PRP and PRF—Subgroups and Divisions When Used in Dentistry
Abstract
:1. Introduction to PRP and PRF Subgroup Divisions
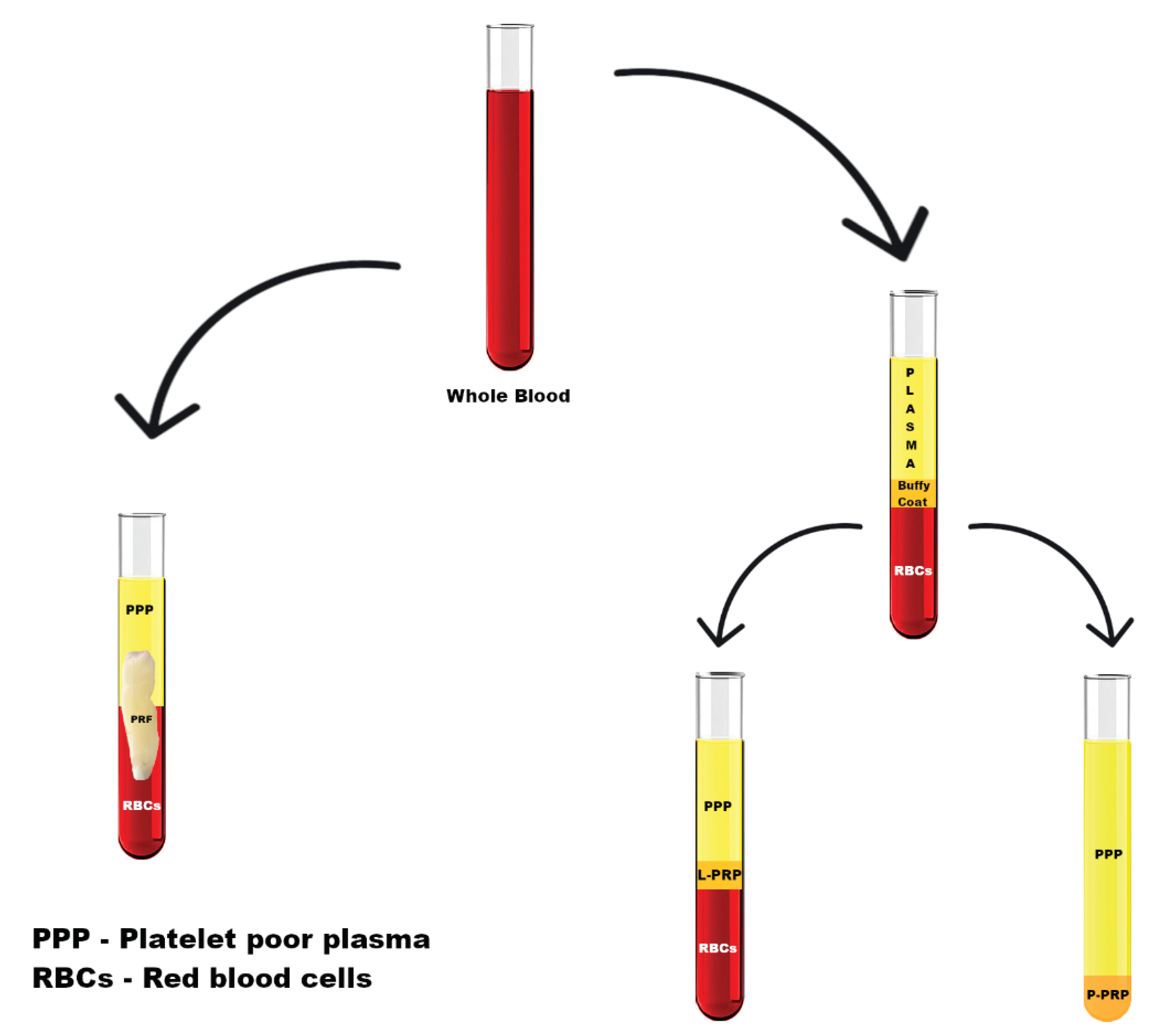
- Platelet-rich plasma (PRP):
- a)
- Pure platelet-rich plasma (P-PRP);
- b)
- Leukocyte- and platelet-rich plasma (L-PRP).
