0.001 |
| Fasting glucose (per 10 mg/dL) | 1.000 (0.999–1.001) | 0.694 |
| Triglyceride (per 10 mg/dL) | 0.999 (0.998–0.999) | <0.001 |
| Total cholesterol (per 10 mg/dL) | 1.002 (1.000–1.005) | 0.034 |
| HDL-C (per 10 mg/dL) | 0.991 (0.988–0.994) | <0.001 |
| LDL-C (per 10 mg/dL) | 0.998 (0.996–1.000) | 0.052 |
| eGFR (per 1 mL/min/1.73 m2) | 1.003 (1.002–1.005) | <0.001 |
| Uric acid (per 1 mg/dL) | 1.053 (1.036–1.070) | <0.001 |
| Betel Nut Chewing Characteristics | FEV1/FVC ≥ 70% (n = 4103) | FEV1/FVC < 70% (n = 1032) | p-Value |
|---|---|---|---|
| Years of chewing betel nut (years) | 14.0 ± 11.1 | 15.1± 10.9 | 0.002 |
| How often do you chew betel nut? | 0.012 | ||
| 1–3 days/month (score = 1) | 7.4 | 4.5 | |
| 1,2 days/week (score = 2) | 15.4 | 11.8 | |
| 3–5 days/week (score = 3) | 16.2 | 13.8 | |
| Everyday (score = 4) | 61.0 | 69.9 | |
| How many betel nuts a day? | 0.005 | ||
| <10 quids (score = 1) | 41.1 | 31.2 | |
| 10–20 quids (score = 2) | 34.2 | 37.4 | |
| 21–30 quids (score = 3) | 11.1 | 13.8 | |
| ≥31 quids (score = 4) | 13.6 | 17.7 | |
| Cumulative dose (year × frequency × daily) | 119.6 ± 132.7 | 148.2 ± 148.2 | 0.001 |
| Variables | Multivariable (FEV1/FVC < 70%) | |
|---|---|---|
| Odds Ratio (95% CI) | p-Value | |
| Years of chewing betel nut (per 1 year) # | 1.008 (1.002–1.014) | 0.012 |
| How often do you chew betel nut? # | ||
| 1–3 days/month (score = 1) | Reference | |
| 1, 2 days/week (score = 2) | 1.245 (0.668–2.323) | 0.490 |
| 3–5 days/week (score = 3) | 1.346 (0.729–2.484) | 0.342 |
| Everyday (score = 4) | 1.793 (1.038–3.097) | 0.036 |
| How many betel nuts a day? # | ||
| <10 quids (score = 1) | Reference | |
| 10–20 quids (score = 2) | 1.404 (1.058–1.862) | 0.019 |
| 21–30 quids (score = 3) | 1.662 (1.130–2.446) | 0.010 |
| ≥31 quids (score = 4) | 1.717 (1.199–2.459) | 0.003 |
| Cumulative dose (per 1 year × frequency × daily) # | 1.001 (1.001–1.002) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Geng, J.-H.; Wu, D.-W.; Chen, S.-C.; Hung, C.-H.; Kuo, C.-H. Betel Nut Chewing Was Associated with Obstructive Lung Disease in a Large Taiwanese Population Study. J. Pers. Med. 2021, 11, 973. https://doi.org/10.3390/jpm11100973
Huang C-H, Geng J-H, Wu D-W, Chen S-C, Hung C-H, Kuo C-H. Betel Nut Chewing Was Associated with Obstructive Lung Disease in a Large Taiwanese Population Study. Journal of Personalized Medicine. 2021; 11(10):973. https://doi.org/10.3390/jpm11100973
Chicago/Turabian StyleHuang, Chao-Hsin, Jiun-Hung Geng, Da-Wei Wu, Szu-Chia Chen, Chih-Hsing Hung, and Chao-Hung Kuo. 2021. "Betel Nut Chewing Was Associated with Obstructive Lung Disease in a Large Taiwanese Population Study" Journal of Personalized Medicine 11, no. 10: 973. https://doi.org/10.3390/jpm11100973
APA StyleHuang, C.-H., Geng, J.-H., Wu, D.-W., Chen, S.-C., Hung, C.-H., & Kuo, C.-H. (2021). Betel Nut Chewing Was Associated with Obstructive Lung Disease in a Large Taiwanese Population Study. Journal of Personalized Medicine, 11(10), 973. https://doi.org/10.3390/jpm11100973






