On the Necessity of a Customized Knee Spacer in Peri-Prosthetic Joint Infection Treatment: 3D Numerical Simulation Results
Abstract
:1. Introduction
2. Materials and Methods
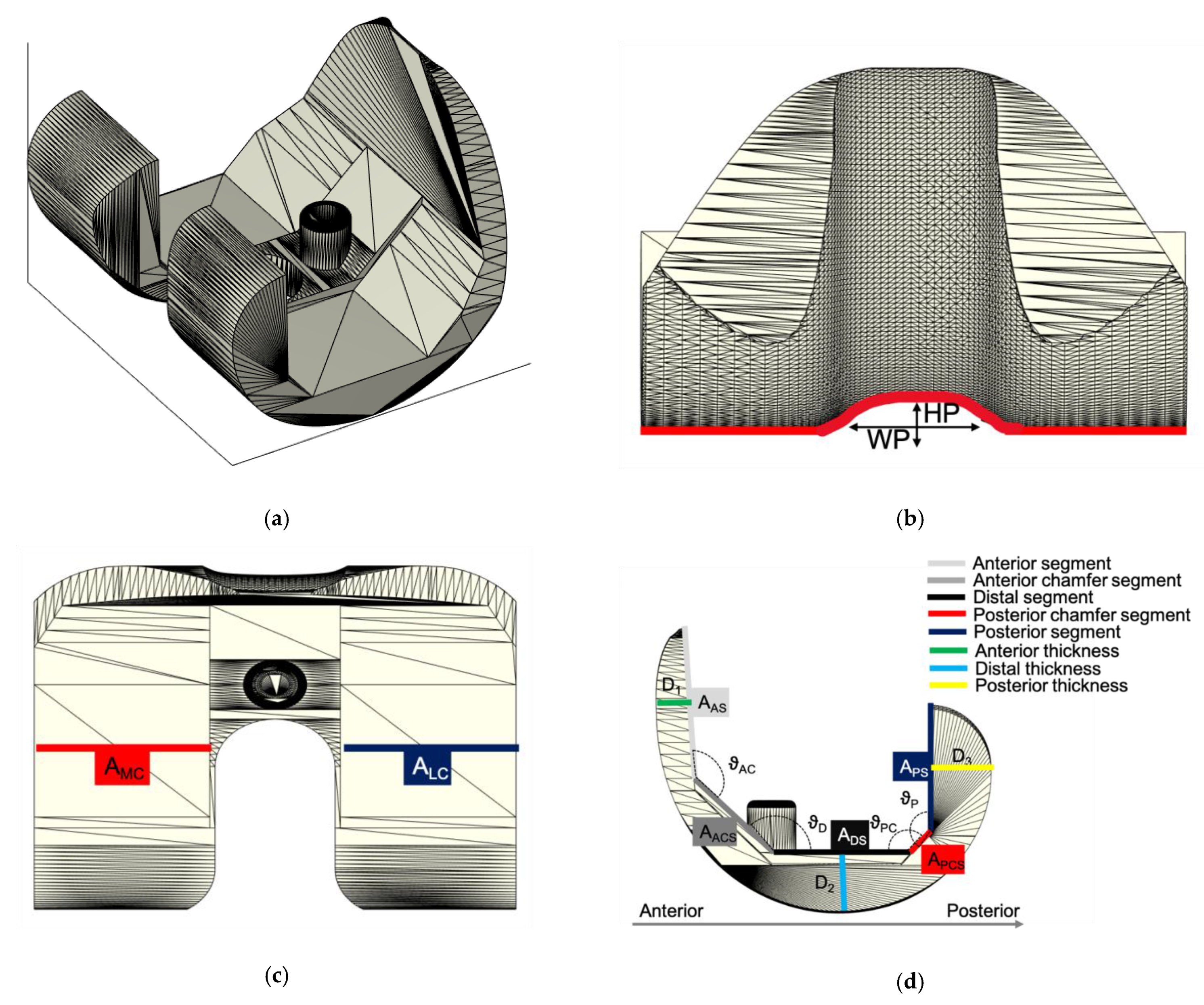
2.1. ALPMMA Spacer 3D Modeling
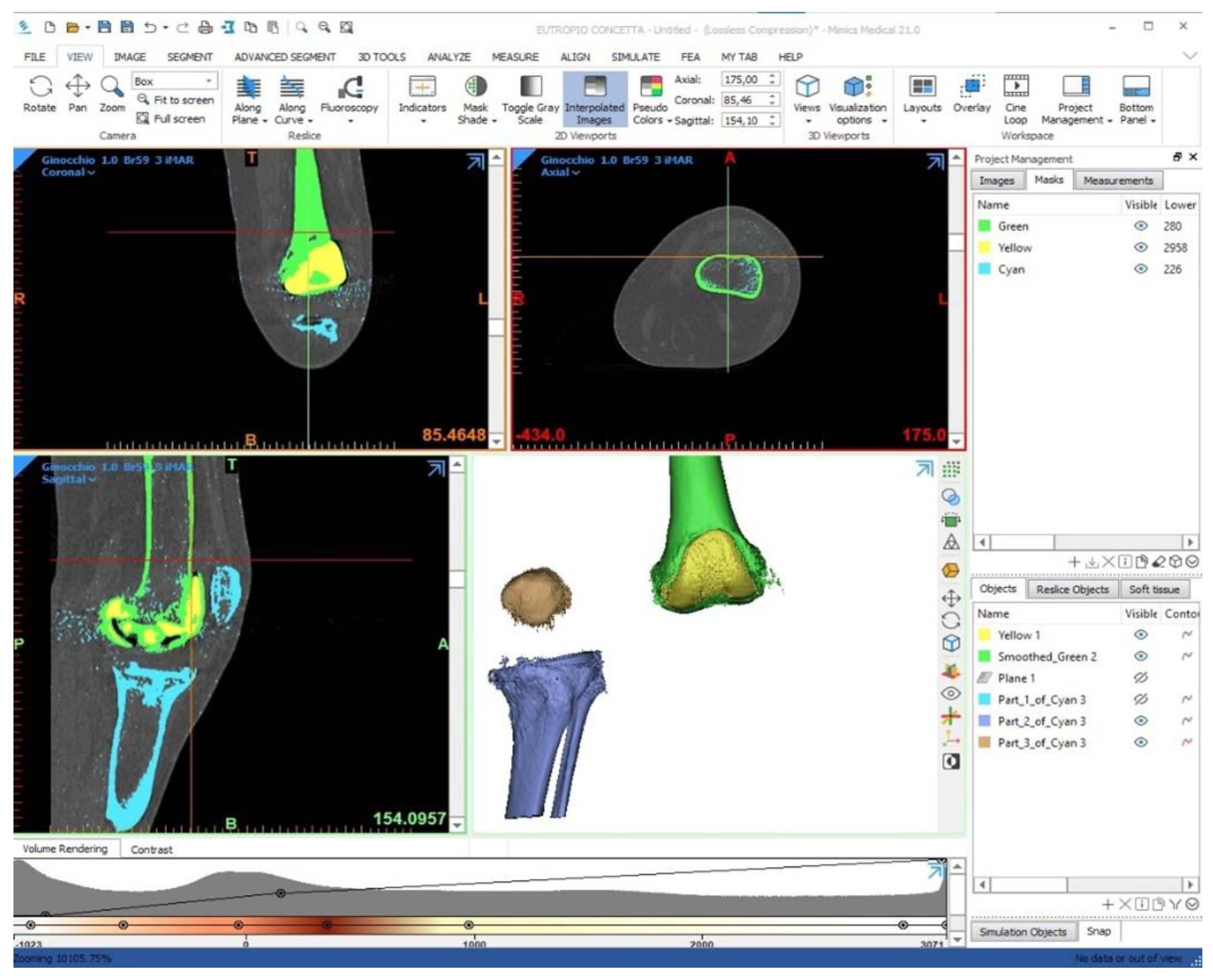
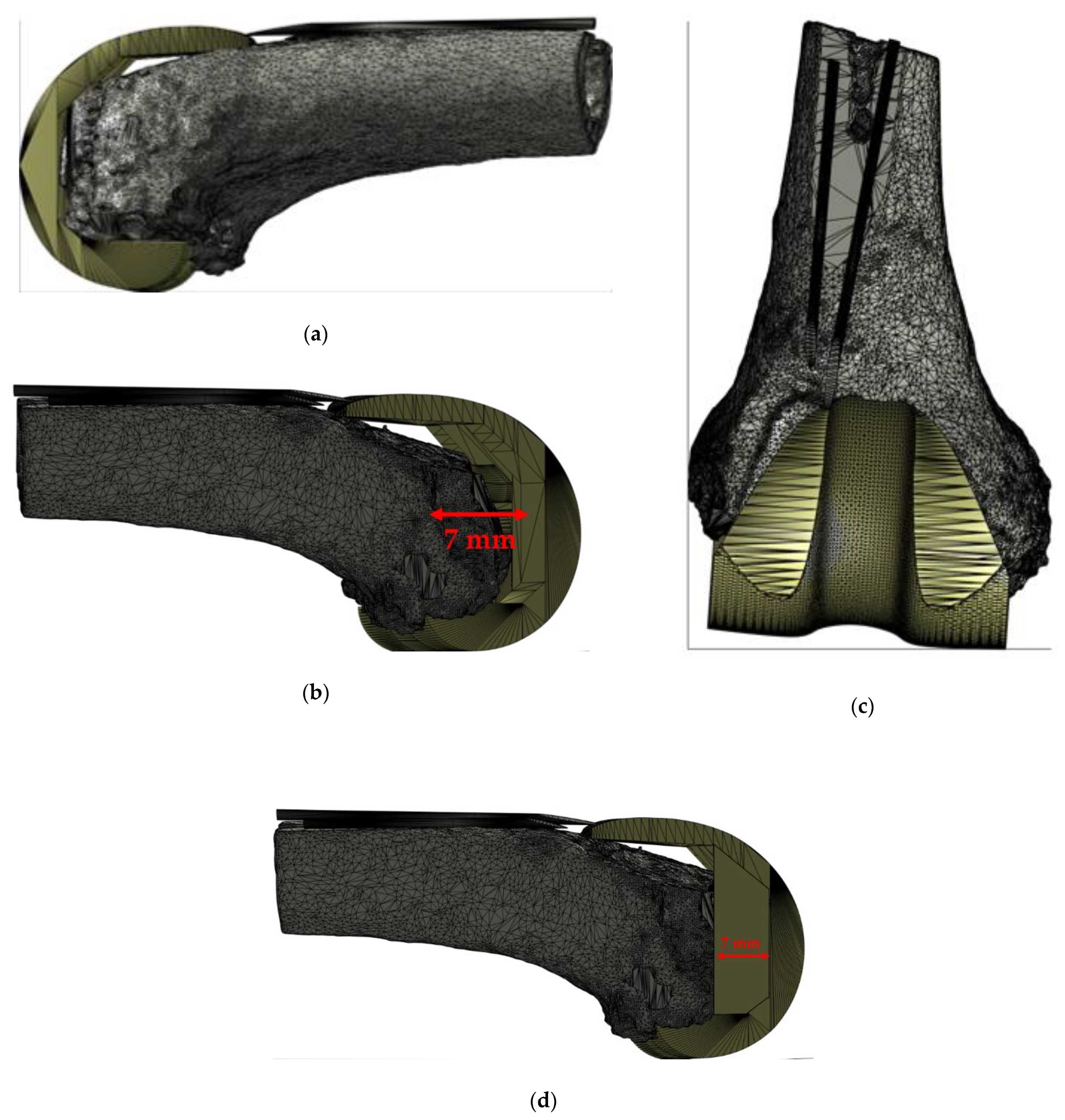
2.2. Patient Data Management “3D Bone Model”
2.3. Patient Characteristics
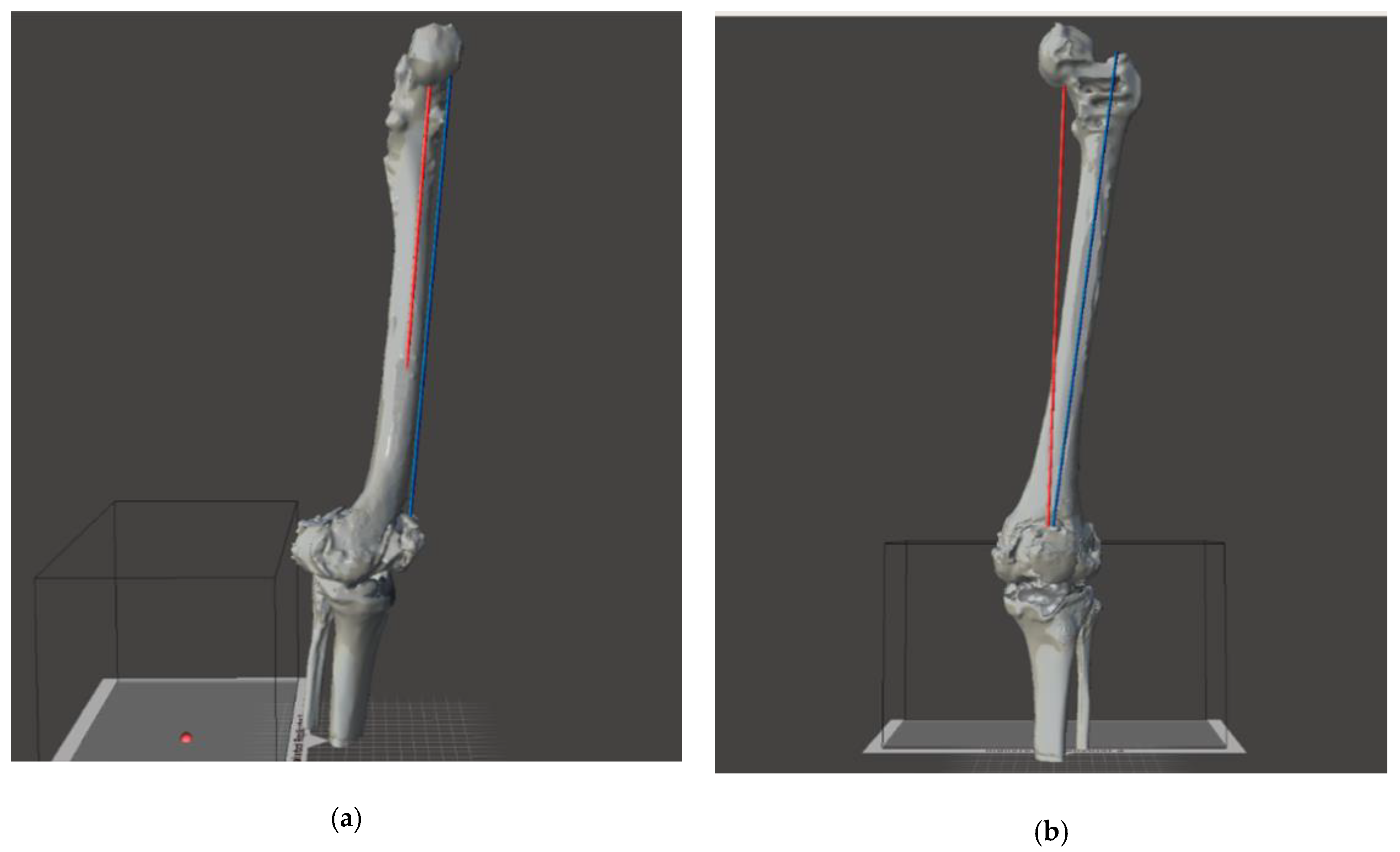
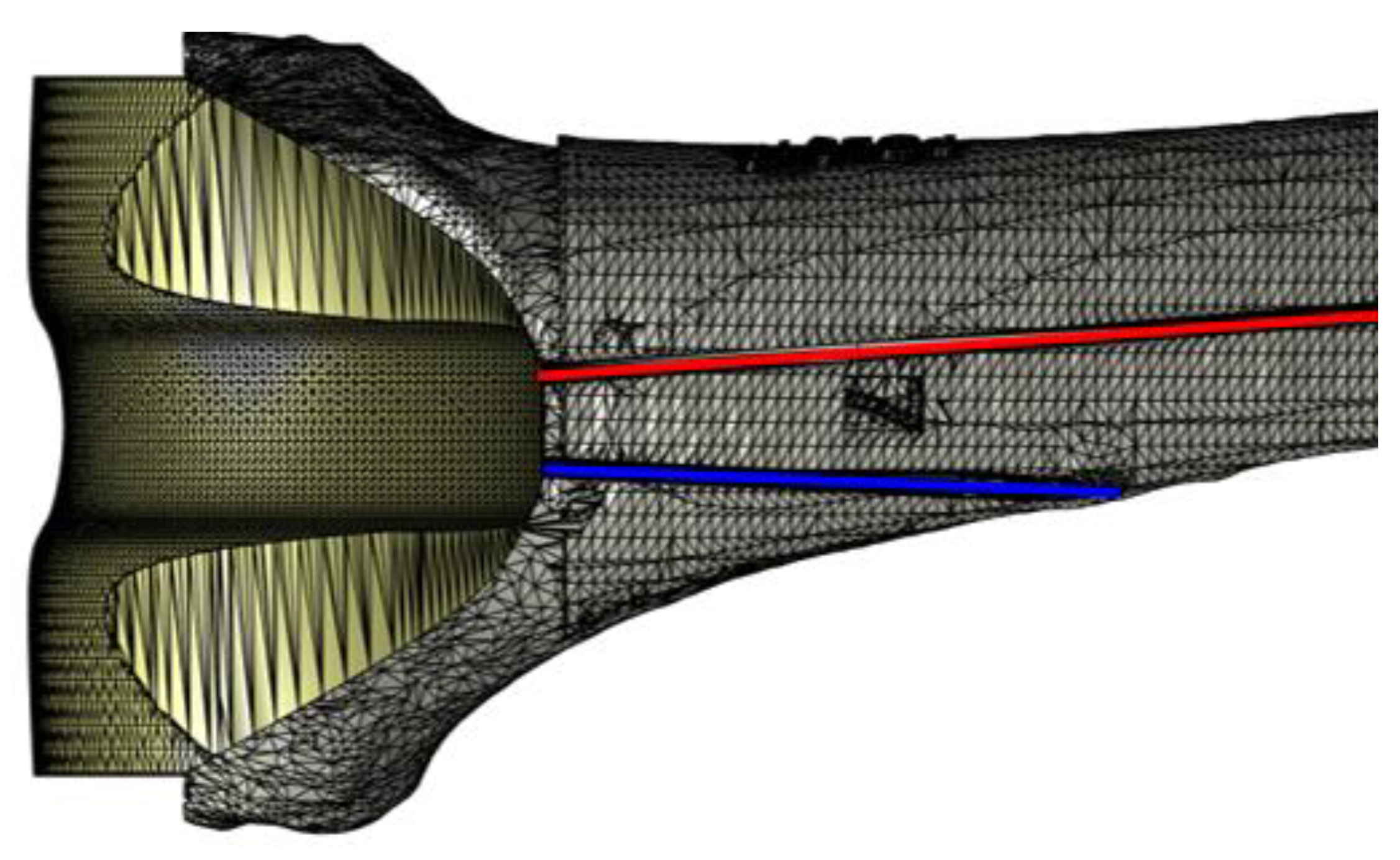
2.4. Virtual Planning
3. Results
3.1. CASE N° 1: “Joint Line Restoration (Femoral Distal Symmetrical Augmentation)”
3.2. CASE N° 2: “Alignment Defect Restoration (Femoral Distal Asymmetrical Augmentation)”
3.3. CASE N° 3: “Rotational Defect Restoration (Femoral Posterior Asymmetrical Augmentation)”
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Hospital Discharge Survey: 2010 Table, Procedures by Selected Patient Characteristics; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013. [Google Scholar]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Havelin, L.I.; Fenstad, A.M.; Salomonsson, R.; Mehnert, F.; Furnes, O.; Overgaard, S.; Pedersen, A.B.; Herberts, P.; Karrholm, J.; Garellick, G. The Nordic Arthroplasty Register Association: A unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009, 80, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Robertsson, O.; Bizjajeva, S.; Fenstad, A.M.; Furnes, O.; Lidgren, L.; Mehnert, F.; Odgaard, A.; Pedersen, A.B.; Havelin, L.I. Knee arthroplasty in Denmark, Norway and Sweden. Acta Orthop. 2010, 81, 82–89. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatolog. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Patel, R. Aseptic Failure in Total Knee Arthroplasty. In Total Knee Arthroplasty; Rodríguez-Merchán, E., Oussedik, S., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Balato, G.; Ascione, T.; Iorio, P.; de Franco, C.; de Matteo, V.; D’Addona, A.; Tammaro, N.; Pellegrino, A. Knee septic arthritis caused by α-hemolytic Streptococcus in a patient with a recent history of knee arthroscopy: A case report. BMC Infect. Dis. 2019, 19, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garfield, K.; Noble, S.; Lenguerrand, E.; Whitehouse, M.R.; Sayers, A.; Reed, M.R.; Blom, A.W. What are the inpatient and day case costs following primary total hip replacement of patients treated for prosthetic joint infection: A matched cohort study using linked data from the National Joint Registry and Hospital Episode Statistics. BMC Med. 2020, 18, 335. [Google Scholar]
- Balato, G.; Ascione, T.; Rosa, D.; Pagliano, P.; Solarino, G.; Moretti, B.; Mariconda, M. Release of gentamicin from cement spacers in two-stage procedures for hip and knee prosthetic infection: An in vivo pharmacokinetic study with clinical follow-up. J. Biol. Regul. Homeost. Agent. 2015, 29 (Suppl. 4), 63–72. [Google Scholar]
- Bozic, K.J.; Ries, M.D. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J. Bone Jt. Surg. 2005, 87, 1746–1751. [Google Scholar] [PubMed]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65. [Google Scholar] [CrossRef]
- Parisi, T.J.; Konopka, J.F.; Bedair, H.S. What is the long-term economic societal effect of periprosthetic infections after THA? A Markov analysis. Clin. Orthop. Relat. Res. 2017, 475, 1891–1900. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Pavlou, G.; Mújica-Mota, R.E.; Toms, A.D. The epidemiology of revision total knee and hip arthroplasty in England and Wales a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Jt. J. 2015, 97, 1076–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, F.S.; Muirhead-Allwood, S.K.; Manktelow, A.R.; and Bacarese-Hamilton, I. Two-stage uncemented revision hip arthroplasty for infection. J. Bone Jt. Surg. Am. 2000, 82, 689–694. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Shih, C.H.; Chang, Y.H.; Lee, M.S.; Yang, W.E.; Shih, H.N. Treatment of deep infection of the hip associated with massive bone loss: Two-stage revision with an antibiotic-loaded interim cement prosthesis followed by reconstruction with allograft. J. Bone Jt. Surg. 2005, 87, 770–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citak, M.; Argenson, J.N.; Masri, B.; Kendoff, D.; Springer, B.; Alt, V.; Baldini, A.; Cui, Q.; Deirmengian, G.; Del Sel, H.; et al. Spacers. J. Arthroplast. 2014, 2, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Hozack, W.J.; Cuckler, J.M.; Booth, R.E., Jr. Two-stage reimplantation of septic total knee arthroplasty. Report of three cases using an antibiotic-PMMA spacer block. J. Arthroplast. 1988, 3, 369–377. [Google Scholar] [CrossRef]
- Masri, B.A.; Duncan, C.P.; Beauchamp, C.P. Long-term elution of antibiotics from bone-cement: An in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J. Arthroplast. 1998, 13, 331–338. [Google Scholar] [CrossRef]
- Hofmann, A.; Kane, K.; Tkach, T.; Plaster, R.; Camargo, M. Treatment of Infected Total Knee Arthroplasty Using an Articulating Spacer. Clin. Orthop. Relat. Res. 1995, 321, 45–54. [Google Scholar] [CrossRef]
- Van Thiel, G.S.; Berend, K.R.; Klein, G.R.; Gordon, A.C.; Lombardi, A.V.; Della Valle, C.J. Intraoperative Molds to Create an Articulating Spacer for the Infected Knee Arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Fehring, T.K.; Odum, S.; Calton, T.F.; Mason, J.B. Articulating versus static spacers in revision total knee arthroplasty for sepsis. Clin. Orthop. 2000, 380, 9–16. [Google Scholar] [CrossRef]
