Influence of Genetic Polymorphisms on Clinical Outcomes of Glatiramer Acetate in Multiple Sclerosis Patients
Abstract
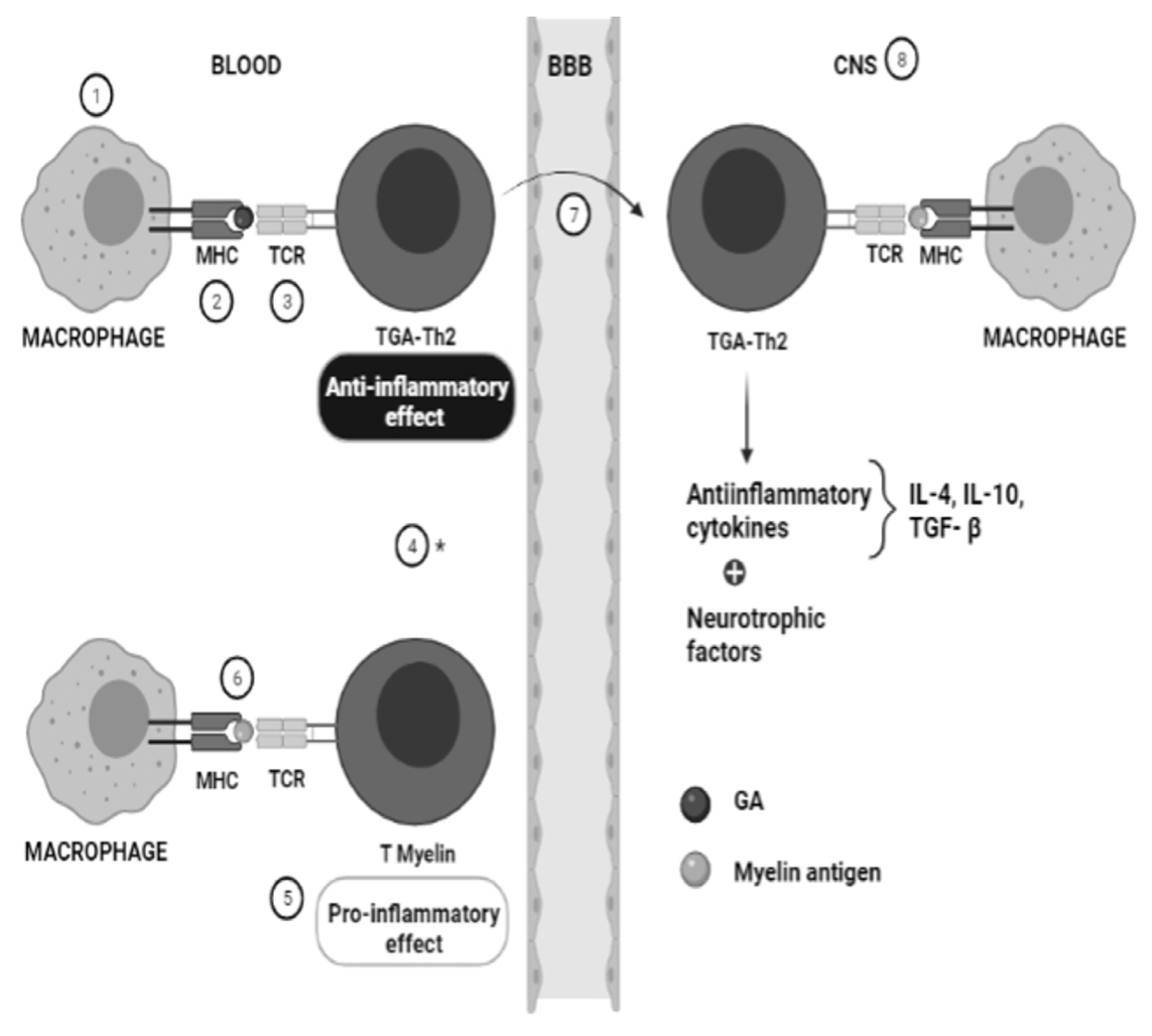
1. Introduction
2. Materials and Methods
3. Pharmacogenetics of Glatiramer Acetate in MS
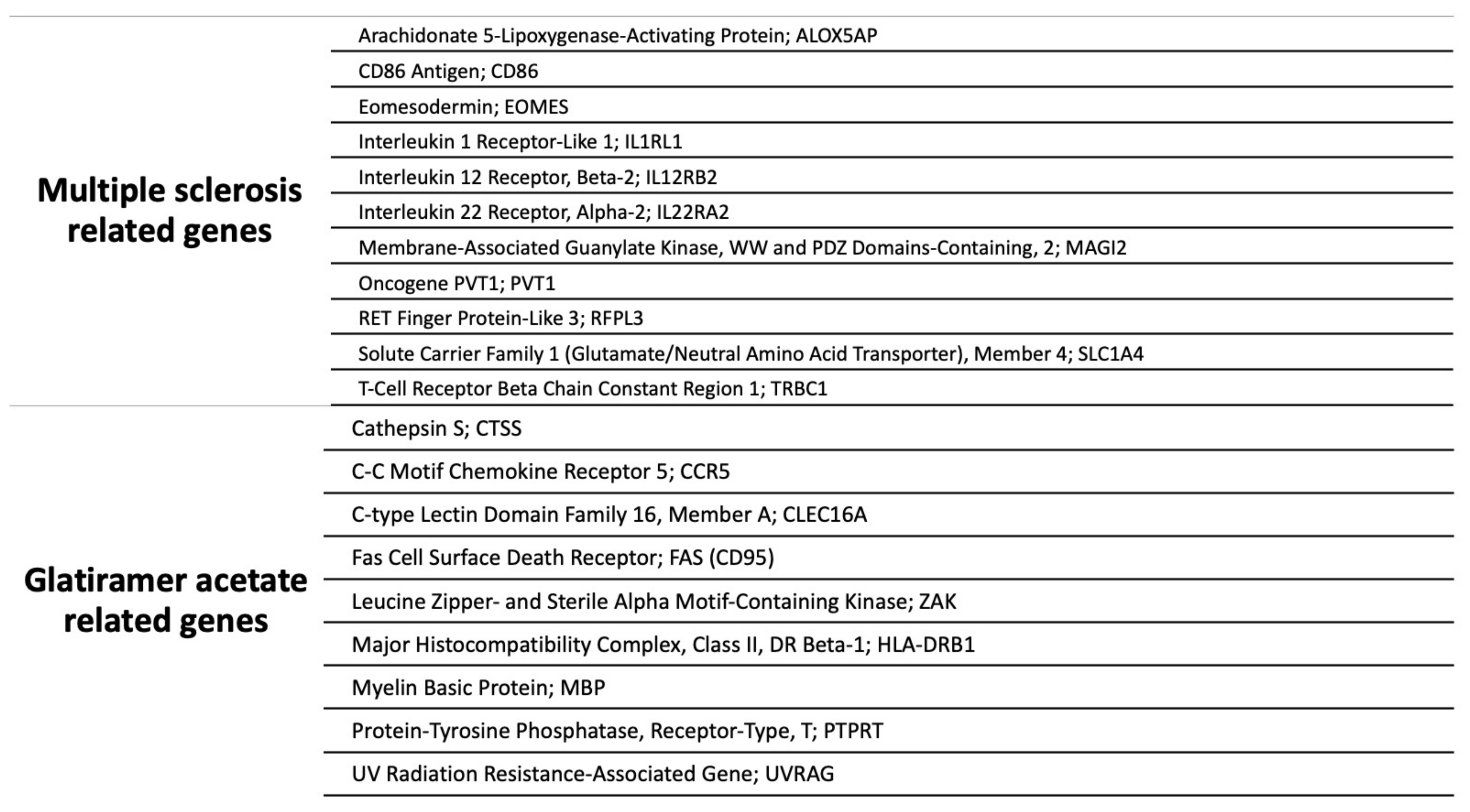
3.1. Multiple Sclerosis Related Genes
3.1.1. Arachidonate 5-Lipoxygenase-Activating Protein; ALOX5AP
3.1.2. CD86 Antigen; CD86
3.1.3. Eomesodermin; EOMES
3.1.4. Interleukin 1 Receptor-like 1; IL1RL1
3.1.5. Interleukin 12 Receptor, Beta-2; IL12RB2
3.1.6. Interleukin 22 Receptor, Alpha-2; IL22RA2
3.1.7. Membrane-Associated Guanylate Kinase, WW and PDZ Domains-Containing, 2; MAGI2
3.1.8. Oncogene PVT1; PVT1
3.1.9. RET Finger Protein-like 3; RFPL3
3.1.10. Solute Carrier Family 1 (Glutamate/Neutral Amino Acid Transporter), Member 4; SLC1A4
3.1.11. T-Cell Receptor Beta Chain Constant Region 1; TRBC1
3.2. Glatiramer Acetate Related Genes
3.2.1. Cathepsin S; CTSS
3.2.2. C-C Motif Chemokine Receptor 5; CCR5
3.2.3. C-Type Lectin Domain Family 16, Member A; CLEC16A
3.2.4. Fas Cell Surface Death Receptor; FAS (CD95)
3.2.5. Leucine Zipper- and Sterile Alpha Motif-Containing Kinase; ZAK
3.2.6. Major Histocompatibility Complex, Class II, DR Beta-1; HLA-DRB1
3.2.7. Myelin Basic Protein; MBP
3.2.8. Protein-Tyrosine Phosphatase, Receptor-Type, T; PTPRT
3.2.9. UV Radiation Resistance-Associated Gene; UVRAG
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ALOX5AP | Arachidonate 5-lipoxygenase-activating protein gene |
| APC | Antigen-presenting cell |
| CCR5 | C-C motif chemokine receptor 5 gene |
| CIS | Clinically isolated syndrome |
| CLEC16A | C-type lectin domain family 16 member A gene |
| CNS | Central nervous system |
| CTSS | Cathepsin S gene |
| EOMES | Eomesodermin gene |
| FORTE | Forty mg Efficacy of glatiramer acetate |
| GA | Glatiramer acetate |
| GALA | Glatiramer Acetate Low-frequency Administration |
| HLA-DRB1 | Major histocompatibility complex class II DR beta-1 gene |
| IL1RL1 | Interleukin 1 receptor-like 1 gene |
| IL22RA2 | Interleukin 22 receptor alpha-2 gene |
| JNK/SAPK1 | C-Jun N-terminal kinase 1/stress-activated protein kinase |
| MAGI2 | Membrane-associated guanylate kinase WW and PDZ domains-containing 2 gene |
| MBP | Myelin basic protein |
| MS | Multiple sclerosis |
| NFĸΒ | Nuclear factor kappa light chain in β cells |
| PPMS | Primary progressive multiple sclerosis |
| PTP | Protein tyrosine phosphatase |
| PTPRT | Protein-tyrosine phosphatase receptor-type T gene |
| PVT1 | Plasmacytoma variant translocation oncogene |
| RFPL3 | RET finger protein-like 3 gene |
| RRMS | Relapsing-remitting multiple sclerosis |
| SLC1A4 | Solute carrier family 1 member 4 gene |
| SPMS | Secondary progressive multiple sclerosis |
| Th2 | T helper type 2 |
| TRBC1 | T-cell receptor beta chain constant region 1 gene |
| UVRAG | UV radiation resistance-associated gene |
| ZAK | Leucine zipper- and sterile alpha motif-containing kinase gene |
References
- Freal, J.E.; Kraft, G.H.; Coryell, J.K. Symptomatic fatigue in multiple sclerosis. Arch. Phys. Med. Rehabil. 1984, 65, 135–138. [Google Scholar]
- Hauser, S.L.; Oksenberg, J.R. The neurobiology of multiple sclerosis: Genes, inflammation, and neurodegeneration. Neuron 2006, 52, 61–76. [Google Scholar] [CrossRef]
- Krupp, L.B.; Alvarez, L.A.; LaRocca, N.G.; Scheinberg, L.C. Fatigue in multiple sclerosis. Arch. Neurol. 1988, 45, 435–437. [Google Scholar] [CrossRef]
- Lucchinetti, C.; Bruck, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Hestvik, A.L. The double-edged sword of autoimmunity: Lessons from multiple sclerosis. Toxins 2010, 2, 856–877. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vecsei, L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Toldi, J.; Vecsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Arslan, G.A.; Tuncer, A.; Karabudak, R.; Dinçel, A.S. Evaluation of Thiol Homeostasis in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders. Front. Neurol. 2021, 12, 716195. [Google Scholar] [CrossRef] [PubMed]