- 2.
- Platelet-rich fibrin (PRF):
- a)
- Pure platelet-rich fibrin (P-PRF);
- b)
- Leukocyte- and platelet-rich fibrin (L-PRF);
- c)
- Injectable PRF (I-PRF).
Molecular Basis of PRP and PRF Methods
2. Materials and Methods
3. Leucocyte Platelet-Rich Fibrin (L-PRF)
4. Use of Platelet-Rich Plasma in Personalized Dentistry
4.1. Description of Pure Platelet-Rich Plasma (P-PRP) Use in Dentistry
4.2. Use of Leucocyte Platelet-Rich Plasma (L-PRP) in Dentistry
5. Injectable Platelet-Rich Fibrin (I-PRF) and Personalized Dental Procedures
6. Regenerative Treatments with the Use of PRP and PRF in Dentistry
6.1. PRP in Gingival Fibroblast Proliferation—Tissue Regeneration
6.2. Regenerative Endodontic Treatment (RET) and Blood Derivatives
6.3. PRP and PRF in Intrabony Defect Treatment
7. Other Use
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varshney, S.; Dwivedi, A.; Pandey, V. Antimicrobial effects of various platelet rich concentrates-vibes from in-vitro studies-a systematic review. J. Oral Biol. Craniofacial Res. 2019, 9, 299–305. [Google Scholar] [CrossRef]
- Mariani, E.; Filardo, G.; Canella, V.; Berlingeri, A.; Bielli, A.; Cattini, L.; Landini, M.P.; Kon, E.; Marcacci, M.; Facchini, A. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy 2014, 16, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Preeja, C.; Arun, S. Platelet-rich fibrin: Its role in periodontal regeneration. Saudi J. Dent. Res. 2014, 5, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Cieslik-Bielecka, A.; Dohan Ehrenfest, D.M.; Lubkowska, A.; Bielecki, T. Microbicidal properties of Leukocyte- and Platelet-Rich Plasma\Fibrin: New Perspectives. J. Biol. Regul. Homeost. Agents 2012, 26, 42–52. [Google Scholar]
- Crisci, A.; De Crescenzo, U.; Crisci, M. Platelet-rich concentrates (L-PRF, PRP) in tissue regeneration: Control of apoptosis and interactions with regenerative cells. J. Clin. Mol. Med. 2018, 1, 1000116. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Yuan, Q.; Mao, L.; Chen, F.-L.; Ji, F.; Cui, Z.-H. Vitamin D deficiency causes insulin resistance by provoking oxidative stress in hepatocytes. Oncotarget 2017, 8, 67605–67613. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilian, O.; Flesch, I.; Wenisch, S.; Taborski, B.; Jork, A.; Schnettler, R.; Jonuleit, T. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur. J. Med. Res. 2004, 9, 337–344. [Google Scholar]
- Fréchette, J.P.; Martineau, I.; Gagnon, G. Platelet-rich plasmas: Growth factor content and roles in wound healing. J. Dent. Res. 2005, 84, 434–439. [Google Scholar] [CrossRef]
- Kour, P.; Pudakalkatti, P.S.; Vas, A.M.; Das, S.; Padmanabhan, S. Comparative Evaluation of Antimicrobial Efficacy of Platelet-rich Plasma, Platelet-rich Fibrin, and Injectable Platelet-rich Fibrin on the Standard Strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans Abstract. Contemp. Clin. Dent. 2017, 8, 538–544. [Google Scholar] [CrossRef]
- Lv, H.; Chen, Y.; Cai, Z.; Lei, L.; Zhang, M.; Zhou, R.; Huang, X. The efficacy of platelet-rich fibrin as a scaffold in regenerative endodontic treatment: A retrospective controlled cohort study. BMC Oral Health 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Ivanovski, S.; Cei, S.; Ducci, F.; Tonetti, M.; Gabriele, M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral Implant. Res. 2006, 17, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.A.; Pham, T.A.V. Effects of platelet-rich plasma on human gingival fibroblast proliferation and migration in vitro. J. Appl. Oral Sci. 2018, 26, e20180077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagl, A.; Bedi, S.; Hassan, K.; AlHumaid, J. Use of platelet-rich plasma for regeneration in non-vital immature permanent teeth: Clinical and cone-beam computed tomography evaluation. J. Int. Med. Res. 2017, 45, 583–593. [Google Scholar] [CrossRef]