- King, J.D.; Hamilton, D.H.; Jacobs, C.A.; Duncan, S.T. The Hidden Cost of Commercial Antibiotic-Loaded Bone Cement: A Systematic Review of Clinical Results and Cost Implications Following Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 3789–3792. [Google Scholar] [CrossRef]
- Amanatullah, D.; Dennis, D.; Oltra, E.G.; Marcelino Gomes, L.S.; Goodman, S.B.; Hamlin, B.; Hansen, E.; Hashemi-Nejad, A.; Holst, D.C.; Komnos, G.; et al. Hip and Knee Section, Diagnosis, Definitions: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2018, 1–9. [Google Scholar] [CrossRef]
- Berger, R.A.; Crossett, L.S.; Jacobs, J.J.; Rubash, H.E. Malrotation causing patellofemoral complications after total knee arthroplasty. Clin. Orthop. Relat. Res. 1998, 356, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Kubicek, J.; Tomanec, F.; Cerny, M.; Vilimek, D.; Kalova, M.; Oczka, D. Recent Trends, Technical Concepts and Components of Computer-Assisted Orthopedic Surgery Systems: A Comprehensive Review. Sensors 2019, 19, 5199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engh, G.A.; Ammeen, D.J. Bone loss with revision total knee arthroplasty: Defect classification and alternatives for reconstruction. Instr. Course Lect. 1999, 48, 167–175. [Google Scholar] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef]
- Wijdicks, C.A.; Griffith, C.; LaPrade, R.F.; Johansen, S.; Sunderland, A.; Arendt, E.A.; Engebretsen, L. Radiographic identification of the primary medial knee structures. J. Bone Jt. Surg. Am. 2009, 1, 521–529. [Google Scholar] [CrossRef]
- Berger, R.A.; Seel, M.J.; Schleiden, M. Determination of femoral component rotation in total knee arthroplasty using computer tomography. Orthop. Trans. 1993, 17, 427. [Google Scholar]