- Ömerhoca, S.; Akkaş, S.Y.; İçen, N.K. Multiple Sclerosis: Diagnosis and Differential Diagnosis. Arch. Neuropsychiatry 2018, 55, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, W.J. Differential diagnosis of multiple sclerosis. Better Explan. Clin. Pract. 2019, 92, 1037–1038. [Google Scholar] [CrossRef]
- Luca, M.; Chisari, C.G.; Zanghì, A.; Patti, F. Early-Onset Alcohol Dependence and Multiple Sclerosis: Diagnostic Challenges. Int. J. Environ. Res. Public Health 2021, 18, 5588. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Patti, F. CSF biomarkers in multiple sclerosis: Beyond neuroinflammation. Neuroimmunol. Neuroinflamm. 2021, 8, 14–41. [Google Scholar] [CrossRef]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Pugliatti, M.; Rosati, G.; Carton, H.; Riise, T.; Drulovic, J.; Vecsei, L.; Milanov, I. The epidemiology of multiple sclerosis in Europe. Eur. J. Neurol. 2006, 13, 700–722. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sorensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Grossman, I.; Avidan, N.; Singer, C.; Goldstaub, D.; Hayardeny, L.; Eyal, E.; Ben-Asher, E.; Paperna, T.; Pe’er, I.; Lancet, D.; et al. Pharmacogenetics of glatiramer acetate therapy for multiple sclerosis reveals drug-response markers. Pharm. Genom. 2007, 17, 657–666. [Google Scholar] [CrossRef]
- Grossman, I.; Knappertz, V.; Laifenfeld, D.; Ross, C.; Zeskind, B.; Kolitz, S.; Ladkani, D.; Hayardeny, L.; Loupe, P.; Laufer, R.; et al. Pharmacogenomics strategies to optimize treatments for multiple sclerosis: Insights from clinical research. Prog. Neurobiol. 2017, 152, 114–130. [Google Scholar] [CrossRef]
- Castro-Borrero, W.; Graves, D.; Frohman, T.C.; Flores, A.B.; Hardeman, P.; Logan, D.; Orchard, M.; Greenberg, B.; Frohman, E.M. Current and emerging therapies in multiple sclerosis: A systematic review. Adv. Neurol. Disord. 2012, 5, 205–220. [Google Scholar] [CrossRef]
- Huisman, E.; Papadimitropoulou, K.; Jarrett, J.; Bending, M.; Firth, Z.; Allen, F.; Adlard, N. Systematic literature review and network meta-analysis in highly active relapsing-remitting multiple sclerosis and rapidly evolving severe multiple sclerosis. BMJ Open 2017, 7, e013430. [Google Scholar] [CrossRef]
- Findling, O.; Sellner, J. Second-generation immunotherapeutics in multiple sclerosis: Can we discard their precursors? Drug Discov. Today 2021, 26, 416–428. [Google Scholar] [CrossRef]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2017, 17, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.K. Pharmacogenetic Biomarkers to Predict Treatment Response in Multiple Sclerosis: Current and Future Perspectives. Mult. Scler. Int. 2017, 2017, 6198530. [Google Scholar] [CrossRef]
- Aharoni, R. The mechanism of action of glatiramer acetate in multiple sclerosis and beyond. Autoimmun. Rev. 2013, 12, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, D.K.; Mehta, R.S.; Ortega, S.B.; Bhoj, V.; Racke, M.K.; Karandikar, N.J. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J. Immunol. 2006, 176, 7119–7129. [Google Scholar] [CrossRef] [PubMed]
- Tsareva, E.; Kulakova, O.; Boyko, A.; Favorova, O. Pharmacogenetics of multiple sclerosis: Personalized therapy with immunomodulatory drugs. Pharm. Genom. 2016, 26, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. 2018, 24, 96–120. [Google Scholar] [CrossRef] [PubMed]
- Boster, A.L.; Ford, C.C.; Neudorfer, O.; Gilgun-Sherki, Y. Glatiramer acetate: Long-term safety and efficacy in relapsing-remitting multiple sclerosis. Expert Rev. Neurother. 2015, 15, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Nair, K.V.; Glajch, J.L.; Ganguly, T.C.; Kantor, D. Two decades of glatiramer acetate: From initial discovery to the current development of generics. J. Neurol. Sci. 2017, 376, 255–259. [Google Scholar] [CrossRef]
- Ganji, A.; Monfared, M.E.; Shapoori, S.; Nourbakhsh, P.; Ghazavi, A.; Ghasami, K.; Mosayebi, G. Effects of interferon and glatiramer acetate on cytokine patterns in multiple sclerosis patients. Cytokine 2019, 126, 154911. [Google Scholar] [CrossRef]