- Rattanasuwan, K.; Rassameemasmaung, S.; Kiattavorncharoen, S.; Sirikulsathean, A.; Thorsuwan, J.; Wongsankakorn, W. Platelet-rich plasma stimulated proliferation, migration, and attachment of cultured periodontal ligament cells. Eur. J. Dent. 2017, 11, 192–195. [Google Scholar] [CrossRef]
- Qiao, J.; Duan, J.; Zhang, Y.; Chu, Y.; Sun, C. The effect of concentrated growth factors in the treatment of periodontal intrabony defects. Futur. Sci. OA 2016, 2, FS136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahabi, S.; Vaziri, S.; Torshabi, M.; Rezaei Esfahrood, Z.J. Effects of plasma rich in growth factors and platelet-rich fibrin on proliferation and viability of human gingival fibroblasts. J. Dent. 2015, 12, 504–512. [Google Scholar]
- Yu, B.; Wang, Z. Effect of concentrated growth factors on beagle periodontal ligament stem cells in vitro. Mol. Med. Rep. 2014, 9, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.; Gronthos, S.; Shi, S.; Bartold, P.M. A method to isolate, purify, and characterize human periodontal ligament stem cells. Methods Mol. Biol. 2017, 1537, 413–427. [Google Scholar] [CrossRef]
- Matsuda, N.; Lin, W.L.; Kumar, N.M.; Cho, M.I.; Genco, R.J. Mitogenic, Chemotactic, and Synthetic Responses of Rat Periodontal Ligament Fibroblastic Cells to Polypeptide Growth Factors In Vitro. J. Periodontol. 1992, 63, 515–525. [Google Scholar] [CrossRef]
- Nishimura, F.; Terranova, V.P. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J. Dent. Res. 1996, 75, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Tysiąc-Miśta, M.; Bulanda, S.; Gruca, O.; Wyszyńska, M.; Kasperski, J. Platelet-rich fibrin—New course in regenerative medicine and dentistry. Protet. Stomatol. 2019, 69, 444–451. [Google Scholar] [CrossRef]
- Crisci, A.; Manfredi, S.; Crisci, M. Fibrin Rich in Leukocyte-Platelets (L-PRF) and Injectable Fibrin Rich Platelets (i-PRF), Two Opportunity in Regenerative Surgery: Review of The Sciences and Literature. IOSR J. Dent. Med. Sci. 2019, 18, 66–79. [Google Scholar] [CrossRef]
- Bolhari, B.; Meraji, N.; Ghorbanzadeh, A.; Sarraf, P.; Moayeri, R. Applications of Fibrin-based products in Endodontics: A Literature Review. Dent. Hypotheses 2019, 10, 85–90. [Google Scholar] [CrossRef]
- Badade, P.S.; Mahale, S.A.; Panjwani, A.A.; Vaidya, P.D.; Warang, A.D. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2016, 27, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Karde, P.A.; Sethi, K.S.; Mahale, S.A.; Khedkar, S.U.; Patil, A.G.; Joshi, C.P. Comparative evaluation of platelet count and antimicrobial efficacy of injectable platelet-rich fibrin with other platelet concentrates: An in vitro study. J. Indian Soc. Periodontol. 2017, 21, 97–101. [Google Scholar] [CrossRef]
- Przadka, P.; Kiełbowicz, Z.; Skrzypczak, P. Autogenne osocze bogatopłytkowe—Rodzaje, sposoby aktywacji i zastosowanie. Med. Weter. 2016, 72, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich Concentrates Differentially Release Growth Factors and Induce Cell Migration In Vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielecki, T.M.; Gazdzik, T.S.; Arendt, J.; Szczepanski, T.; Król, W.; Wielkoszynski, T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: An in vitro study. J. Bone Jt. Surg.—Ser. B 2007, 89, 417–420. [Google Scholar] [CrossRef]
- Yang, L.C.; Hu, S.W.; Yan, M.; Yang, J.J.; Tsou, S.H.; Lin, Y.Y. Antimicrobial Activity of Platelet-Rich Plasma and Other Plasma Preparations Against Periodontal Pathogens. J. Periodontol. 2015, 86, 310–318. [Google Scholar] [CrossRef]