- Moreland, J.R.; Bassett, L.W.; Hanker, G.J. Radiographic analysis of the axial alignment of the lower extremity. J. Bone Jt. Surg. Am. 1987, 69, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Mose, K. Methods of measuring in Legg-Calvé-Perthes disease with special regard to the prognosis. Clin. Orthop. Relat. Res. 1980, 150, 103–109. [Google Scholar] [CrossRef]
- Pei, G.-X.; Yan, Y.-B. Current status and progress of digital orthopaedics in China. J. Orthop. Transl. 2014, 2, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Karhade, A.V.; Schwab, J.H.; Bedair, H.S. Development of Machine Learning Algorithms for Prediction of Sustained Postoperative Opioid Prescriptions After Total Hip Arthroplasty. J. Arthroplast. 2019, 34, 2272–2277. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymku´c, S.; Gajewska, E.P.; Klucznik, T.; Molga, K.; Dittwald, P.; Startek, M.; Grzybowski, B.A. Computer-Assisted Synthetic Planning: The End of the Beginning. Angew. Chem. Int. Ed. 2016, 55, 5904–5937. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, R.D.; Laycock, K.A. Image-guided surgery. In Biomedical Photonics Handbook: Therapeutics and Advanced Biophotonics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; Volume 3, pp. 219–238. [Google Scholar]
- Swienckowski, J.J.; Bono, F.S.; Spagnuolo, M.W. Unicompartmental replacement arthroplasty: A review. Minerva Ortop. E Traumatol. 2005, 56, 49–63. [Google Scholar]
- Lin, H.H.; Lo, L.J. Three-dimensional computer-assisted surgical simulation and intraoperative navigation in orthognathic surgery: A literature review. J. Formos. Med. Assoc. 2015, 114, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Angibaud, L.; Jung, A.; Hamad, C.; Bertrand, F.; Liu, D.; Huddleston, J. Accuracy of a computer-assisted surgical system for total knee arthroplasy: A review of surgical parameters on 4000+ clinical cases. J. Orthop. Res. 2017, 99, 20. [Google Scholar]