- Johnson, K.P.; Brooks, B.R.; Cohen, J.A.; Ford, C.C.; Goldstein, J.; Lisak, R.P.; Myers, L.W.; Panitch, H.S.; Rose, J.W.; Schiffer, R.B. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995, 45, 1268–1276. [Google Scholar] [CrossRef]
- Comi, G.; Filippi, M.; Wolinsky, J.S. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann. Neurol. 2001, 49, 290–297. [Google Scholar]
- Fusco, C.; Andreone, V.; Coppola, G.; Luongo, V.; Guerini, F.; Pace, E.; Florio, C.; Pirozzi, G.; Lanzillo, R.; Ferrante, P.; et al. HLA-DRB1*1501 and response to copolymer-1 therapy in relapsing-remitting multiple sclerosis. Neurology 2001, 57, 1976–1979. [Google Scholar] [CrossRef]
- Bovis, F.; Kalincik, T.; Lublin, F.; Cutter, G.; Malpas, C.; Horakova, D.; Havrdova, E.K.; Trojano, M.; Prat, A.; Girard, M.; et al. Treatment Response Score to Glatiramer Acetate or Interferon Beta-1a. Neurology 2021, 96, e214–e227. [Google Scholar] [CrossRef]
- Drew, L. Pharmacogenetics: The right drug for you. Nature 2016, 537, S60–S62. [Google Scholar] [CrossRef] [PubMed]
- Zarzuelo-Romero, M.J.; Pérez-Ramírez, C.; Carrasco-Campos, M.I.; Sánchez-Martín, A.; Calleja Hernández, M.A.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Therapeutic Value of Single Nucleotide Polymorphisms on the Efficacy of New Therapies in Patients with Multiple Sclerosis. J. Pers. Med. 2021, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Campos, M.I.; Pérez-Ramírez, C.; Macías-Cortés, E.; Puerta-García, E.; Sánchez-Pozo, A.; Arnal-García, C.; Barrero-Hernández, F.J.; Calleja-Hernández, M.; Jiménez-Morales, A.; Cañadas-Garre, M. Pharmacogenetic Predictors of Response to Interferon Beta Therapy in Multiple Sclerosis. Mol. Neurobiol. 2021, 58, 4716–4726. [Google Scholar] [CrossRef]
- Martínez-Aguilar, L.; Pérez-Ramírez, C.; Maldonado-Montoro, M.D.M.; Carrasco-Campos, M.I.; Membrive-Jiménez, C.; Martínez-Martínez, F.; García-Collado, C.; Calleja-Hernández, M.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Effect of genetic polymorphisms on therapeutic response in multiple sclerosis relapsing-remitting patients treated with interferon-beta. Mutat. Res. Rev. Mutat. Res. 2020, 785, 108322. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.J.; Keating, G.M. Glatiramer acetate: A review of its use in relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs 2010, 70, 1545–1577. [Google Scholar] [CrossRef]
- McGraw, C.A.; Lublin, F.D. Interferon beta and glatiramer acetate therapy. Neurotherapeutics 2013, 10, 2–18. [Google Scholar] [CrossRef]
- Aharoni, R. Immunomodulation neuroprotection and remyelination—The fundamental therapeutic effects of glatiramer acetate: A critical review. J. Autoimmun. 2014, 54, 81–92. [Google Scholar] [CrossRef]
- From, R.; Eilam, R.; Bar-Lev, D.D.; Levin-Zaidman, S.; Tsoory, M.; LoPresti, P.; Sela, M.; Arnon, R.; Aharoni, R. Oligodendrogenesis and myelinogenesis during postnatal development effect of glatiramer acetate. Glia 2014, 62, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Hocevar, K.; Ristic, S.; Peterlin, B. Pharmacogenomics of Multiple Sclerosis: A Systematic Review. Front. Neurol. 2019, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Mahurkar, S.; Suppiah, V.; O’Doherty, C. Pharmacogenomics of interferon beta and glatiramer acetate response: A review of the literature. Autoimmun. Rev. 2014, 13, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Vandenbroeck, K. Pharmacogenomics and multiple sclerosis: Moving toward individualized medicine. Curr. Neurol. Neurosci. Rep. 2011, 11, 484–491. [Google Scholar] [CrossRef]
- Yandava, C.N.; Kennedy, B.P.; Pillari, A.; Duncan, A.M.; Drazen, J.M. Cytogenetic and radiation hybrid mapping of human arachidonate 5-lipoxygenase-activating protein (ALOX5AP) to chromosome 13q12. Genomics 1999, 56, 131–133. [Google Scholar] [CrossRef]