- Pham, T.A.V.; Tran, T.T.P.; Luong, N.T.M. Antimicrobial Effect of Platelet-Rich Plasma against Porphyromonas gingivalis. Int. J. Dent. 2019, 2019, 7329103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Ameer, L.A.; Raheem, Z.J.; Abdulrazaq, S.S.; Ali, B.G.; Nasser, M.M.; Khairi, A.W.A. The anti-inflammatory effect of the platelet-rich plasma in the periodontal pocket. Eur. J. Dent. 2018, 11, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Moojen, D.J.F.; Everts, P.A.M.; Schure, R.M.; Overdevest, E.P.; Van Zundert, A.; Knape, J.T.A.; Castelein, R.M.; Creemers, L.B.; Dhert, W.J.A. Antimicrobial activity of platelet-leukocyte gel against staphylococcus aureus. J. Orthop. Res. 2008, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Intravia, J.; Allen, D.A.; Durant, T.J.S.; McCarthy, M.B.R.; Russell, R.; Beitzel, K.; Cote, M.P.; Dias, F.; Mazzocca, A.D. In vitro evaluation of the anti-bacterial effect of two preparations of platelet rich plasma compared with Cefazolin and whole blood. Muscles Ligaments Tendons J. 2014, 4, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Prax, M.; Bekeredjian-Ding, I.; Krut, O. Microbiological Screening of Platelet Concentrates in Europe. Transfus. Med. Hemotherapy 2019, 46, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.J.; Wicaksana, A.; Davidson, D.; Spratt, D.; Mosahebi, A. An evaluation of the bacteriostatic effect of platelet-rich plasma. Int. Wound J. 2021, 18, 448–456. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-PRF or PRP. Int. J. Mol. Sci. 2017, 18, 331. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Santhakumar, M.; Yayathi, S.; Retnakumari, N. A clinicoradiographic comparison of the effects of platelet-rich fibrin gel and platelet-rich fibrin membrane as scaffolds in the apexification treatment of young permanent teeth. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 65–70. [Google Scholar] [CrossRef]
- Nikolidakis, D.; Jansen, J.A. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng.-Part B Rev. 2008, 14, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Hojjati, S.; Sattari, M.; Ghasemi, T.; Ahmadi, R.; Mashayekhi, A. Effect of platelet-rich plasma concentrations on the proliferation of periodontal cells: An in vitro study. Eur. J. Dent. 2016, 10, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creeper, F.; Ivanovski, S. Effect of autologous and allogenic platelet-rich plasma on human gingival fibroblast function. Oral Dis. 2012, 18, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meng, H.X.; Tang, J.M.; Li, S.L.; Tang, Y.; Chen, Z.B. The effect of different platelet-rich plasma concentrations on proliferation and differentiation of human periodontal ligament cells in vitro. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 8411–8413. [Google Scholar] [CrossRef]
- Soffer, E.; Ouhayoun, J.P.; Dosquet, C.; Meunier, A.; Anagnostou, F. Effects of platelet lysates on select bone cell functions. Clin. Oral Implant. Res. 2004, 15, 581–588. [Google Scholar] [CrossRef]
- Hsu, C.W.; Yuan, K.; Tseng, C.C. The negative effect of platelet-rich plasma on the growth of human cells is associated with secreted thrombospondin-1. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, 185–192. [Google Scholar] [CrossRef]
- Okuda, K.; Kawase, T.; Momose, M.; Murata, M.; Saito, Y.; Suzuki, H.; Wolff, L.F.; Yoshie, H. Platelet-Rich Plasma Contains High Levels of Platelet-Derived Growth Factor and Transforming Growth Factor-β and Modulates the Proliferation of Periodontally Related Cells In Vitro. J. Periodontol. 2003, 74, 849–857. [Google Scholar] [CrossRef]
- Creeper, F.; Lichanska, A.M.; Marshall, R.I.; Seymour, G.J.; Ivanovski, S. The effect of platelet-rich plasma on osteoblast and periodontal ligament cell migration, proliferation and differentiation. J. Periodontal Res. 2009, 44, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, M.H.; Gul, M.; Ghafoor, R.; Badar, S.B. Management of immature necrotic permanent teeth with regenerative endodontic procedures—A review of literature. J. Pak. Med. Assoc. 2019, 69, 1514–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.M.; Yang, S.F.; Zhao, J.H.; Chang, Y.C. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J. Endod. 2010, 36, 1628–1632. [Google Scholar] [CrossRef]