- Hernandez, D.; Garimella, R.; Eltorai, A.E.M.; Daniels, A.H. Computer-assisted orthopaedic surgery. Orthop. Surg. 2017, 9, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Linden-van der Zwaag, H.M.J.; Wolterbeek, R.; Nelissen, R.G.H.H. Computer assisted orthopedic surgery; its influence on prosthesis size in total knee replacement. Knee 2008, 15, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.H.; Audia, P.G. Self-enhancement and learning from performance feedback. Acad. Manag. Rev. 2012, 37, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Beghdadi, A.; Larabi, M.C.; Bouzerdoum, A.; Iftekharuddin, K.M. A survey of perceptual image processing methods. Signal. Process. Image Commun. 2013, 28, 811–831. [Google Scholar] [CrossRef]
- Shukla, K.N.; Potnis, A.; Dwivedy, P. A Review on Image Enhancement Techniques. Int. J. Eng. Appl. Comput. Sci. 2017, 2, 232–235. [Google Scholar] [CrossRef]
- Shamir, A. Asurvey on mesh segmentation techniques. Comput. Graph. Forum 2008, 27, 1539–1556. [Google Scholar] [CrossRef]
- Lv, J.; Chen, X.; Huangy, J.; Bao, H. Semi-supervised mesh segmentation and labeling. Eur. Symp. Geom. Process. 2012, 31, 2241–2248. [Google Scholar] [CrossRef] [Green Version]
- Mesejo, P.; Ibáñez, Ó.; Cordón, Ó.; Cagnoni, S. A survey on image segmentation using metaheuristic-based deformable models: State of the art and critical analysis. Appl. Sof. Comput. J. 2016, 44, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhan, Y.; Dewan, M.; Huang, J.; Metaxas, D.N.; Zhou, X.S. Deformable segmentation via sparse shape representation. Med. Image Comput. Comput. Assist. Interv. 2011, 14, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, M.; Lymburner, L.; Volk, M. The comparison index: A tool for assessing the accuracy of image segmentation. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 311–321. [Google Scholar] [CrossRef]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. SegNet: A Deep Convolutional Encoder-Decoder Architecture for Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, M.T.; Friederich, N.F.; Becker, R.; Karlsson, J. Personalised medicine in knee arthroplasty: We need more science! Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1357–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, K.K.; Kuo, F.C.; Horcajada, J.P.; Hughes, H.; Cavagnaro, L.; Marculescu, C.; McLaren, A.; Nodzo, S.R.; Riccio, G.; Sendi, P.; et al. General Assembly, Treatment, Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S225–S237. [Google Scholar] [CrossRef]
- Anemüller, R.; Belden, K.; Brause, B.; Citak, M.; Del Pozo, J.L.; Frommelt, L.; Gehrke, T.; Hewlett, A.; Higuera, C.A.; Hughes, H.; et al. Hip and Knee Section, Treatment, Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S463–S475. [Google Scholar] [CrossRef]
- Aalirezaie, A.; Abolghasemian, M.; Busato, T.; Dennis, D.; Ghazavi, M.; Holst, D.C.; Kelly, M.; Kissin, Y.D.; Kuijpers, M.; Lange, J.; et al. Hip and Knee Section, Treatment, Two-Stage Exchange: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S439–S443. [Google Scholar] [CrossRef] [PubMed]
- Abdel, M.P.; Barreira, P.; Battenberg, A.; Berry, D.J.; Blevins, K.; Font-Vizcarra, L.; Frommelt, L.; Goswami, K.; Greiner, J.; Janz, V.; et al. Hip and Knee Section, Treatment, Two-Stage Exchange Spacer-Related: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S427–S438. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D.; INFORM Team. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.C.; Berger, R.A.; Petrella, A.J.; Karmas, A.; Rubash, H.E. Optimizing femoral component rotation in total knee arthroplasty. Clin. Orthop. Relat. Res. 2001, 392, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Balato, G.; Balato, M.; Papa, D. Spaziatore di Ginocchio. Italian Patent 102020000005524, 24 May 2020. [Google Scholar]