- Safizadeh, B.; Hoshyar, R.; Mehrpour, M.; Eftekhar, M.; Salimi, V.; Yazdani, S.; Bijari, B.; Khodakhah, F.; Tavakoli-Yaraki, M. The role of expression and activity of 15-Lipoxygenase isoforms and related cytokines in patients with Multiple Sclerosis and healthy controls. J. Neuroimmunol. 2018, 325, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Feldman, A.; Gurevich, M. Molecular profiling of glatiramer acetate early treatment effects in multiple sclerosis. Dis. Markers 2009, 27, 63–73. [Google Scholar] [CrossRef]
- Ross, C.J.; Towfic, F.; Shankar, J.; Laifenfeld, D.; Thoma, M.; Davis, M.; Weiner, B.; Kusko, R.; Zeskind, B.; Knappertz, V.; et al. A pharmacogenetic signature of high response to Copaxone in late-phase clinical-trial cohorts of multiple sclerosis. Genome Med. 2017, 9, 50. [Google Scholar] [CrossRef]
- Reeves, R.H.; Patch, D.; Sharpe, A.H.; Borriello, F.; Freeman, G.J.; Edelhoff, S.; Disteche, C. The costimulatory genes Cd80 and Cd86 are linked on mouse chromosome 16 and human chromosome 3. Mamm Genome 1997, 8, 581–582. [Google Scholar] [CrossRef]
- Freeman, G.J.; Borriello, F.; Hodes, R.J.; Reiser, H.; Gribben, J.G.; Ng, J.W.; Kim, J.; Goldberg, J.M.; Hathcock, K.; Laszlo, G. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J. Exp. Med. 1993, 178, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Fraussen, J.; Claes, N.; Van Wijmeersch, B.; van Horssen, J.; Stinissen, P.; Hupperts, R.; Somers, V. B cells of multiple sclerosis patients induce autoreactive proinflammatory T cell responses. Clin. Immunol. 2016, 173, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Hussien, Y.; Sanna, A.; Soderstrom, M.; Link, H.; Huang, Y.M. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J. Neuroimmunol. 2001, 121, 102–110. [Google Scholar] [CrossRef]
- Yi, C.H.; Terrett, J.A.; Li, Q.Y.; Ellington, K.; Packham, E.A.; Armstrong-Buisseret, L.; McClure, P.; Slingsby, T.; Brook, J.D. Identification, mapping, and phylogenomic analysis of four new human members of the T-box gene family: EOMES, TBX6, TBX18, and TBX19. Genomics 1999, 55, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Parnell, G.P.; Gatt, P.N.; Krupa, M.; Nickles, D.; McKay, F.C.; Schibeci, S.D.; Batten, M.; Baranzini, S.; Henderson, A.; Barnett, M.; et al. The autoimmune disease-associated transcription factors EOMES and TBX21 are dysregulated in multiple sclerosis and define a molecular subtype of disease. Clin. Immunol. 2014, 151, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Raveney, B.J.E.; Sato, W.; Takewaki, D.; Zhang, C.; Kanazawa, T.; Lin, Y.; Okamoto, T.; Araki, M.; Kimura, Y.; Sato, N.; et al. Involvement of cytotoxic Eomes-expressing CD4(+) T cells in secondary progressive multiple sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Kulakova, O.; Bashinskaya, V.; Kiselev, I.; Baulina, N.; Tsareva, E.; Nikolaev, R.; Kozin, M.; Shchur, S.; Favorov, A.; Boyko, A.; et al. Pharmacogenetics of glatiramer acetate therapy for multiple sclerosis: The impact of genome-wide association studies identified disease risk loci. Pharmacogenomics 2017, 18, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Dale, M.; Nicklin, M.J. Interleukin-1 receptor cluster: Gene organization of IL1R2, IL1R1, IL1RL2 (IL-1Rrp2), IL1RL1 (T1/ST2), and IL18R1 (IL-1Rrp) on human chromosome 2q. Genomics 1999, 57, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Yagami, A.; Orihara, K.; Morita, H.; Futamura, K.; Hashimoto, N.; Matsumoto, K.; Saito, H.; Matsuda, A. IL-33 mediates inflammatory responses in human lung tissue cells. J. Immunol. 2010, 185, 5743–5750. [Google Scholar] [CrossRef]
- Ahmadi, M.; Fathi, F.; Fouladi, S.; Alsahebfosul, F.; Manian, M.; Eskandari, N. Serum IL-33 Level and IL-33, IL1RL1 Gene Polymorphisms in Asthma and Multiple Sclerosis Patients. Curr. Mol. Med. 2019, 19, 357–363. [Google Scholar] [CrossRef]
- Carpintero, R.; Burger, D. IFNbeta and glatiramer acetate trigger different signaling pathways to regulate the IL-1 system in multiple sclerosis. Commun. Integr. Biol. 2011, 4, 112–114. [Google Scholar] [CrossRef][Green Version]
- Morton, S.M.; Bocaccio, I.; Depetris, D.; Mattei, M.; Dessein, A. Assignment of IL12RB2 to human chromosome 1p31.3-->p31.2 between D1S230 and D1S198. Cytogenet. Genome Res. 1997, 79, 282–283. [Google Scholar] [CrossRef]