- Narang, I.; Mittal, N.; Mishra, N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: A clinical study. Contemp. Clin. Dent. 2015, 6, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, V.Y.; Johns, D.A.; Maroli, R.K.; Sekar, M.; Chandrasekaran, R.; Karthikeyan, S.; Renganathan, S.K. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: A triple blind randomized clinical trial. J. Clin. Diagn. Res. 2017, 11, ZC34–ZC39. [Google Scholar] [CrossRef]
- Murray, P.E. Platelet-rich plasma and platelet-rich fibrin can induce apical closure more frequently than blood-clot revascularization for the regeneration of immature permanent teeth: A meta-analysis of clinical efficacy. Front. Bioeng. Biotechnol. 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Mittal, N. A comparative evaluation of natural and artificial scaffolds in regenerative endodontics: A clinical study. Saudi Endod. J. 2016, 6, 9–15. [Google Scholar] [CrossRef]
- Bindal, P.; Kasim, N.H.A.; Ramasamy, T.S.; Dabbagh, A.; Moharamzadeh, K.; Chai, W.L. Dental Pulp Tissue Engineering and Regenerative Endodontic Therapy; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081009673. [Google Scholar]
- Bezgin, T.; Yilmaz, A.D.; Celik, B.N.; Kolsuz, M.E.; Sonmez, H. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. J. Endod. 2015, 41, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, V.Y.; Johns, D.A.; Vidyanath, S.; Kumar, M.R. Platelet Rich Fibrin in the revitalization of tooth with necrotic pulp and open apex. J. Conserv. Dent. 2012, 15, 395–398. [Google Scholar] [PubMed] [Green Version]
- Annunziata, M.; Piccirillo, A.; Perillo, F.; Cecoro, G.; Nastri, L.; Guida, L. Enamel matrix derivative and autogenous bone graft for periodontal regeneration of intrabony defects in humans: A systematic review and meta-analysis. Materials 2019, 12, 2634. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Chug, A.; Mahesh, L.; Singh, S.; Singh, K. Optimal management of intrabony defects: Current insights. Clin. Cosmet. Investig. Dent. 2019, 11, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.S.; Feng, Z.H.; Wu, G.F.; Bai, S.Z.; Dong, Y.; Chen, F.M.; Zhao, Y.M. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, C.; Huang, S.; Wu, X.; Zhao, Y.; Pan, C.; Wang, H.; Liu, J.; Li, Q.; Kou, Y. Efficacy of Adjunctive Bioactive Materials in the Treatment of Periodontal Intrabony Defects: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2018, 2018, 8670832. [Google Scholar] [CrossRef] [PubMed]
- Goyal, J.; Sachdeva, S.; Salaria, S.K.; Vakil, N.; Mittal, A. Comparative assessment of periodontal regeneration in periodontal intraosseous defects treated with PepGen P-15 unaided or in blend with platelet-rich fibrin: A clinical and high-resolution computed tomography scan-assisted volumetric analysis. J. Indian Soc. Periodontol. 2020, 24, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, Z.; Ali, J.; Baboota, S.; Talegaonkar, S.; Ahmad, Z.; Sahni, J.K. Treatment modalities and evaluation models for periodontitis. Int. J. Pharm. Investig. 2012, 2, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dassatti, L.; Manicone, P.F.; Lauricella, S.; Pastorino, R.; Filetici, P.; Nicoletti, F.; D’Addona, A. A comparative scanning electron microscopy study between the effect of an ultrasonic scaler, reciprocating handpiece, and combined approach on the root surface topography in subgingival debridement. Clin. Exp. Dent. Res. 2020, 6, 470–477. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.; Ma, Z.; Shi, H.; Zhang, Y.; Wu, M.; Cui, W. Clinical effectiveness of Er: YAG lasers adjunct to scaling and root planing in non-surgical treatment of chronic periodontitis: A meta-analysis of Randomized Controlled Trials. Med. Sci. Monit. 2018, 24, 7090–7099. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Garg, V.; Kanoriya, D.; Singhal, S. 1.2% Rosuvastatin Versus 1.2% Atorvastatin Gel Local Drug Delivery and Redelivery in Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Placebo-Controlled Clinical Trial. J. Periodontol. 2016, 87, 756–762. [Google Scholar] [CrossRef]