- Rasmussen, L.S.; Gisvold, S.E.; Wisborg, T. Ethics Committee approval for observational studies. Acta Anaesthesiol. Scand. 2014, 58, 1047–1048. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balato, M.; Petrarca, C.; de Matteo, V.; Lenzi, M.; Festa, E.; Sellitto, A.; Campi, J.; Zarrelli, M.; Balato, G. On the Necessity of a Customized Knee Spacer in Peri-Prosthetic Joint Infection Treatment: 3D Numerical Simulation Results. J. Pers. Med. 2021, 11, 1039. https://doi.org/10.3390/jpm11101039
Balato M, Petrarca C, de Matteo V, Lenzi M, Festa E, Sellitto A, Campi J, Zarrelli M, Balato G. On the Necessity of a Customized Knee Spacer in Peri-Prosthetic Joint Infection Treatment: 3D Numerical Simulation Results. Journal of Personalized Medicine. 2021; 11(10):1039. https://doi.org/10.3390/jpm11101039
Chicago/Turabian StyleBalato, Marco, Carlo Petrarca, Vincenzo de Matteo, Marco Lenzi, Enrico Festa, Andrea Sellitto, Jessica Campi, Mauro Zarrelli, and Giovanni Balato. 2021. "On the Necessity of a Customized Knee Spacer in Peri-Prosthetic Joint Infection Treatment: 3D Numerical Simulation Results" Journal of Personalized Medicine 11, no. 10: 1039. https://doi.org/10.3390/jpm11101039
APA StyleBalato, M., Petrarca, C., de Matteo, V., Lenzi, M., Festa, E., Sellitto, A., Campi, J., Zarrelli, M., & Balato, G. (2021). On the Necessity of a Customized Knee Spacer in Peri-Prosthetic Joint Infection Treatment: 3D Numerical Simulation Results. Journal of Personalized Medicine, 11(10), 1039. https://doi.org/10.3390/jpm11101039