- Koch, M.A.; Thomas, K.R.; Perdue, N.R.; Smigiel, K.S.; Srivastava, S.; Campbell, D.J. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity 2012, 37, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Mondal, S.; Jana, A.; Pahan, K. Interleukin-12 (IL-12), but not IL-23, induces the expression of IL-7 in microglia and macrophages: Implications for multiple sclerosis. Immunology 2014, 141, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Presnell, S.R.; Parrish-Novak, J.; Kindsvogel, W.; Jaspers, S.; Chen, Z.; Dillon, S.R.; Gao, Z.; Gilbert, T.; Madden, K.; et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc. Natl. Acad. Sci. USA 2001, 98, 9511–9516. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, H.; Guerreiro-Cacais, A.O.; Bedri, S.K.; Linnerbauer, M.; Linden, M.; Abdelmagid, N.; Tandre, K.; Hollins, C.; Irving, L.; Glover, C.; et al. IL-22 Binding Protein Promotes the Disease Process in Multiple Sclerosis. J. Immunol. 2019, 203, 888–898. [Google Scholar] [CrossRef]
- Wood, J.D.; Yuan, J.; Margolis, R.L.; Colomer, V.; Duan, K.; Kushi, J.; Kaminsky, Z.; Kleiderlein, J.J.; Sharp, A.H.; Ross, C.A. Atrophin-1, the DRPLA gene product, interacts with two families of WW domain-containing proteins. Mol. Cell. Neurosci. 1998, 11, 149–160. [Google Scholar] [CrossRef]
- Bierzynska, A.; Soderquest, K.; Dean, P.; Colby, E.; Rollason, R.; Jones, C.; Inward, C.D.; McCarthy, H.J.; Simpson, M.A.; Lord, G.M.; et al. MAGI2 Mutations Cause Congenital Nephrotic Syndrome. J. Am. Soc. Nephrol. 2017, 28, 1614–1621. [Google Scholar] [CrossRef]
- Wu, X.; Hepner, K.; Castelino-Prabhu, S.; Do, D.; Kaye, M.B.; Yuan, X.J.; Wood, J.; Ross, C.; Sawyers, C.L.; Whang, Y.E. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc. Natl. Acad. Sci. USA 2000, 97, 4233–4238. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.; Adams, J.M. Chromosome 8 breakpoint far 3′ of the c-myc oncogene in a Burkitt’s lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. 1986, 5, 2845–2851. [Google Scholar] [CrossRef] [PubMed]
- Eftekharian, M.M.; Ghafouri-Fard, S.; Soudyab, M.; Omrani, M.D.; Rahimi, M.; Sayad, A.; Komaki, A.; Mazdeh, M.; Taheri, M. Expression Analysis of Long Non-coding RNAs in the Blood of Multiple Sclerosis Patients. J. Mol. Neurosci. 2017, 63, 333–341. [Google Scholar] [CrossRef]
- Zeni, P.F.; Mraz, M. LncRNAs in adaptive immunity: Role in physiological and pathological conditions. RNA Biol. 2021, 18, 619–632. [Google Scholar] [CrossRef]
- Timasheva, Y.R.; Nasibullin, T.R.; Tuktarova, I.A.; Erdman, V.V.; Galiullin, T.R.; Zaplakhova, O.V.; Bakhtiiarova, K.Z.; Mustafina, O.E. The analysis of association between multiple sclerosis and genetic markers identified in genome-wide association studies. Zhurnal Nevrol Psikhiatr Im S S Korsakova 2020, 120, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont, J.; Nikolaev, S.I.; Perrier, A.L.; Guo, S.; Cartier, L.; Sorce, S.; Laforge, T.; Aubry, L.; Khaitovich, P.; Peschanski, M.; et al. Evolutionary forces shape the human RFPL1,2,3 genes toward a role in neocortex development. Am. J. Hum. Genet. 2008, 83, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Damseh, N.; Simonin, A.; Jalas, C.; Picoraro, J.A.; Shaag, A.; Cho, M.T.; Yaacov, B.; Neidich, J.; Al-Ashhab, M.; Juusola, J.; et al. Mutations in SLC1A4, encoding the brain serine transporter, are associated with developmental delay, microcephaly and hypomyelination. J. Med. Genet. 2015, 52, 541–547. [Google Scholar] [CrossRef]
- Heimer, G.; Marek-Yagel, D.; Eyal, E.; Barel, O.; Oz Levi, D.; Hoffmann, C.; Ruzzo, E.K.; Ganelin-Cohen, E.; Lancet, D.; Pras, E.; et al. SLC1A4 mutations cause a novel disorder of intellectual disability, progressive microcephaly, spasticity and thin corpus callosum. Clin. Genet. 2015, 88, 327–335. [Google Scholar] [CrossRef]