- Sezgin, Y.; Uraz, A.; Taner, I.L.; Çulhaoğlu, R. Effects of platelet-rich fibrin on healing of intra-bony defects treated with anorganic bovine bone mineral. Braz. Oral Res. 2017, 31, e15. [Google Scholar] [CrossRef] [Green Version]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: Intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Tewari, S.; Narula, S.C.; Sharma, R.K.; Tanwar, N. Platelet-rich fibrin along with a modified minimally invasive surgical technique for the treatment of intrabony defects: A randomized clinical trial. J. Periodontal Implant. Sci. 2019, 49, 355–365. [Google Scholar] [CrossRef]
- Panda, S.; Doraiswamy, J.; Malaiappan, S.; Varghese, S.S.; Del Fabbro, M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2016, 7, 13–26. [Google Scholar] [CrossRef]
- Du, J.; Mei, S.; Guo, L.; Su, Y.; Wang, H.; Liu, Y.; Zhao, Z.; Wang, S.; Liu, Y. Platelet-rich fibrin/aspirin complex promotes alveolar bone regeneration in periodontal defect in rats. J. Periodontal Res. 2018, 53, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 25, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef] [PubMed]
| Authors | Substance/Division into Groups | Effects |
|---|---|---|
| Lv et al. [11] | PRF | No significant difference between PRF and the technique of inducing bleeding. |
| Narang et al. [51] | Blood clot (group II) PRF (group III) PRP transferred to collagen (group IV) | Periapical healing No statistically significant difference between groups II and IV; the best result was found for group III (98%). Apical closure No statistically significant difference between groups II–IV. Root lengthening No statistically significant difference between groups II and IV; the best result was found for group II (99%). Dental wall thickness No statistically significant difference between groups II and IV despite the better result in group 2; the best result was found for group III (60%). |
| Shivashankar et al. [52] | PRF (group A) Induced bleeding (group B) PRP (group C) | After 3 months: No statistically significant difference between groups A–C. After 12 months: The best results in group C. |
| Murray [53] | PRP PRF BCR (blood clot) | After 12 months: Periapical lesion No statistically significant difference between the groups. Apical closure Higher effectiveness of PRP and PRF than BCR. Root lengthening No statistically significant difference between the groups. Dental wall thickness No statistically significant difference between the groups. |
| Sharma et al. [54] | Blood clot (group I) PRF (group II) Collagen (group III) PLGA (group IV) | Periapical healing Statistically significant difference between groups II–IV; the best result was found for group II (75%), while the worst result was for group IV. Apical closure, root lengthening, dentinal wall thickening No statistically significant difference between the groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF—Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. https://doi.org/10.3390/jpm11100944
Pietruszka P, Chruścicka I, Duś-Ilnicka I, Paradowska-Stolarz A. PRP and PRF—Subgroups and Divisions When Used in Dentistry. Journal of Personalized Medicine. 2021; 11(10):944. https://doi.org/10.3390/jpm11100944
Chicago/Turabian StylePietruszka, Paulina, Izabela Chruścicka, Irena Duś-Ilnicka, and Anna Paradowska-Stolarz. 2021. "PRP and PRF—Subgroups and Divisions When Used in Dentistry" Journal of Personalized Medicine 11, no. 10: 944. https://doi.org/10.3390/jpm11100944
APA StylePietruszka, P., Chruścicka, I., Duś-Ilnicka, I., & Paradowska-Stolarz, A. (2021). PRP and PRF—Subgroups and Divisions When Used in Dentistry. Journal of Personalized Medicine, 11(10), 944. https://doi.org/10.3390/jpm11100944