- Beall, S.S.; Biddison, W.E.; McFarlin, D.E.; McFarland, H.F.; Hood, L.E. Susceptibility for multiple sclerosis is determined, in part, by inheritance of a 175-kb region of the TcR V beta chain locus and HLA class II genes. J. Neuroimmunol. 1993, 45, 53–60. [Google Scholar] [CrossRef]
- Hockertz, M.K.; Paty, D.W.; Beall, S.S. Susceptibility to relapsing-progressive multiple sclerosis is associated with inheritance of genes linked to the variable region of the TcR beta locus: Use of affected family-based controls. Am. J. Hum. Genet. 1998, 62, 373–385. [Google Scholar] [CrossRef][Green Version]
- Deussing, J.; Roth, W.; Rommerskirch, W.; Wiederanders, B.; von Figura, K.; Peters, C. The genes of the lysosomal cysteine proteinases cathepsin B, H, L, and S map to different mouse chromosomes. Mamm. Genome 1997, 8, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Foti Cuzzola, V.; Palella, E.; Celi, D.; Barresi, M.; Giacoppo, S.; Bramanti, P.; Marino, S. Pharmacogenomic update on multiple sclerosis: A focus on actual and new therapeutic strategies. Pharm. J 2012, 12, 453–461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, R.; Paxton, W.A.; Choe, S.; Ceradini, D.; Martin, S.R.; Horuk, R.; MacDonald, M.E.; Stuhlmann, H.; Koup, R.A.; Landau, N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 1996, 86, 367–377. [Google Scholar] [CrossRef]
- Rottman, J.B.; Ganley, K.P.; Williams, K.; Wu, L.; Mackay, C.R.; Ringler, D.J. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am. J. Pathol. 1997, 151, 1341–1351. [Google Scholar]
- Lynch, E.A.; Heijens, C.A.W.; Horst, N.F.; Center, D.M.; Cruikshank, W.W. Cutting edge: IL-16/CD4 preferentially induces Th1 cell migration: Requirement of CCR5. J. Immunol. 2003, 171, 4965–4968. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Fukuda, K.; Doi, Y.; Sugino, M.; Kimura, F.; Hanafusa, T.; Ikemoto, T.; Shimizu, A. Expression of TH1/TH2-related chemokine receptors on peripheral T cells and correlation with clinical disease activity in patients with multiple sclerosis. Eur. Neurol. 2004, 52, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, R.A.; Hestvik, A.L.; Holmoy, T.; Maghazachi, A.A. Expression and functional activity of chemokine receptors in glatiramer acetate-specific T cells isolated from multiple sclerosis patient receiving the drug glatiramer acetate. Hum. Immunol. 2011, 72, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Tsareva, E.Y.; Kulakova, O.G.; Boyko, A.N.; Shchur, S.G.; Lvovs, D.; Favorov, A.V.; Gusev, E.I.; Vandenbroeck, K.; Favorova, O.O. Allelic combinations of immune-response genes associated with glatiramer acetate treatment response in Russian multiple sclerosis patients. Pharmacogenomics 2012, 13, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Hakonarson, H.; Grant, S.F.; Bradfield, J.P.; Marchand, L.; Kim, C.E.; Glessner, J.T.; Grabs, R.; Casalunovo, T.; Taback, S.P.; Frackelton, E.C.; et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 2007, 448, 591–594. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Rijvers, L.; Melief, M.J.; van Langelaar, J.; van der Vuurst de Vries, R.M.; Wierenga-Wolf, A.F.; Koetzier, S.C.; Priatel, J.J.; Jorritsma, T.; van Ham, S.M.; Hintzen, R.Q.; et al. The Role of Autoimmunity-Related Gene CLEC16A in the B Cell Receptor-Mediated HLA Class II Pathway. J. Immunol. 2020, 205, 945–956. [Google Scholar] [CrossRef]
- Zuvich, R.L.; Bush, W.S.; McCauley, J.L.; Beecham, A.H.; De Jager, P.L.; the International Multiple Sclerosis Genetics Consortium; Ivinson, A.J.; Compston, A.; Hafler, D.A.; Hauser, S.L.; et al. Interrogating the complex role of chromosome 16p13.13 in multiple sclerosis susceptibility: Independent genetic signals in the CIITA-CLEC16A-SOCS1 gene complex. Hum. Mol. Genet. 2011, 20, 3517–3524. [Google Scholar] [CrossRef]
- Inazawa, J.; Itoh, N.; Abe, T.; Nagata, S. Assignment of the human Fas antigen gene (Fas) to 10q24.1. Genomics 1992, 14, 821–822. [Google Scholar] [CrossRef]
- Haas, J.; Fritzsching, B.; Trubswetter, P.; Korporal, M.; Milkova, L.; Fritz, B.; Vobis, D.; Krammer, P.H.; Suri-Payer, E.; Wildemann, B. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J. Immunol. 2007, 179, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Schumacher, M.; Pfaff, D.; Schumacher, K.; Jarius, S.; Balint, B.; Wiendl, H.; Haas, J.; Wildemann, B. Fine-tuning of regulatory T cell function: The role of calcium signals and naive regulatory T cells for regulatory T cell deficiency in multiple sclerosis. J. Immunol. 2013, 190, 4965–4970. [Google Scholar] [CrossRef] [PubMed]
- Häusler, D.; Hajiyeva, Z.; Traub, J.W.; Zamvil, S.S.; Lalive, P.H.; Bruck, W.; Weber, M.S. Glatiramer acetate immune modulates B-cell antigen presentation in treatment of MS. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e698. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.A.; Callow, M.G.; Waldbaum, L.; Thomas, S.; Ruggieri, R. MRK, a mixed lineage kinase-related molecule that plays a role in gamma-radiation-induced cell cycle arrest. J. Biol. Chem. 2002, 277, 13873–13882. [Google Scholar] [CrossRef]
- Liu, T.C.; Huang, C.J.; Chu, Y.C.; Wei, C.C.; Chou, C.C.; Chou, M.Y.; Chou, C.K.; Yang, J.J. Cloning and expression of ZAK, a mixed lineage kinase-like protein containing a leucine-zipper and a sterile-alpha motif. Biochem. Biophys. Res. Commun. 2000, 274, 811–816. [Google Scholar] [CrossRef]
- Hasson, T.; Kolitz, S.; Towfic, F.; Laifenfeld, D.; Bakshi, S.; Beriozkin, O.; Shacham-Abramson, M.; Timan, B.; Fowler, K.D.; Birnberg, T.; et al. Functional effects of the antigen glatiramer acetate are complex and tightly associated with its composition. J. Neuroimmunol. 2016, 290, 84–95. [Google Scholar] [CrossRef]
- Von Salome, J.; Gyllensten, U.; Bergstrom, T.F. Full-length sequence analysis of the HLA-DRB1 locus suggests a recent origin of alleles. Immunogenetics 2007, 59, 261–271. [Google Scholar] [CrossRef]
- Lundberg, A.S.; McDevitt, H.O. Evolution of major histocompatibility complex class II allelic diversity: Direct descent in mice and humans. Proc. Natl. Acad. Sci. USA 1992, 89, 6545–6549. [Google Scholar] [CrossRef]
- Gross, R.; Healy, B.C.; Cepok, S.; Chitnis, T.; Khoury, S.J.; Hemmer, B.; Weiner, H.L.; Hafler, D.A.; De Jager, P.L. Population structure and HLA DRB1 1501 in the response of subjects with multiple sclerosis to first-line treatments. J. Neuroimmunol. 2011, 233, 168–174. [Google Scholar] [CrossRef]
- Saxe, D.F.; Takahashi, N.; Hood, L.; Simon, M.I. Localization of the human myelin basic protein gene (MBP) to region 18q22→qter by in situ hybridization. Cytogenet. Genome Res. 1985, 39, 246–249. [Google Scholar] [CrossRef]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. 2006, 63, 1945–1961. [Google Scholar] [CrossRef]
- Glatiramer Acetate. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- McAndrew, P.E.; Frostholm, A.; White, R.A.; Rotter, A.; Burghes, A.H. Identification and characterization of RPTP rho, a novel RPTP mu/kappa-like receptor protein tyrosine phosphatase whose expression is restricted to the central nervous system. Mol. Brain Res. 1998, 56, 9–21. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Parsons, D.W.; Bardelli, A.; Sager, J.; Szabo, S.; Ptak, J.; Silliman, N.; Peters, B.A.; van der Heijden, M.S.; et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science 2004, 304, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Perelman, B.; Dafni, N.; Naiman, T.; Eli, D.; Yaakov, M.; Feng, T.L.; Sinha, S.; Weber, G.; Khodaei, S.; Sancar, A.; et al. Molecular cloning of a novel human gene encoding a 63-kDa protein and its sublocalization within the 11q13 locus. Genomics 1997, 41, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Hao, Z.; Itsumi, M.; Abouelkheer, Y.; Brenner, D.; Gao, Y.; Wakeham, A.; Hong, C.; Li, W.Y.; Sylvester, J.; et al. Autophagy-independent functions of UVRAG are essential for peripheral naive T-cell homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
| Gene | Year | N | Ethnicity | Polymorphism | Overall Response Rate | PMID | ||
|---|---|---|---|---|---|---|---|---|
| p-Value | OR (95% CI) | Genotype Associated | ||||||
| ALOX5AP | 2017 | 639 | Multinational * | rs10162089 | 0.008 | 1.56 | T | 28569182 |
| 532 | Multinational * | rs10162089 | 0.032 | 1.58 | T | |||
| CD86 | 2007 | 35 | Belgium, Canada, The Netherlands, Italy, and the UK | rs1129055 | 0.022 | 6.28 (1.3–30.3) | C | 17622942 |
| 48 | Belgium, Canada, The Netherlands, Italy, and the UK | rs2001791 | 0.062 | 8.3 (0.9–77.0) | T | |||
| CLEC16A | 2017 | 296 | Russian | rs6498169 | 0.025 | 2.38 (1.08–5.27) | A | 29095108 |
| CTSS | 2007 | 43 | Belgium, Canada, The Netherlands, Italy, and the UK | rs2275235 | 0.014 | 11.59 (1.6–81.9) | G | 17622942 |
| 47 | Belgium, Canada, The Netherlands, Italy, and the UK | rs1415148 | 0.009 | 6.85 (1.6–29.2) | A | |||
| EOMES | 2017 | 296 | Russian | rs2371108 | 0.018 | 2.00 (1.09–3.66) | T | 29095108 |
| FAS | 2007 | 47 | Belgium, Canada, The Netherlands, Italy, and the UK | rs982764 | 0.050 | 2.97 (1.0–8.8) | C | 17622942 |
| IL1RL1 | 2007 | 48 | Belgium, Canada, The Netherlands, Italy, and the UK | rs956730 | 0.025 | 5.81 (1.2–27.1) | A | 17622942 |
| IL12RB2 | 2007 | 34 | United States of America | rs946685 | 0.027 | 0.24 (0.07–0.85) | G | 17622942 |
| IL22RA2 | 2017 | 296 | Russian | rs202573 | 0.008 | 2.08 (1.18–7.41) | GG | 29095108 |
| HLA-DRB1 | 2011 | 332 | United States of America | rs3135388 | 0.015 | 2.7 (1.2–6.0) | AA | 21115201 |
| MAGI2 | 2017 | 639 | Multinational * | rs16886004 | 0.002 | 2.15 | A | 28569182 |
| 532 | Multinational * | rs16886004 | <0.001 | 5.56 | A | |||
| MBP | 2007 | 32 | Belgium, Canada, The Netherlands, Italy, and the UK | rs470929 | 0.040 | 5.3(1.1–25.9) | T | 17622942 |
| 2017 | 639 | Multinational * | rs1789084 | 0.036 | 0.7 | T | 28569182 | |
| PTPRT | 2017 | 639 | Multinational * | rs1117602254 | 0.004 | 0.21 | C | 28569182 |
| 532 | Multinational * | rs1117602254 | 0.016 | 0.28 | C | |||
| PVT1 | 2017 | 296 | Russian | rs2114358 | 0.005 | 2.77 (1.33–5.77) | A | 29095108 |
| RFPL3 | 2017 | 532 | Multinational * | rs1789084 | 0.010 | 0.57 | C | 28569182 |
| 532 | Multinational * | rs73166319 | <0.001 | 0.12 | C | |||
| SLC1A4 | 2017 | 639 | Multinational * | rs759458 | <0.001 | 3.31 | G | 28569182 |
| 532 | Multinational * | rs759458 | 0.049 | 1.86 | G | |||
| TRBC | 2007 | 31 | Belgium, Canada, The Netherlands, Italy, and the UK | rs71878 | 0.015 | 6.8 (1.45–31.9) | C | 17622942 |
| UVRAG | 2017 | 639 | Multinational * | rs80191572 | 0.002 | 0.20 | A | 28569182 |
| 532 | Multinational * | rs80191572 | <0.001 | 0.12 | A | |||
| ZAK | 2017 | 639 | Multinational * | rs139890339 | <0.001 | 0.05 | C | 28569182 |
| 532 | Multinational * | rs139890339 | 0.011 | 0.14 | C | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarzuelo-Romero, M.J.; Pérez-Ramírez, C.; Cura, Y.; Carrasco-Campos, M.I.; Marangoni-Iglecias, L.M.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Influence of Genetic Polymorphisms on Clinical Outcomes of Glatiramer Acetate in Multiple Sclerosis Patients. J. Pers. Med. 2021, 11, 1032. https://doi.org/10.3390/jpm11101032
Zarzuelo-Romero MJ, Pérez-Ramírez C, Cura Y, Carrasco-Campos MI, Marangoni-Iglecias LM, Ramírez-Tortosa MC, Jiménez-Morales A. Influence of Genetic Polymorphisms on Clinical Outcomes of Glatiramer Acetate in Multiple Sclerosis Patients. Journal of Personalized Medicine. 2021; 11(10):1032. https://doi.org/10.3390/jpm11101032
Chicago/Turabian StyleZarzuelo-Romero, María José, Cristina Pérez-Ramírez, Yasmín Cura, María Isabel Carrasco-Campos, Luciana María Marangoni-Iglecias, María Carmen Ramírez-Tortosa, and Alberto Jiménez-Morales. 2021. "Influence of Genetic Polymorphisms on Clinical Outcomes of Glatiramer Acetate in Multiple Sclerosis Patients" Journal of Personalized Medicine 11, no. 10: 1032. https://doi.org/10.3390/jpm11101032
APA StyleZarzuelo-Romero, M. J., Pérez-Ramírez, C., Cura, Y., Carrasco-Campos, M. I., Marangoni-Iglecias, L. M., Ramírez-Tortosa, M. C., & Jiménez-Morales, A. (2021). Influence of Genetic Polymorphisms on Clinical Outcomes of Glatiramer Acetate in Multiple Sclerosis Patients. Journal of Personalized Medicine, 11(10), 1032. https://doi.org/10.3390/jpm11101032







